Abstract
Impulsive personalities are considered to have a general impairment in cognitive flexibility and cortical inhibition. To examine this hypothesis we used a trial by trial Stroop task in impulsive and non impulsive patients with Parkinson’s disease (PD) and recorded errors and reaction times (RT). We tested 28 impulsive PD (PD+ICB) and 24 non impulsive PD (PD-ICB) patients prior to and after dopaminergic medication. These results were compared with 24 age matched normal controls. We found an increased error rate in all PD patients prior to their usual medication which resolved after medication. Furthermore patients on medication showed enhanced cognitive flexibility and shorter RT. There was no difference between non impulsive and impulsive PD patients. This suggests that the impulsive behaviours may not affect response inhibition tasks and the response inhibition required in the Stroop test does not engage the same processes that differentiate impulsive and non-impulsive PD patients, which likely involve mesolimbic dopamine.
Introduction
The Stroop Colour Word test is a simple but reliable and well researched test for examining cognitive flexibility. The task requires participants to respond to the ink colour and suppress the more familiar word identity. Whilst responses in congruent settings are relatively automatic, incongruency between the letters and ink colour requires keen attention and leads to slower responses.
Impairment in the Stroop test has been described in patients with frontal lobe damage, drug abusers [1], patients with schizophrenia [2] and patients with Parkinson’s disease (PD) [3]. However, an item by item Stroop test has never been used in PD patients who have developed impulsive compulsive behaviours (ICB) such as pathological gambling, compulsive shopping, hypersexuality, and binge eating. These patients have poorer working memory [4] but it is unclear whether fast cognitive updating as required in the Stroop test will be also impaired. As dopaminergic medications are strongly implicated in the development of ICBs [5], we tested all patients before and after dopaminergic medication to assess its effect on cognitive flexibility. We anticipated that PD patients with ICBs (PD+ICB) would perform worse than non impulsive PD (PD-ICB) patients and normal controls on a task that requires inhibition of competing responses. We also thought that all patients would show improvement in cognitive flexibility, reflecting an improved ability to respond to changing task demands, after dopaminergic medication.
Patients and methods
Twenty-four PD-ICB, 28 PD+ICB patients and 24 normal controls were tested on an item by item Stroop test. The presence of ICBs was defined using proposed criteria [5, 6]. Most PD+ICB patients had more than 1 addictive behaviour, which is in line with the hypothesis that all ICBs share common risk factors regardless of their type of impulsive compulsive behaviour [7]. The ICBs included compulsive sexual behaviour (13 patients), pathological gambling (11 patients), compulsive buying (8 patients), punding (4 patients) and kleptomania (1 patient). None of the patients was clinically depressed at the time of testing and only 4 out of 28 PD+ICB patients and 2 out of 24 PD-ICB patients were taking antidepressant medications. See table 1. All patients were recruited from the National Hospital for Neurology and Neurosurgery Queen Square, London. Normal controls were mainly recruited from amongst the patient’s partners. Participants who provided written informed consent to protocols approved by the UCLH Trust local ethics committee were included. Patients who scored under 27/30 points on the Mini Mental State Examination were excluded. Testing was done in a quiet environment either in the patient’s home or in a hotel room using a laptop computer and a microphone. The patient groups were matched for disease duration, motor disability and medication.
Table 1.
