Abstract
Adenosine receptors are a member of the large family of seven transmembrane spanning G protein coupled receptors (GPCR). The four adenosine receptor subtypes – A1, A2a, A2b, A3 – exert their effects via the activation of one or more heterotrimeric G proteins resulting in the modulation of intracellular signaling. Numerous studies over the past decade have documented the complexity of GPCR signaling at the level of protein-protein interactions as well as through signaling crosstalk. With respect to adenosine receptors the activation of one receptor subtype can have profound direct effects in one cell type, but little or no effect in other cells. There is significant evidence that the compartmentation of subcellular signaling plays a physiological role in the fidelity of GPCR signaling. This compartmentation is evident at the level of the plasma membrane in the form of membrane microdomains such as caveolae and lipid rafts. This review will summarize and critically assess our current understanding of the role of membrane microdomains in regulating adenosine receptor signaling.
Keywords: Adenosine receptors, Lipid rafts, Caveolae, Caveolin, cholesterol
1. Introduction
Adenosine, a purine nucleoside catabolite of ATP, exerts numerous effects in mammalian organ systems. Adenosine can modulate cell metabolism via several mechanisms, with the most direct being its rephosphorylation to AMP via adenosine kinase to help restore/maintain ATP levels. Adenosine however is best known for regulating cell function via the activation of four distinct purinergic P1 adenosine receptor (AR) subtypes – A1, A2a, A2b, A3 – which are part of the large family of seven transmembrane spanning G protein coupled receptors (GPCR) (1,2). The A1 and A2a subtypes are high-affinity receptors, whereas A2bAR and A3AR are low affinity receptors. Thus adenosine can exert physiological effect under basal conditions as well as conditions of stress and inflammation when extracellular adenosine levels increase.
Adenosine receptors couple to multiple G proteins and activate various intracellular signaling pathways (3,4). Many cell types express multiple adenosine receptor subtypes, but in some cell types activation of these receptors exerts few effects, while in others the same receptors produce profound effects. For example A1AR activation decreases cAMP in adipocytes [5] and increases intracellular calcium in smooth muscle cells [6,7], but in cardiac ventricular myocytes A1AR appears to exert little, if any, direct effects on these parameters [8,9]. Interestingly in cardiomyocytes A1AR significantly reduces these same parameters during β1-adrenergic receptor stimulation resulting in the well known A1AR anti-adrenergic effect [8,9]. There are also numerous reports that adenosine receptors can heterodimerize to alter cell signaling [10–13]. These observations, as well as similar reports on other receptors, indicate that GPCR signaling is very complex, and multiple mechanisms appear to be capable of controlling the fidelity of signaling.
2. Membrane microdomains
One mechanism proposed for the regulation of subcellular signaling is compartmentation at the level of cell membranes. These membrane microdomains, more commonly referred to as lipid rafts, are highly enriched in glycosylphosphatidylinositol (GPI)-anchored proteins, sphingolipids, and cholesterol imparting on them less fluidity as well as being relatively resistant to solubilization by non-ionic detergents, such as Triton X-100, at cold temperatures [14–18]. A specialized type of lipid raft is characterized by the structural protein caveolin which imparts a flask-shaped invagination (50 –100 nm) of the membrane. These microdomains are referred to as caveolae. There are three isoforms of caveolin, referred to as caveolin-1, -2, and -3, which exhibit cell-specific expression patterns. For example caveolin-1 is highly expressed in endothelial cells, but has little, if any expression in cardiac ventricular myocytes, whereas the opposite expression profile is seen for caveolin-3 [15, 19].
In addition to directly binding cholesterol caveolin modulates signal transduction by serving as a scaffold for numerous proteins, some of which possess caveolin binding motifs [20–25]. Numerous second messengers such as heterotrimeric G proteins, eNOS, extracellular regulated mitogen activated protein kinase (ERK), PKC isoforms, and adenylyl cyclase have been shown to be localized and/or concentrated in caveolae and lipid rafts. There have also been numerous reports that several GPCR are present in caveolae [25–28]. The co-localization of GPCR and second messengers in microdomains may permit the rapid and selective activation or deactivation of intracellular signaling as well as controlling its compartmentation.
Lipid rafts and caveolae can be isolated by various techniques. Due to their high concentration of cholesterol and resistance to non-ionic detergents these membrane microdomains can be isolated by differential centrifugation. The two most commonly cited methods based on these principles are the techniques of Song et al [20] and Smart et al [29]. The former method relies on membrane solubilization with high pH (9.0) sodium carbonate; the resulting homogenate is separated into multiple fractions (10–12) using discontinuous sucrose gradient centrifugation. The method of Smart et al [29] utilizes Percoll gradient centrifugation followed by Optiprep gradient separation of caveolae membranes from bulk plasma membranes. A supplemental approach for verifying the localization of proteins in caveolae is to perform co-immunoprecipitation studies in caveolin-enriched fractions.
3. Adenosine receptors and membrane microdomains
Over the past decade significant evidence has accumulated that adenosine receptor signaling may be regulated via membrane microdomains. Such regulation may include localization of adenosine receptors in caveolae or lipid rafts, modulation of signaling, and trafficking. The initial evidence for this concept appears to be observations by Andersson-Forsman and Gustafsson in 1985 that ecto-5'-nucleotidase (CD73) in various types of guinea-pig smooth muscle was localized in caveolae [30]. Several subsequent studies have provided additional evidence for such a localization [31–33]. Ecto-5'-nucleotidase, a GPI-anchored protein dephosphorylates extracellular AMP to adenosine, and since adenosine is rapidly catabolized to inosine, close proximity of extracellular adenosine to adenosine receptors would provide optimal receptor activation. Such a mechanism was proposed by Anderson in 1993 [14]. There are also reports that concentrative nucleoside transporters-1 and -3 are located in lipid rafts/caveolae [34,35]. Finally it has been reported that all four human adenosine receptor subtypes contain portions of the caveolin binding motif [36]. This review will provide a critical analysis of the evidence for adenosine receptor subtype localization in membrane microdomains in various cell types.
