Abstract
A decrease of brain allopregnanolone biosynthesis may play a role in emotion, impulsive behavior, and anxiety spectrum disorders by decreasing GABAergic neurotransmission. In male mice, four weeks of social isolation induces behavioral dysfunctions such as aggression, fear, and anxiety-like behavior associated with a decrease in allopregnanolone biosynthesis in selected corticolimbic structures comprising the basolateral amygdala (BLA), olfactory bulb, hippocampus, and medial prefrontal cortex. Importantly, no decrease in allopregnanolone biosynthesis has been found in the striatum and cerebellum. Given the importance of the amygdaloid complex in emotional behavior, we hypothesized that this brain area may play a pivotal role in decreasing social isolation-induced aggression. Thus, socially isolated mice were directly infused with S-norfluoxetine (S-NFLX) or pregnanolone (an analog of allopregnanolone) into the BLA and striatum. When S-NFLX (2.5, 3.75, and 5 nmol/0.2 μl) or pregnanolone (1.25, 2.5, 3.75, and 5.0 nmol/0.2 μl) is directly infused into the BLA, these agents dose-dependently reduced aggression (S-NFLX up to 93% and pregnanolone up to 96%) of a socially isolated mouse to a same-sex intruder. However, S-NFLX (3.75 and 5 nmol) infused directly into the striatum failed to alter aggression. Allopregnanolone content in the BLA after S-NFLX (3.75 nmol) infusion was increased by 3-fold and in the hippocampus, by 80%. Allopregnanolone levels did not change in the olfactory bulb or in the frontal cortex of the same mice. S-NFLX (3.75 nmol) infused into the striatum failed to increase the levels of allopregnanolone.
These results suggest that S-NFLX, acting as a selective brain steroidogenic stimulant (SBSS), increases corticolimbic allopregnanolone levels and regulates aggression, which underscore the pivotal role of the BLA and hippocampus in the regulation of aggressiveness in socially isolated mice.
Keywords: Allopregnanolone, 5α-reductase type I, selective brain steroidogenic stimulants (SBSSs), aggressive behavior, GABAA receptors, social isolation, anxiety, PTSD
1. Introduction
Cortical, hippocampal, and amygdala principal glutamatergic neurons synthesize allopregnanolone, a neurosteroid that positively and allosterically modulates GABA action at post- and extra-synaptic GABAA receptors (Puia et al., 1990; 1991; Belelli and Lambert 2005; Lambert et al., 2009). These GABAA receptors are located on the same dendrites or cell bodies of the cortical, hippocampal, and amygdala pyramidal and pyramidal-like neurons that produce allopregnanolone (Agis-Balboa et al., 2006; 2007). Glutamatergic neuron-produced allopregnanolone has the physiological function of maintaining the strength of GABAergic neurotransmission (Pinna et al., 2000; Puia et al., 2003; Hosie et al., 2006) by reaching GABAA receptors at the intracellular site of the receptors (Akk et al., 2005) or by an autocrine mechanism (Agis Balboa et al., 2006) and regulates several emotional and cognitive behaviors (Pibiri et al., 2008; Pinna et al., 2008; Frye, 2009). Exogenously applied allopregnanolone has several pharmacological properties, including anticonvulsive (Belelli et al., 1989; Matsumoto et al., 2003), anxiolytic (Reddy and Kulkami, 1997; Barbaccia et al., 2001; Zimmerberg and Kjunski, 2004; Khisti et al., 2000; Mohler, 2006), antiaggressive (Pinna et al., 2003; 2008), and sedative effects (Norberg et al., 1987).
The importance of allopregnanolone biosynthesis on brain function is highlighted by accumulating evidence that by decreasing GABAergic neurotransmission, decreased brain allopregnanolone levels play a role in several affective and emotional disorders, including impulsive behavior, anxiety spectrum disorders, PTSD, and depression (Rasmusson et al., 2006; Nappi et al., 2001; Ströhle et al., 2002; Pearlstein, 2010; Uzunova et al., 1998; reviewed in Uzunova et al., 2006; Pinna et al., 2006a).