| Controls | PD+ICB | PD-ICB | t value except * and ** |
p-value | |
|---|---|---|---|---|---|
| Participants (no.) | 24 | 28 | 24 | ||
| Age (yrs) | 57.8 ± 10.7 | 54.6 ± 9.2 | 64,2 ±10.1 | F = 7.0 ** | = 0.002 |
| Gender (male) | 14 | 21 | 21 | χ 2 = 5.3 * | =0.071 |
| At disease onset | - | 44.5 ± 8.7 | 52.5 ± 9.6 | t = 3.1 | =0.03 |
|
| |||||
| Disease duration (yrs) | - | 10.1 ± 5.5 | 11.7 ± 7.2 | t = 0.88 | =0.39 |
| Education (yrs) | 13.2 ± 2.9 | 13.4 ± 3.0 | 14.7 ± 3.6 | F = 1.7 ** | =0.18 |
|
| |||||
| LEU dose(mg/day) | - | 832 ± 425 | 821 ± 400 | t = 0.1 | =0.9 |
| DA (patients) | - | 14 | 16 | χ 2 = 1.4 | =0.27 |
| Antidepressants (patients) |
- | 4 | 2 | χ 2 = 0.45 | =0.4 |
|
| |||||
| UPDRS on | - | 15.5 ± 8.3 | 14.4 ± 5.8 | t = 0.5 | =0.6 |
| UPDRS off | - | 27.3 ± 9.1 | 26.8 ± 6.7 | t = 0.2 | =0.8 |
| Improvement in UPRDS (%) |
- | 43.2 | 46.2 | ||
|
| |||||
| Gambling | - | 11 | - | ||
| Hypersexuality | - | 13 | - | ||
| Shopping | - | 8 | - | ||
| Punding | - | 4 | - | ||
| Kleptomania | - | 1 | - | ||
UPDRS = Unified Parkinson’s Disease Rating Scale; LEU = L-dopa equivalent units; DA = dopamine agonists. All values are mean ± SD.
= Chi-square
= F value.
PD patients were tested in either an “on” or “off” medication state in a counterbalanced order. Results were compared with 24 normal controls who were matched to the PD+ICB group.
Patients who were tested “off” first performed the task between 8.00am and 9.00am and had not taken their medication for at least 12 hours. They were then retested in their “on medication” state 1 hour after taking their first dopaminergic medication of the day. Patients who were tested ‘on medication’ first did this task usually in mid-morning at a similar time of the day when their symptoms were well controlled. They were re visited on the following day prior to their medication for the second test, again between 8.00am and 9.00 am. All patients had an excellent L-dopa response which was assessed by the UPDRS (part 3) motor score. Levodopa equivalent units (LEU) were calculated as described previously [6].
To account for age related differences in performance we used an item by item Stroop test consisting of four colours (green, red, blue or yellow) and measured reaction time for each trial separately. Each word appeared centrally on a black background. Participants were asked to name the colour of the word as quickly as possible and had a maximum of 4 sec to respond. Sixteen trials were recorded, 8 were congruent and 8 incongruent in a pseudo randomized order, giving four possible patterns of testing namely incongruent followed by incongruent trial, congruent by incongruent, incongruent by congruent and congruent by congruent trials (Fig 1C). A standard microphone (Logitech) was used for recording responses at 6 Khz. Reaction time (RT) was computed by finding significant (p<0.01) deviations of the recorded variance in the speech signal, relative to a 200 ms initial baseline.
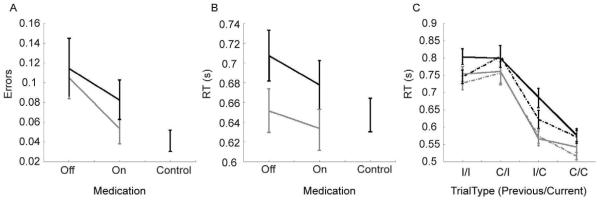
Fig. 1.
Behavioural results. A. Error rates for each subject group. Black=PD+ICB, grey=PD-ICB. B. Reaction times. C. Reaction times for switch and non-switch trials in the patient group. Solid lines=off medication, dotted lines=on medication, I=incongruent, C=congruent.
Statistical analysis
A mixed model ANOVA was performed. The dependent variable was either the error rate or the reaction time, averaged by condition. Condition (off vs. on and 1st and 2nd session in normal controls) were modelled as within subject factors and group (PD-ICB, PD+ICB and normal controls) was modelled as a between subject factor. Subject was included as a random factor. Since there was a significant age-difference between the groups we have added age as a cofactor in all analyses.
Results
We found a significant effect of age between the 3 groups (F(2,74)=7.0, p=0.002). Post hoc analysis revealed that the PD-ICB group was older than the PD+ICB (p=0.001) and a trend to be older than the control group (p=0.058). We found a significant effect of age of onset between the patient groups (t49=3.1, p=0.03). There was no difference in the LEU dose, disease duration and UPDRS motor score (part 3), across the groups (Table 1).