3.1 A1 adenosine receptors and membrane microdomains
The adenosine receptor subtype first reported to be localized in lipid rafts/caveolae, and for which the most evidence exists for such a localization, is the A1 adenosine receptor (A1AR). Our laboratory reported in 2000 that A1AR were concentrated in caveolae of adult rat ventricular cardiomyocytes [37]. Using the caveolae isolation methods of Smart et al [29] the caveolin-3 buoyant low-density fraction contained < 0.4% of the protein found in the initial postnuclear supernatant, but was > 7-fold enriched in cholesterol compared to the bulk plasma membrane (PM). As shown in Figure 1A the caveolae membranes (CM) were enriched in caveolin-3 and eNOS, but were devoid of transferrin receptors (TR) and clathrin. Figure 1B illustrates that under basal conditions (Buffer) the majority of the A1AR immunoreactivity (using a rabbit polyclonal antibody raised against the third extracellular domain of the rat A1AR receptor gene (amino acids 163–176)) was located in the caveolae membranes. Cardiomyocytes were treated with adenosine deaminase (ADA) to exclude possible stimulation of the A1 receptor by endogenous adenosine.
Figure 1.
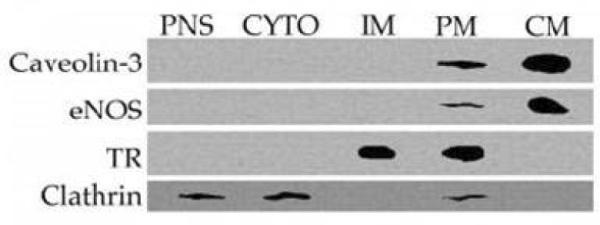
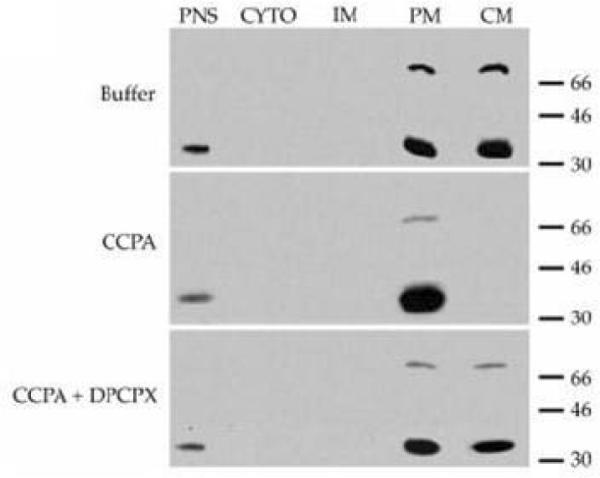
Cardiomyocyte A1AR localization in caveolae membranes (CM). Figure 1A illustrates that caveolin-3 and eNOS in adult rat ventricular myocytes are enriched in CM compared to bulk plasma membranes (PM). CM are devoid of transferrin receptors (TR) which are located in intracellular membranes (IM) and clathrin. Figure 1B indicates that in unstimulated myocytes (Buffer) the majority of A1AR immunoreactivity is in CM vs PM. After stimulation with the A1 agonist CCPA (200 nM, 15 min) A1AR immunoreactivity was only present in PM. Treatment with the A1AR antagonist DPCPX (200 nM) + CCPA blocked the translocation of A1AR. PNS, postnuclear supernatant; CYTO, cytosol. This research was originally published in Journal of Biological Chemistry, Lasley et al. Activated cardiac adenosine A1 receptors translocate out of caveolae. 2000;275: 4417–4421. © the American Society for Biochemistry and Molecular Biology.
Treatment of myocytes with the A1 agonist 2-chloro-N6-cyclopentyadenosine (CCPA, 200 nM, 15 min) resulted in the loss of all A1AR immunoreactivity from caveolin-3 enriched fractions to the bulk plasma membrane fraction, an effect that was prevented by prior treatment of the myocytes with the A1AR antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, 200 nM). Adenosine A1AR were co-immunoprecipitated with caveolin-3 in unstimulated cells but not after agonist exposure. Using immunofluorescence we have observed similar staining patterns for caveolin-3 (Figure 2A) and A1AR (Figure 2B) in unstimulated rat cardiomyocytes (unpublished observations).
Figure 2.
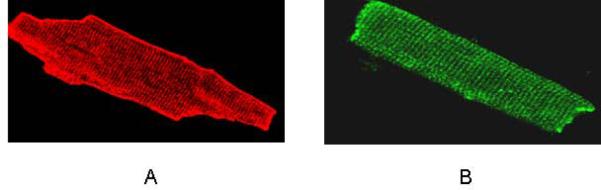
Immunofluorescence evidence of colocalization of caveolin-3 (A) and A1AR (B) in adult rat ventricular myocytes. Myocytes were fixed with paraformaldehyde, permeabilized and incubated with either a mouse monoclonal antibody for caveolin-3 (A) or rabbit polyclonal anti-A1AR (B) followed by the appropriate fluorescently labeled secondary antibody.