Animal models of these disorders associated with a downregulation of allopregnanolone biosynthesis, such as the bulbectomized rat, a recognized rodent model of depression, and the socially isolated mouse model of anxiety spectrum disorders, including impulsivity and PTSD, have been investigated to study the beneficial behavioral actions of several steroidogenic antidepressants and GABAergic drugs (Matsumoto et al., 1999; Uzunova et al., 2003; 2004; 2006; Pinna et al., 2000; 2006a; 2006b; 2009; Barbaccia, 2004; Pibiri et al., 2008). For instance in our laboratory, socially isolated mice show increased aggression toward intruders, anxiety-like behavior, exaggerated contextual fear conditioning, and decreased sedative action of barbiturates and benzodiazepines (Matsumoto et al., 1999; Pinna et al., 2003; 2006a, 2006b; 2009; Guidotti et al., 2001; Pibiri et al., 2008). These behavioral abnormalities are associated with a decrease in 5α-reductase type I (the rate-limiting step enzyme in allopregnanolone biosynthesis) and allopregnanolone content in glutamatergic neurons of several corticolimbic regions, including the basolateral amygdala (BLA), the hippocampus, and the medial prefrontal cortex (Matsumoto et al., 1999; Serra et al., 2000; Dong et al., 2001; Pinna et al., 2003; Agis Balboa et al., 2007, Pibiri et al., 2008). Importantly, no decrease in allopregnanolone or 5α-reductase has been found in GABAergic neurons of the striatum and cerebellum (Agis-Balboa et al., 2007; Pibiri et al., 2008; Pinna et al., 2008).
Previous studies have shown that socially isolated mice given systemic injections of the selective brain steroidogenic stimulant (SBSS), S-norfluoxetine (S-NFLX), or other steroidogenic antidepressants show a dose-dependent reduction in aggressive behavior, normalization of contextual fear conditioning, and attenuation of anxiety-like behavior (Pinna et al., 2003; Pinna et al., 2006a; Pibiri et al., 2008). These behavioral effects of S-NFLX were associated with a normalization of social isolation-induced reduction of corticolimbic allopregnanolone content rather than by increases of selective serotonin reuptake inhibition (Pinna et al., 2003; 2004; 2009; Pinna, 2010). These studies, however, did not investigate the neuronal circuitries whereby S-NFLX’s neurosteroidogenic action results in decreased aggression.
Several previous studies have shown that allopregnanolone has anxiolytic effects when microinfused into the amygdala and the medial prefrontal cortex but not in the hippocampus (Engin and Treit, 2007). Likewise, allopregnanolone showed a strong antianxiety action in the conflict test and the elevated-plus maze when microinfused into the central nucleus of the amygdala, suggesting that the amygdaloid nucleus could be involved in the mechanisms underlying the anxiolytic-like action of allopregnanolone (Akwa et al., 1999). Interestingly, inhibiting 5α-reductase in the amygdala by administering fenasteride resulted in attenuation of the antianxiety and antidepressive action of allopregnanolone (Walf et al., 2006). More recent results showed that microinfusion of allopregnanolone in the central region of the amygdala exerts antidepressant-like effects, however, infusion of allopregnanolone into the nucleus accumbens and medial prefrontal cortex fails to produce antidepressant effects (Shirayama et al., 2010).
Thus we hypothesized that by microinfusing S-NFLX into the BLA, pharmacological stimulation of local allopregnanolone biosynthesis would alleviate aggression whereas microinjection of S-NFLX into the striatum would fail to reduce aggression in socially isolated mice. Consequently, to investigate the circuitry involved in the antiaggressive effects of S-NFLX in socially isolated mice and elucidate whether allopregnanolone biosynthesis is involved, we microinfused S-NFLX directly into the BLA and into the striatum. BLA was chosen because of the pivotal role of this key corticolimbic area in the regulation of emotional disorders and also because this area expresses a dramatic decrease of allopregnanolone levels following protracted social isolation (Agis-Balboa et al., 2007; Pibiri et al., 2008). On the other hand, striatal allopregnanolone levels are unchanged after social isolation and this brain area seems to play only a marginal role in aggressive behavior and other emotional behaviors (Agis-Balboa et al., 2007). Following direct microinfusion of S-NFLX or allopregnanolone, we measured both aggressive behavior and allopregnanolone content in various corticolimbic areas.
2. Materials and Method
2.1 Animals and Tissue Preparation
Adult male Swiss–Webster mice (Harlan Breeders) (18- to 20-g body weight) maintained under a 12-h dark/light cycle with food and water ad libitum were used for all experiments. Mice were housed either in groups of five per cage (24 × 17 × 12 cm) or individually (socially isolated) in a cage of the same size for a time periods of four weeks preceding surgery, followed by one week of recovery before behavioral and biochemical studies (Pinna et al., 2003). The vivarium temperature was kept near 24°C and the humidity near 65%.
All of the animal procedures used in our research were approved by the University of Illinois at Chicago Animal Care Committee.
2.2 Dissection of brain regions
Immediately after decapitation, brains were frozen and the frontal portion cut into 1-mm-thick slices using a Jacobovitz brain slicer (Zivic Miller). The slices obtained from 1.18 to 0.14 anterior to bregma were mounted on a coverslip kept at 4°C and disks (1.5-mm diameter) were punched out from these slices, including the striatum and the frontoparietal somatosensory cortex. Similarly, the slices obtained at 1.06–2.06mm posterior to the bregma were used to punch out disks (1.5-mm diameter), including the dorsal hippocampus and BLA.