Analysis of Stroop test
We compared the PD-ICB and PD+ICB groups off and on medication to normal controls, pair-wise (Bonferroni corrected for 4 comparisons). For errors (Fig. 1A) we found a main effect of group for PD-ICB patients off medication vs. normal controls (F(1, 34)=7.18, p=0.037) and ICB off medication vs. normal controls (F(1, 36)=8.25, p=0.022). Thus, off medication all patients made more errors than normal controls, but on medication there was no difference between patients and normal controls (p > 0.05). In all cases there were significant effects of congruency, i.e. whether the trial was congruent or incongruent (p<0.01). There were no other significant effects or interactions. For RT there were no significant differences between groups (Fig. 1B).
Comparing PD-ICB and PD+ICBs on and off medication showed a main effect of congruency (F(1, 164)=51.82, p<0.001) and an effect of medication (F(1, 164)=3.89, p=0.050), but no main effect of group (F(1, 39)=0.21, p=0.649) on the error rates. There were no significant interactions (p>0.535). For RT there were no significant main effects or interactions (p>0.153).
The data for the PD-ICB and PD+ICB subjects was then split depending on whether the trial followed a trial of the same type, or switched (i.e. congruent followed by congruent, or congruent followed by incongruent, etc.) to examine cognitive flexibility (Fig. 1C). Thus, in addition to a main effect of congruency we included an effect of switch vs. nonswitch, which reflected the previous trial type. For errors there was no main effect of group (F(1, 32)=0.04, p=0.847) and the main effect of medication just missed significance (F1(1, 1402)=3.66, p=0.056). There was a main effect of congruency (F(1, 1402)=15.69, p<0.001) and a switch by congruent interaction (F(1, 1402)=13.85, p<0.001). For RT there was no significant effect of group (F(1, 35)=2.35, p=0.135), but there was a main effect of medication (F(1,1406)=7.41, p=0.007) and a switch by congruent interaction (F(1, 1406)=5.69, p=0.017).
Discussion
We found a significant Stroop interference effect in all participants. Furthermore we demonstrated that all PD patients made more errors than normal controls when off medication. We found that in their “on state” patients had a shorter RT on switching behaviour between congruent and incongruent trials, in keeping with previous studies [8, 9]. Our findings are also consistent with previous studies showing improvement of Stroop performance in PD patients with and without deep brain stimulation [9,10].
There was no difference in RT between PD patients and normal controls in keeping with previous studies [10]. Further patients on medication showed a trend to be slower in RT in congruent trials followed by incongruent trials compared to incongruent trials followed by incongruent trials (Fig. 1). This might be explained by an increased awareness caused by the previous conflicting trial. We found no difference in error rates between the patient groups which implies that the inability to suppress automatic responses and the inability to suppress, for example, the urge to gamble depend on different processes and neural systems. Our findings are also in line with two other studies which have shown no impairment on the Frontal Assessment Battery scores in PD patients with pathological gambling compared to those without ICBs [11,12]. Thus, ICBs seem to be unimpaired in tasks that are mediated by frontal cortex, as for example occurs also with response suppression tasks [13]. Our results are also consistent with another study done in PD patients with pathological gambling, which showed impairment in a risk assessment task but not in other cognitive domains [14]. Our study extends the current literature and demonstrates there is no difference in cognitive flexibility between PD controls and patients with impulse control disorders irrespective of the type.
Furthermore performance of the Stroop test might not trigger mesolimbic dopamine release and could fail to activate limbic and reward centres of the brain which are known to be abnormal in PD+ICB patients. In line with this notion is the finding that there was no correlation between amygdala activation and Stroop performance [15]. Since cognitive performance may vary during the day [16], we tested patients in their “off” condition on average at about 8.30 am and patients of the “on” group at a similar time point, on average at 10.30 am.
There are however some limitations in our study. Patients who were tested first’ on’ then’ off’ were tested on separate days, whereas patients tested first off and then on were tested on the same day. Thus, the test-retest interval differed between the two groups and conceivably might have influenced the results. However, our significant results are within-subject effects comparing off vs. on, and they did not depend on the order of testing.
Future work using an emotionally charged Stroop test, which is more likely to activate the limbic system, could potentially demonstrate differences between the two PD groups.
Acknowledgement
The authors wish to thank the patients and families who participated in the study. This work was supported in part by the Intramural Research Program of the NIH, NIMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Additional references included at request of reviewers.