Our observations regarding A1AR concentration in caveolin-3 enriched fractions were subsequently reported by two other groups [38,39]. Cavalli et al [38], who solubilized rat myocardial membranes with both the sodium carbonate method of Song et al [20] and also Triton X-100, observed that A1AR co-immunoprecipitated with caveolin-3, and A1AR immunofluorescence co-localized with caveolin-3 immunofluorescence in cardiomyocytes, similar to our observations in Figure 2. Garg et al [39] reported that A1AR could be co-immunoprecipitated with caveolin-3 in adult rat ventricular cardiomyocytes. Thus using all two well-recognized techniques of isolating caveolae and lipid rafts, as well as co-immunoprecipitation and immunofluorescence, myocardial A1AR appear to be concentrated in caveolae under basal conditions.
Concurrent with our observations Murthy and Makhlouf [40] provided the initial evidence that A1AR stimulation could modulate signaling in caveolae. These authors, using the sodium carbonate method of Song et al [20] to isolate caveolae in rabbit intestinal smooth muscle cells, reported that Gαq/11, Gαi1/2, and Gαi3 were present in caveolin-3 enriched fractions as well as heavier fractions. The A1 agonist cyclopentyladenosine (CPA, 1μM, 10 min) selectively activated Gαi3 and resulted in a significant increase in the amount of Gαi3 (as well as Gβγ) that could be immunoprecipitaed with caveolin-3 in both whole cell lysates and caveolin-enriched fractions. A1 agonist treatment in these cells was associated with increased PLC-β activity, and pretreatment with CPA or acetylcholine significantly blunted the subsequent response to CPA. The authors concluded that this blunted response was due to receptor desensitization as well as binding of Gαi3 to caveolin-3. Interestingly the authors observed that CPA had no effect on phosphatidylinositol 4, 5-bisphosphate (PIP2) levels in Triton X-100 soluble fractions but decreased by 34% in Triton X-100 insoluble fractions. Since caveolin is relatively resistant to Triton solubilization, the authors concluded that PLC-β activity was increased in caveolar fractions.
Despite the multiple observations that cardiac A1AR are localized in caveolae membranes, the role that this specific localization plays in cardiac A1AR signaling remains unclear. Adenosine A1 receptor activation in normal cardiomyocytes exerts no direct effects on contractility, intracellular calcium, or cAMP [8,9]. Thus A1AR enrichment in cardiomyocyte caveolae under basal conditions could provide highly localized A1AR signaling in these cells. However in only one of the above studies was the localization of A1AR determined after agonist exposure. We observed that A1AR immunoreactivity translocated from caveolar to bulk plasma membranes, but not intracellular membranes, after A1 agonist exposure (15 min) (37). This observation suggests that cardiomyocyte A1AR do not rapidly internalize, but it is not clear what role this change in localization plays in A1AR signaling. In normal myocardium A1AR stimulation exerts a potent inhibition of β1-adrenergic contractile and biochemical responses (referred to as the A1 anti-adrenergic effect). β-adrenergic receptors, adenylyl cyclase, as well as Gαs and Gαi, are localized, at least to some extent, in caveolae in adult cardiomyocytes [41]. However the β2-adrenergic receptor is localized in caveolae to a much greater extent than β1-adrenergic receptors, which are distributed more widespread in the plasma membrane. The differential distributions of the β-adrenergic receptor subtypes in adult cardiomyocytes is consistent with their different functional effects. Activation of cardiac β2-adrenergic receptors is associated with little increases in total intracellular calcium and cAMP levels and exerts only small increases in contractility, whereas β1-adrenergic receptor stimulation produces significant increases in all of these parameters [42]. Our observations indicate that the cardiomyocyte A1AR translocates out of caveolae upon agonist exposure (37), but there have been no reports on the localization of cardiac β1-adrenergic receptors after agonist exposure. Thus the role of receptor localization in or movement out of membrane microdomains in the cardiac A1AR anti-adrenergic effect remains uncertain.
Although our observations that A1AR move out of caveolae after agonist exposure appear to be consistent with the A1AR anti-adrenergic effect, there is additional evidence that cardiac A1AR may modulate signaling in caveolae membranes and/or detergent resistant membranes. Garg et al [39] reported that A1AR and the pore-forming subunit of sarcolemmal KATP channels, Kir6.2, could be co-immunoprecipitated with caveolin-3 in cardiomyocytes. These authors also observed that the cholesterol reducing agent methyl-β-cyclodextrin (MβCD) blunted A1AR agonist-mediated activation of KATP channels. Interestingly MβCD had no effect on the KATP channel activation by the KATP channel opener pinacidil. We reported that A1AR agonist treatment of adult rat cardiomyocytes decreased phosphorylation of the mitogen activated protein kinases(MAPK) p38 and ERK in Triton X-100 insoluble membranes, but had no effect on these kinases in Triton soluble membranes [43]. Most recently Yang et al [44] reported that brief exposure of adult rat cardiomyocytes to an A1 agonist increased translocation of PKC-ε and PKC-δ into caveolin-3 enriched fractions. These observations suggest that although A1AR appear to translocate out of cardiomyocyte caveolae after agonist stimulation, A1AR signaling still occurs in these microdomains.