2.3 Microinjections of pregnanolone and S-NFLX
Using a stereotaxic apparatus (Kopf Instruments, Tujunga, CA), mice were bilaterally implanted with 7-mm stainless-steel guide cannulae (26G; Plastics One) under sodium pentobarbital (50 mg/kg i.p.) anesthesia. Guides were directed toward the BLA (from bregma, AP −1.4mm; ML +/− 3.1mm; DV −3.7mm) or the striatum (from bregma AP +1.3 mm; ML +/− 1.8 mm; DV −2.0mm). The cannulae were affixed to the skull with dental acrylic and jeweler’s screws. After post-surgery recovery of 5–7 days, the mice received an infusion either S-NFLX (gift from Eli Lilly) (2.5 nmol, 3.75 nmol, or 5 nmol/0.2 ul) with a control of saline or 5β-pregnan-3α-ol-20-one (pregnanolone, an analog of allopregnanolone, from steraloids) (1.25 nmol, 2.5 nmol, 3.75, or 5.0 nmol in 0.2 ul) with a control of 10% (2-hydroxypropyl)-β-cyclodextrin (Aldrich) via a 33G stainless steel cannula attached to a 10 μl Hamilton syringe via FEP-tubing (CMA/Microdialysis AB, Stockholm, Sweden), which extended 1.0 mm beyond the tip of the guide cannula. Drug administration was controlled by a microinjection pump (CMA/100) that delivered 1 μl to the injection site at a rate of 0.2 μl/min. The internal cannula was left in place for one minute after the end of infusion. The behavioral test was run 15–20 min after infusion.
2.4 Behavioral testing
2.4.1 Resident–Intruder Test
To test the aggressive behavior of resident male socially isolated mice, an intruder mouse of the same gender was placed in a resident home cage (24 × 17 × 12) and resident–intruder interactions were videotaped for 10 min. The aggressive behavior of resident socially isolated mice was characterized by an initial pattern of exploratory activity around the intruder, which was followed by rearing and tail rattle, accompanied in few seconds by wrestling and/or a violent biting attack (Pinna et al., 2003; 2005). The number of these attacks and/or amount of wrestling during the 10 min observation period was recorded.
2.4.2 Locomotion Measures
A computerized AccuScan 12 Animal Activity Monitoring System (Columbus Instruments, Columbus, OH) assisted by Versamax software (AccuScan Instruments, Columbus, OH) was used to quantitatively monitor locomotor activity in mice, as described (Pinna et al., 1997). Each activity cage consisted of a Perspex box (20 × 20 × 20 cm) surrounded by horizontal and vertical infrared sensor beams. The interruptions per 10 min of the horizontal sensors were taken as a measure of horizontal activity whereas those of vertical sensors measured rearing activity. Activity was recorded from mice for 10 min beginning 20 min after infusion of vehicle, or 3.75 nmol pregnanolone or S-NFLX.
2.5 Histological control
The precise locations of the guide cannulae and infusion site were determined at the end of behavioral experiments in mice that were killed. Brains were removed and sectioned in coronal sections for tissue dissection. The trajectory of the cannulae was also determined. Only mice in which the cannulae were positioned in the BLA or striatum according to the coordinates of the experiment were considered for biochemical and data analyses.
2.6 Measurement of Brain Neurosteroid Content
Extraction, derivatization, and GC-MS analyses of allopregnanolone were performed with minor modifications as described (Uzunov et al., 1996; Pinna et al 2000; 2003). (i) The tissues of interest were homogenized in 10 vol. of distilled water containing 2–5 fmol/ml [3H]allopregnanolone (New England Nuclear) to monitor the HPLC retention profile and deuterium-labeled allopregnanolone (allopregnanolone-17,21,21,21-D4) (Steraloids) was used as an internal standard. The supernatants were extracted with ethyl acetate and after lyophilization were purified with HPLC as described (Pinna et al., 2000). (ii) The HPLC fractions containing allopregnanolone were derivatized with heptafluorobutyric acid anhydride (HFBA) (Supelco) and subjected to GC-mass fragmentography analysis.
Mass fragmentography analysis of derivatized allopregnanolone was performed in the standard electron impact (EI) mode. The detection limit was ≈10 fmol; the standard curve was linear between 5 and 105 fmol. For quantification, the m/z ion-monitoring mode was 496 for HFBA-allopregnanolone, 500 for HFBA-D-allopregnanolone.
2.7 Statistical Analyses
Data are given as means ± SEMs unless otherwise indicated. Comparisons between the control group and each of the treatment groups were performed by one-way ANOVA followed by Dunnett’s test or Student’s t test, as indicated in the figure legend.
3. Results
3.1 Body weight
Body weight failed to change following installation of cannulae and during the entire experiment procedure between the treatment groups (not shown).