- [1].Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. J Addict Dis. 2002;21(1):61–74. doi: 10.1300/j069v21n01_06. [DOI] [PubMed] [Google Scholar]
- [2].Barch DM, Carter CS, Cohen JD. Factors influencing Stroop performance in schizophrenia. Neuropsychology. 2004 Jul;18(3):477–84. doi: 10.1037/0894-4105.18.3.477. [DOI] [PubMed] [Google Scholar]
- [3].Hsieh YH, Chen KJ, Wang CC, Lai CL. Cognitive and motor components of response speed in the stroop test in Parkinson’s disease patients. Kaohsiung J Med Sci. 2008 Apr;24(4):197–203. doi: 10.1016/S1607-551X(08)70117-7. [DOI] [PubMed] [Google Scholar]
- [4].Djamshidian A, Jha A, O’Sullivan S, Silveira-Moriyama L, Jacobson C, Lees A, et al. Risk and learning in impulsive and non impulsive patients with Parkinson’s disease. Mov Disord. 2010 Oct 15;25(13):2203–10. doi: 10.1002/mds.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Voon V, Thomsen T, Miyasaki JM, de Souza M, Shafro A, Fox SH, et al. Factors associated with dopaminergic drug-related pathological gambling in Parkinson disease. Arch Neurol. 2007 Feb;64(2):212–6. doi: 10.1001/archneur.64.2.212. [DOI] [PubMed] [Google Scholar]
- [6].Evans AH, Katzenschlager R, Paviour D, O’Sullivan JD, Appel S, Lawrence AD, et al. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004 Apr;19(4):397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- [7].Torta DM, Castelli L. Reward pathways in Parkinson’s disease: clinical and theoretical implications. Psychiatry Clin Neurosci. 2008 Apr;62(2):203–13. doi: 10.1111/j.1440-1819.2008.01756.x. [DOI] [PubMed] [Google Scholar]
- [8].Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41(11):1431–41. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- [9].Jahanshahi M, Ardouin CM, Brown RG, Rothwell JC, Obeso J, Albanese A, et al. The impact of deep brain stimulation on executive function in Parkinson’s disease. Brain. 2000 Jun;123(Pt 6):1142–54. doi: 10.1093/brain/123.6.1142. [DOI] [PubMed] [Google Scholar]
- [10].Fera F, Nicoletti G, Cerasa A, Romeo N, Gallo O, Gioia MC, et al. Dopaminergic modulation of cognitive interference after pharmacological washout in Parkinson’s disease. Brain Res Bull. 2007 Sep 14;74(1-3):75–83. doi: 10.1016/j.brainresbull.2007.05.009. [DOI] [PubMed] [Google Scholar]
- [11].Siri C, Cilia R, De Gaspari D, Canesi M, Meucci N, Zecchinelli AL, et al. Cognitive status of patients with Parkinson’s disease and pathological gambling. J Neurol. Feb;257(2):247–52. doi: 10.1007/s00415-009-5301-5. [DOI] [PubMed] [Google Scholar]
- [12].Voon V, Thomsen T, Miyasaki JM, de Souza M, Shafro A, Fox SH, et al. Factors associated with dopaminergic drug-related pathological gambling in Parkinson disease. Arch Neurol. 2007 Feb;64(2):212–6. doi: 10.1001/archneur.64.2.212. [DOI] [PubMed] [Google Scholar]
- [13].Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999 Nov 11;402(6758):179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- [14].Rossi M, Gerschcovich ER, de Achaval D, Perez-Lloret S, Cerquetti D, Cammarota A, et al. Decision-making in Parkinson’s disease patients with and without pathological gambling. Eur J Neurol. Jan;17(1):97–102. doi: 10.1111/j.1468-1331.2009.02792.x. [DOI] [PubMed] [Google Scholar]
- [15].Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007 Jun 1;61(11):1306–9. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].West R, Murphy KJ, Armilio ML, Craik FI, Stuss DT. Effects of time of day on age differences in working memory. J Gerontol B Psychol Sci Soc Sci. 2002 Jan;57(1):P3–10. doi: 10.1093/geronb/57.1.p3. [DOI] [PubMed] [Google Scholar]