Finally there is evidence supporting an interaction between caveolin-3 and A1AR in diseased myocardium. It has been reported that cardiomyocyte-specific constitutive A1AR overexpression results in hypertrophy and dilatation [45]. The results of a preliminary report indicate that this cardiac pathology is associated with decreased expression of caveolin-3 and altered localization of caveolin [46]. Interestingly caveolin-3 KO mice also develop heart failure [47]. The significance of these interactions between A1AR expression and caveolin-3 levels in the observed cardiac pathology remains to be determined.
There are additional reports that A1AR may localize in and/or modulate signaling in lipid rafts/caveolae. Ginés et al [48] reported in 2001 that exposure of a porcine kidney epithelial cell line (LLC-PK1) to the A1 agonist N6-(R)-phenylisopropyl-adenosine (PIA, 100 nM, 30 min) was associated with the translocation of A1 receptors from high-density, caveolin-1 devoid fractions to lower density fractions, including caveolin-1 enriched fractions. Fractions were isolated via the detergent-free sodium carbonate method of Song et al [20]. The authors concluded that this was the mechanism by which A1 receptors were internalized in this cell type. Although the authors did not test whether the LLC-PK1 A1AR could be co-immunoprecipitated with caveolin-1, they did show that a GST-fusion protein, containing the C-terminal domain of the A1 receptor, could interact with caveolin-2. In addition the authors stated that the C terminal cytoplasmic tail of the A1 receptor contains a sequence similar to the caveolin binding domain. The primary question concerning these observations is what role this redistribution into caveolin-1 enriched fractions plays in A1AR-mediated effects on cAMP and inositol phosphate production as well as solute transport in renal epithelial cells. Thus how these observations relate to epithelial cell function remain unanswered.
The results of a subsequent study by this laboratory are consistent with the hypothesis that A1AR in DDT1MF-2 cells internalize via caveolae [49]. Using immunogold staining it was observed that under control conditions nearly all A1AR were in non-caveolin containing membranes, but within 15 min exposure to 50 nM PIA ~ 90% of immunogold staining was present in caveolin containing membranes. Continued exposure to PIA resulted in partial loss of caveolin co-localization with A1AR increasing in intracellular vesicles, such that by 19 hours all A1AR immunogold staining was in intracellular vesicles. Treatment with filipin and MβCD, but not hyperosmolar sucrose or acetic acid, blocked the internalization of A1AR. Filipin and MβCD reduce membrane cholesterol levels which are concentrated in lipid rafts and caveolae, whereas the latter two agents disrupt internalization via clathrin-coated pits. These same investigators had previously reported that in the DDT1MF-2 smooth muscle cell line the A1AR appeared to form clusters in specific locations in the plasma membrane within 5 minutes after exposure to the A1 agonist PIA (50 nM) [50]. These observations, in conjunction with the authors' previous observations regarding A1AR trafficking, indicate that initial movement into caveolae plays a role in the internalization and desensitization in DDT1MF-2 smooth muscle cells. However since there are varying reports on the time-scale of A1AR internalization and desensitization [36,51] the role of caveolae in A1AR trafficking may be cell-specific.
The above information indicates that there is significant evidence for localization and/or translocation of A1AR into and out of caveolae in muscle cells as well as epithelial cells. There is also evidence that A1AR signaling may occur in caveolin-enriched membranes. However the specific role that these observations play in the physiological effects of A1AR under normal conditions and in diseased tissue remains to be elucidated.
3.2 A2a adenosine receptors and membrane microdomains
Adenosine A2a receptors (A2aAR) couple primarily to Gαs, although there are some reports that A2aAR signal via Gαs-independent mechanisms. One of the most recognized signaling effects of A2aAR is the stimulation of adenylyl cyclase activity resulting in increased intracellular cAMP levels. There are multiple reports in the literature that both Gαs and adenylyl cyclase are present or are concentrated in lipid rafts and/or caveolae in various cell types [18, 25, 26]. In contrast there are a very limited number of reports indicating the localization of A2aAR in lipid rafts or caveolae. In fact there appears to be only one study to date providing evidence for A2aAR localization in these membrane microdomains. Mojsilovic-Petrovic et al [52] reported that A2aAR were present in lipid rafts in embryonic Sprague Dawley rat spinal cord neurons. This conclusion was based on the analysis of lipid rafts generated using Triton X-100 and discontinuous sucrose gradient centrifugation. Light fraction 2, which contained the lipid raft marker Thy-1, contained some immunoreactivity for A2aAR based on co-immunoprecipitation with anti-tyrosine kinase B receptor (TrkB) using a mouse monoclonal anti-A2aAR. However the complete distribution of A2aAR in the 10 collected fractions was not shown and co-immunoprecipitation of the A2aAR with a lipid raft marker was not shown. In addition functional evidence of A2aAR localization in lipid rafts was not provided. Given these limitatiqons it is not clear to what extent A2aAR are actually localized in lipid rafts in these cells.
Assaife-Lopes et al [53] provided some functional evidence for A2aAR modulation of signaling in lipid rafts in cultured rat embryonic cortical neurons. The A2aAR agonist CGS21680 (20 nM, 30 min) increased the localization of TrkB in lipid rafts to a greater extent than BDNF, an effect that was blocked by the A2aAR antagonist ZM24135. This effect was not altered by the clathrin-dependent endocytosis inhibitor monodansyl-cadaverine (MDC), but was blocked after treatment of cells with the cholesterol reducing agent MβCD. Although the authors did not determine whether A2aAR themselves localized in lipid rafts or translocated into/out of lipid rafts after activation, they did observe that MβCD treatment had no effect on A2aAR density and Kd values.
The results of two additional studies suggest that disrupting lipid rafts or reducing cholesterol itself can alter A2aAR signaling. Kamata et al [54] reported that a portion of Gαs in human erythrocyte membranes was localized in detergent resistant lipid rafts, and this localization was reversibly lost after brief lidocaine treatment. Lidocaine, which disassembled lipid rafts in these membranes without reducing cholesterol levels, reversibly blocked the effects of NECA on cAMP accumulation and phosphorylation of adducin (a membrane protein). Lam et al [55] reported that cholesterol reduction with MβCD in mouse colon epithelial cells potentiated A2aAR activation of a basolateral K+ channel thus increasing the driving force for anion secretion. Interestingly, neither filipin, which also decreases membrane cholesterol levels, nor sphingomyelinase, which disrupts lipid rafts via degrading sphingomyelin, mimicked the effects of MβCD. In addition the potentiating effects of A2aAR stimulation were absent in cells obtained from caveolin-1 knockout mice. The authors concluded that membrane cholesterol, but not the presence of lipid rafts or caveolae, modulated the A2aAR effect on basolateral anion secretion. The contrasting conclusions from these two studies raise questions concerning the localization of A2aAR in membrane microdomains and/or suggest that this may be cell-specific.
Thus in contrast to the significant evidence indicating that A1AR are linked to caveolae and/or lipid rafts, the support for a similar localization of the A2aAR is equivocal. The consistent finding with respect to A2aAR however is that membrane cholesterol levels do appear to modulate A2aAR signaling. Charalambous et al [56] appear to have an explanation for how this may occur. They provided evidence in PC12 cells (rat pheochromocytoma cell line), embryonic rat striatal neurons, and HEK cells (expressing tagged receptors) that A2aAR stimulation with agonist or antagonist for time periods up to 1.5 hours did not internalize. Studies in HEK cells expressing yellow fluorescence protein (YFP)-tagged A2aAR utilizing fluorescence recovery after photobleaching (FRAP) indicated that the receptor was restricted in lateral mobility independent of agonist or antagonist binding, whereas A2aAR agonist stimulation did reduce mobility of the D2-dopamine/A2aAR hetero-oligomer. The authors determined that this limited mobility of the A2aAR was due not to binding of the C-terminus to cytoskeletal actin, but rather to membrane cholesterol levels (which were reduced by filipin and MβCD). Cholesterol reduction did not alter A2aAR binding characteristics, as recently reported by Assaife-Lopes et al [53], but it did reduce the ability of the receptor to couple to Gs and thus to increase cAMP levels, without altering the ability of the A2aAR to stimulate Gs-independent ERK phosphorylation. This elegant study, demonstrating the role of cholesterol in regulation of A2aAR signaling, may help explain the inconclusive observations regarding A2aAR and membrane microdomains. Consistent with these observations Lyman et al [57] subsequently concluded that cholesterol stabilized helix II of the apo configuration of the human A2aAR.
3.3 A2bAR and A3AR and membrane microdomains
To date there is one report supporting possible A2bAR localization in lipid rafts or caveolae membrane microdomains, but there is no such evidence for A3AR. Sitaraman et al [58] studied A2bAR trafficking in T84 epithelial cells and Caco2-BBE cells stably transfected with GFP-A2bAR. Membranes were isolated by the methods of Smart et al [28]. In the T84 cell line under basal conditions the majority of the A2bAR signal was found in the postnuclear supernatant with very little signal in the caveolar or plasma membrane fractions. After stimulation with adenosine (100 μM, 5 min) there was a 2-fold increase in A2bAR in caveolin-1 enriched fractions in both basolateral and apical membranes. However the significance of this translocation was not addressed further, and the vast majority of the A2bAR translocation (7–8 fold increase) occurred to the bulk plasma membrane. Since co-immunoprecipitation studies with caveolin-1 and A2bAR were not performed, it is difficult to determine whether A2bAR actually translocated to caveolae. In contrast to these observations it has been reported that A2bAR in unstimulated transfected HEK293 cells were localized primarily in the plasma membrane and rapidly (t1/2 < 4 min) internalized, an effect that was blocked with arrestin antisense [59]. This early internalization appeared to be occurring via endosomes. Although both of these studies were conducted in cells transfected with A2bAR, these differences support the notion that adenosine receptor trafficking and signaling appear to be cell-type specific.
Although there is no evidence for A3AR localization in caveolae or lipid rafts, there is a report that these receptors may be organized in membrane microdomains. Cordeaux et al [60] studied CHO cells transfected with human A3AR. Using fluorescence correlation spectroscopy (FCS) they observed that following exposure (2.5 nM, 10 min, 22°C) to a fluorescent A3 agonist, ABEA-X-BY630, there appeared to be two populations of agonist-occupied receptors based on membrane diffusion coefficients. They speculated that the population with the slowest mobility could have been A3 receptor-agonist complexes in caveolae or clathrin coated pits. In fact there is evidence that A3AR may internalize via clathrin-coated pits [61].
4. Concluding remarks
Adenosine receptors are ubiquitous, but their effects are often cell-specific. The localization of adenosine receptors in membrane microdomains also appears to be cell- and receptor subtype-specific. There appears to be significant evidence that A1AR are localized in ventricular cardiomyocyte caveolae under basal conditions, and there are several reports that A1AR modulates signaling in these microdomains. In contrast in renal epithelial cells and smooth muscle cells A1AR appear to translocate into caveolae after agonist stimulation an effect that could be related to A1AR internalization and desensitization. Although it appears that cholesterol levels stabilize the apo-A2aAR and modulate receptor signaling, evidence that this receptor localizes in caveolae or lipid rafts is not conclusive and in some cases is contradictory. There is little, if any, evidence to date that A2bAR and A3AR are located in lipid rafts or caveolae.
Despite the evidence, or lack thereof, supporting the localization of the four adenosine receptor subtypes in membrane microdomains, much work remains to be conducted to understand the significance of these observations. For example, although there is little support for A2bAR and A3AR in lipid rafts or caveolae, all four human receptors appear to contain the caveolin binding motifs. Given the differences in expression levels of adenosine receptor subtypes in various tissues, their localization in lipid rafts/caveolae may also be cell-specific. Since receptors may move in and out of these microdomains only under certain conditions their localization must be examined both in the presence and absence of agonists and antagonists. Since ecto-5'-nucleotidase appears to be localized in lipid rafts the effects that endogenous adenosine exerts on receptor localization must be recognized. In order to better understand the physiological significance of adenosine receptor localization in lipid rafts/caveolae the expression of receptors and their signaling must be examined in both these membrane microdomains as well as non-rafts and other subcellular compartments. Investigators must also recognize the limitations of the methods to isolate membrane microdomains, as well as the limitations of commercially available adenosine receptor antibodies. Finally, given the significant evidence that adenosine receptors modulate cellular responses to stress, such as catecholamine stimulation, oxidative stress, and ischemia-reperfusion, the role of membrane microdomains in modulating adenosine receptor signaling must be examined under these conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fredholm BB. Adenosine receptors as drug targets. Exp. Cell Res. 2010;316:1284–8. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Trincavelli ML, Daniele S, Martini C. Adenosine receptors: what we know and what we are learning. Curr. Top. Med. Chem. 2010;10:860–77. doi: 10.2174/156802610791268756. [DOI] [PubMed] [Google Scholar]
- [3].Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 2003;15:813–27. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- [4].Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol. Ther. 2007;114:208–21. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- [5].Trost T, Stock K. Effects of adenosine derivatives on cAMP accumulation and lipolysis in rat adipocytes and on adenylate cyclase in adipocyte plasma membranes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1977;299:33–40. doi: 10.1007/BF00508634. [DOI] [PubMed] [Google Scholar]
- [6].Ethier MF, Madison JM. Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am. J. Respir. Cell. Mol. Biol. 2006;35:496–502. doi: 10.1165/rcmb.2005-0290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gerwins P, Fredholm BB. Stimulation of adenosine A1 receptors and bradykinin receptors, which act via different G proteins, synergistically raises inositol 1,4,5-trisphosphate and intracellular free calcium in DDT1 MF-2 smooth muscle cells. Proc. Natl. Acad. Sci. USA. 1992;89:7330–7334. doi: 10.1073/pnas.89.16.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fenton RA, Moore ED, Fay FS, Dobson JG., Jr. Adenosine reduces the Ca2+ transients of isoproterenol-stimulated rat ventricular myocytes. Am. J. Physiol. 1991;261:C1107–14. doi: 10.1152/ajpcell.1991.261.6.C1107. [DOI] [PubMed] [Google Scholar]
- [9].Narayan P, Mentzer RM, Jr., Lasley RD. Phosphatase inhibitor cantharidin blocks adenosine A(1) receptor anti-adrenergic effect in rat cardiac myocytes. Am. J. Physiol. (Heart Circ. Physiol.) 2000;278:H1–7. doi: 10.1152/ajpheart.2000.278.1.H1. [DOI] [PubMed] [Google Scholar]
- [10].Dasgupta S, Ferré S, Kull B, Hedlund P, Finnman V, Ahlberg S, Arenas E, Fredholm BB, Fuxe K. Adenosine A2A receptors modulate the binding characteristics of dopamine D2 receptors in stably cotransfected fibroblast cells. Eur. J. Pharmacol. 1996;316:325–331. doi: 10.1016/s0014-2999(96)00665-6. [DOI] [PubMed] [Google Scholar]
- [11].Ginés S, Hillion J, Torvinen M, Le Crom S, Casado V, Canela EI, Rondin S, Lew JY, Watson S, Zoli M, Agnati L, Vernier P, Lluis C, Ferré S, Fuxe K, Franco R. Dopamine D1 and adenosine A1 receptors assemble into functionally interacting heteromeric complexes. Proc. Natl. Acad. Sci. USA. 2000;97:8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, Neve K, Fuxe K, Agnati LF, Woods AS, Ferré S, Lluis C, Bouvier M, Franco R. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Biol. Chem. 2003;278:46741–9. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- [13].Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J. Neurosci. 2006;26:2080–7. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Anderson RGW. Caveolae: where incoming and outgoing messengers meet. Proc. Natl. Acad. Sci. USA. 1993;90:10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ferruccio G, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- [16].Zajchowski LD, Robbins SM. Lipid rafts and little caves. Compartmentalized signalling in membrane microdomains. Eur. J. Biochem. 2002;269:737–52. doi: 10.1046/j.0014-2956.2001.02715.x. [DOI] [PubMed] [Google Scholar]
- [17].Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat. Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- [18].Thomas CM, Smart EJ. Caveolae structure and function. J. Cell. Mol. Med. 2008;12:796–809. doi: 10.1111/j.1582-4934.2008.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Song KS, Scherer PE, Tang ZL, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells: caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 1996;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- [20].Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J.Biol. Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- [21].Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain: implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- [22].Toya Y, Schwencke C, Couet J, Lisanti MP, Ishikawa Y. Inhibition of adenylyl cyclase by caveolin peptides. Endocrinology. 1998;139:2025–2031. doi: 10.1210/endo.139.4.5957. [DOI] [PubMed] [Google Scholar]
- [23].Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- [24].Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz DS, Lisanti MP. Caveolin-mediated regulation of signaling along the p42/p44 MAP kinase cascade in vivo: a role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–211. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- [25].Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am. J. Physiol. 1998;275:L843–L851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- [26].Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br. J. Pharmacol. 2004;143:235–45. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Becher A A, McIlhinney RA. Consequences of lipid raft association on G-protein-coupled receptor function. Biochem. Soc. Symp. 2005;72:151–64. doi: 10.1042/bss0720151. [DOI] [PubMed] [Google Scholar]
- [28].Paila YD, Chattopadhyay A. Membrane cholesterol in the function and organization of G-protein coupled receptors. Subcell. Biochem. 2010;51:439–66. doi: 10.1007/978-90-481-8622-8_16. [DOI] [PubMed] [Google Scholar]
- [29].Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc. Natl. Acad. Sci. USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Andersson-Forsman C, Gustafsson LE. Cytochemical localization of 5'-nucleotidase in the enteric ganglia and in smooth muscle cells of the guinea-pig. J. Neurocytol. 1985;14:551–62. doi: 10.1007/BF01200797. [DOI] [PubMed] [Google Scholar]
- [31].Kittel A, Bacsy E. Ecto-ATPases and 5'-nucleotidases in the caveolae of smooth muscle. Enzyme-histochemical evidence may indicate a role for caveolae in neurotransmission. Cell Biol. Int. 1994;18:875–879. doi: 10.1006/cbir.1994.1124. [DOI] [PubMed] [Google Scholar]
- [32].Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J. Clin. Invest. 1997;99:2588–601. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Abedinpour P, Jergil B B. Isolation of a caveolae-enriched fraction from rat lung by affinity partitioning and sucrose gradient centrifugation. Anal. Biochem. 2003;313:1–8. doi: 10.1016/s0003-2697(02)00561-4. [DOI] [PubMed] [Google Scholar]
- [34].Duflot S, Calvo M, Casado FJ, Enrich C, Pastor-Anglada M. Concentrative nucleoside transporter (rCNT1) is targeted to the apical membrane through the hepatic transcytotic pathway. Exp. Cell Res. 2002;281:77–85. doi: 10.1006/excr.2002.5641. [DOI] [PubMed] [Google Scholar]
- [35].Errasti-Murugarren E, Molina-Arcas M, Casado FJ, Pastor-Anglada M. The human concentrative nucleoside transporter-3 C602R variant shows impaired sorting to lipid rafts and altered specificity for nucleoside-derived drugs. Mol. Pharmacol. 2010;78:157–65. doi: 10.1124/mol.110.063552. [DOI] [PubMed] [Google Scholar]
- [36].Mundell S, Kelly E. Adenosine receptor desensitization and trafficking. Biochim. Biophys. Acta. 2010 doi: 10.1016/j.bbamem.2010.06.007. [Epub ahead of print] In Press. [DOI] [PubMed] [Google Scholar]
- [37].Lasley RD, Narayan P, Uittenbogaard A, Smart EJ. Activated cardiac adenosine A1 receptors translocate out of caveolae. J. Biol. Chem. 2000;275:4417–4421. doi: 10.1074/jbc.275.6.4417. [DOI] [PubMed] [Google Scholar]
- [38].Cavalli A, Eghbali M, Minosyan TY, Stefani E, Philipson KD. Localization of sarcolemmal proteins to lipid rafts in the myocardium. Cell Calcium. 2007;42:313–22. doi: 10.1016/j.ceca.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Garg V, Jiao J, Hu K. Regulation of ATP-sensitive K+ channels by caveolin enriched microdomains in cardiac myocytes. Cardiovasc. Res. 2009;82:51–58. doi: 10.1093/cvr/cvp039. [DOI] [PubMed] [Google Scholar]
- [40].Murthy KS, Makhlouf GM. Heterologous desensitization mediated by G protein-specific binding to caveolin. J Biol Chem. 2000;275:30211–9. doi: 10.1074/jbc.M002194200. [DOI] [PubMed] [Google Scholar]
- [41].Insel PA, Head BP, Ostrom RS, Patel HH, Swaney JS, Tang CM, Roth DM. Caveolae and lipid rafts: G protein-coupled receptor signaling microdomains in cardiac myocytes. Ann. N.Y. Acad. Sci. 2005;1047:166–172. doi: 10.1196/annals.1341.015. [DOI] [PubMed] [Google Scholar]
- [42].Xiao RP, Zhu W, Zheng M, Chakir K, Bond R, Lakatta EG, Cheng H. Subtype-specific beta-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol. Sci. 2004;25:358–65. doi: 10.1016/j.tips.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [43].Ballard-Croft C, Locklar AC, Keith BJ, Mentzer RM, Jr., Lasley RD. Oxidative stress and adenosine A1 receptor activation differentially modulate subcellular cardiomyocyte MAPKs. Am. J. Physiol. (Heart Circ. Physiol.) 2008;294:H263–H271. doi: 10.1152/ajpheart.01067.2007. [DOI] [PubMed] [Google Scholar]
- [44].Yang Z, Sun W, Hu K. Adenosine A(1) receptors selectively target protein kinase C isoforms to the caveolin-rich plasma membrane in cardiac myocytes. Biochimica Biophysica Acta. 2009;1793:1868–1875. doi: 10.1016/j.bbamcr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [45].Funakoshi H, Chan TO, Good JC, Libonati JR, Piuhola J, Chen X, MacDonnell SM, Lee LL, Herrmann DE, Zhang J, Martini J, Palmer TM, Sanbe A, Robbins J, Houser SR, Koch WJ, Feldman AM. Regulated overexpression of the A1-adenosine receptor in mice results in adverse but reversible changes in cardiac morphology and function. Circulation. 2006;114:2240–50. doi: 10.1161/CIRCULATIONAHA.106.620211. [DOI] [PubMed] [Google Scholar]
- [46].Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J. Biol. Chem. 2002;277:38988–97. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- [47].Cheskis E, Chung PH, DeGeorge BR, Feldman EW, Funakoshi H, Jackson W, Jasmin J-F, Koch WJ, Lisanti MP, Ma X-L, Myers VD, Song J, Wagner J, Wang Y, Whitaker-Menezes D, Yelamarty RV, Zhang J, Cheung JY, Chan TO, Feldman AM. Over-expression of the A1-Adenosine Receptor Alters Cardiac Function by Inhibiting Production of Caveolin-3. Circulation. 2009;120:S874. Abstract. [Google Scholar]
- [48].Ginés S, Ciruela F, Burgueño J, Casadó V, Canela EI, Mallol J, Lluís C, Franco R. Involvement of caveolin in ligand-induced recruitment and internalization of A(1) adenosine receptor and adenosine deaminase in an epithelial cell line. Mol. Pharmacol. 2001;59:1314–23. [PubMed] [Google Scholar]
- [49].Escriche M, Burgueño J, Ciruela F, Canela E, Mallol J, Enrich C, Lluís C, Franco R. Ligand-induced caveolae-mediated internalization of A1 adenosine receptors: morphological evidence of endosomal sorting and receptor recycling. Exp. Cell Res. 2003;285:72–90. doi: 10.1016/s0014-4827(02)00090-3. [DOI] [PubMed] [Google Scholar]
- [50].Ciruela F, Saura C, Canela E, Mallol J, Lluís C, Franco R. Ligand-induced phosphorylation, clustering, and desensitization of A1 adenosine receptors. Mol. Pharmacol. 1997;52:788–797. doi: 10.1124/mol.52.5.788. [DOI] [PubMed] [Google Scholar]
- [51].Klaasse EC, Ijzerman AP, de Grip WJ, Beukers MW. Internalization and desensitization of adenosine receptors. Purinergic Signal. 2008;4:21–37. doi: 10.1007/s11302-007-9086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mojsilovic-Petrovic J, Jeong GB, Crocker A, Arneja A, David S, Russell DS, Kalb RG. Protecting motor neurons from toxic insult by antagonism of adenosine A2a and Trk receptors. J. Neurosci. 2006;26:9250–63. doi: 10.1523/JNEUROSCI.1856-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Assaife-Lopes N, Sousa VC, Pereira DB, Ribeiro JA, Chao MV, Sebastião AM. Activation of adenosine A2A receptors induces TrkB translocation and increases BDNF-mediated phospho-TrkB localization in lipid rafts: implications for neuromodulation. J. Neurosci. 2010;30:8468–8480. doi: 10.1523/JNEUROSCI.5695-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [54].Kamata K, Manno S, Ozaki M, Takakuwa Y. Functional evidence for presence of lipid rafts in erythrocyte membranes: Gsalpha in rafts is essential for signal transduction. Am. J. Hematol. 2008;83:371–5. doi: 10.1002/ajh.21126. [DOI] [PubMed] [Google Scholar]
- [55].Lam RS, Nahirney D, Duszyk M. Cholesterol-dependent regulation of adenosine A(2A) receptor-mediated anion secretion in colon epithelial cells. Exp. Cell Res. 2009;315:3028–35. doi: 10.1016/j.yexcr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- [56].Charalambous C, Gsandtner I, Keuerleber S, Milan-Lobo L, Kudlacek O, Freissmuth M, Zezula J. Restricted collision coupling of the A2A receptor revisited: evidence for physical separation of two signaling cascades. J. Biol. Chem. 2008;283:9276–9288. doi: 10.1074/jbc.M706275200. [DOI] [PubMed] [Google Scholar]
- [57].Lyman E, Higgs C, Kim B, Lupyan D, Shelley JC, Farid R, Voth GA. A role for a specific cholesterol interaction in stabilizing the Apo configuration of the human A(2A) adenosine receptor. Structure. 2009;17:1660–8. doi: 10.1016/j.str.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sitaraman SV, Wang L, Wong M, Bruewer M, Hobert M, Yun CH, Merlin D, Madara JL. The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and ezrin upon agonist stimulation. J. Biol. Chem. 2002;277:33188–95. doi: 10.1074/jbc.M202522200. [DOI] [PubMed] [Google Scholar]
- [59].Mundell SJ, Matharu AL, Kelly E, Benovic JL. Arrestin isoforms dictate differential kinetics of A2B adenosine receptor trafficking. Biochemistry. 2000;39:12828–36. doi: 10.1021/bi0010928. [DOI] [PubMed] [Google Scholar]
- [60].Cordeaux Y, Briddon SJ, Alexander SP, Kellam B B, Hill SJ. Agonist-occupied A3 adenosine receptors exist within heterogeneous complexes in membrane microdomains of individual living cells. FASEB J. 2008;22:850–60. doi: 10.1096/fj.07-8180com. [DOI] [PubMed] [Google Scholar]
- [61].Marchese A, Paing M, Temple B, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]