3.2 S-norfluoxetine or pregnanolone microinfusion into the BLA decreases aggressive behavior
Histological analyses confirmed that the socially isolated mice included in the data analyses of aggressive behavior were properly implanted with cannulae in the BLA. The levels of aggression towards a same sex intruder of socially isolated mice that were implanted with guide cannulae into the BLA or the striatum were established before drug treatment and the mice were divided into groups that had similar levels of aggressiveness prior to drug microinfusions.
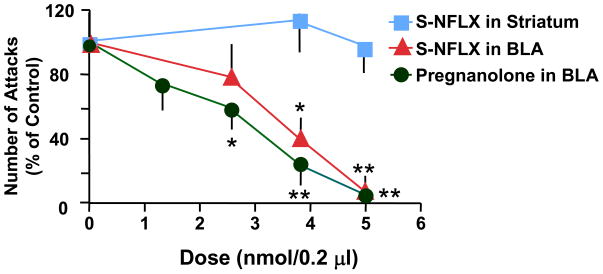
S-NFLX microinfused bilaterally into the BLA dose-dependently reduced the number of attacks by a socially isolated mouse towards an intruder (Figure 1). The dose of 2.5 nmol/0.2 μl of S-NFLX decreased aggression to 21% of control value, and the dose of 3.75 nmol/0.2 μl decreased aggression to 59% of control, whereas 5 nmol/0.2 μl decreased aggression to 93% of control levels (Figure 1).
Figure 1.
Dose-related inhibition of aggressive behavior (% of vehicle-treated mice) elicited by microinfusions of S-NFLX and pregnanolone in socially isolated male mice that have been implanted bilaterally with guide cannulas into the BLA or striatum. Each value is the mean ± SEM of 4–8 mice. Drugs were microinfused 15–20 min before the test. *, P < 0.05; **, P < 0.01, when pregnanolone- or NFLX-treated mice were compared with vehicle-treated mice, one-way ANOVA followed by Dunnett’s test.
A bilateral microinfusion of S-NFLX in the BLA appeared to be necessary to successfully decrease the levels of aggression of socially isolated mice. When socially isolated mice were microinfused with S-NFLX (3.75 nmol/0.2 μl) either in the right or left BLA, the number of attacks on an intruder was not changed (54 ± 8 for the S-NFLX-treated and 61 ± 12 for the vehicle microinfused, n=3–5).
We next assessed whether the effect of S-NFLX on aggression is associated with the neurosteroidogenic effect of this drug. To this end, mice were microinfused bilaterally with pregnanolone into the BLA. Figure 1 shows that pregnanolone dose dependently (1.25 to 5.0 nmol/0.2 μl) reduced the number of attacks by a socially isolated mouse on an intruder.
3.3 Aggression after S-NFLX microinfusion into the striatum
S-NFLX bilaterally infused into the striatum did not alter the aggressive behavior of socially isolated mice. Figure 1 shows that microinfusion of 3.75 nmol/0.2 μl with S-NFLX resulted in aggression that was 113 ± 30 % of control and 5 nmol/0.2 μl resulted in aggression that was 95 ± 26% of control responses.
3.4 Allopregnanolone content in several corticolimbic areas after S-NFLX microinfusion into the BLA
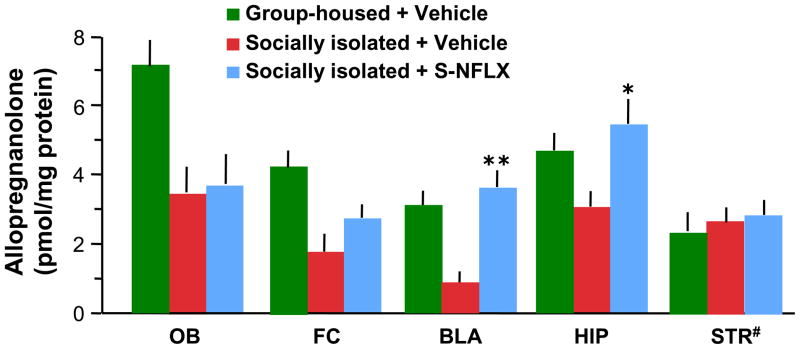
Several corticolimbic areas were dissected in mice decapitated 15 min after bilateral S-NFLX (3.75 nmol/0.2 μl) or vehicle microinfusion into the BLA or striatum, including the olfactory bulb, frontal cortex, hippocampus, BLA, and striatum. In these studies, we confirmed that social isolation elicits a decrease of allopregnanolone levels in all brain regions studied (Pibiri et al., 2008)., The striatum consistently failed to show a reduction of allopregnanolone levels (Figure 2). Importantly, in Figure 2 we show that the allopregnanolone content in the BLA of socially isolated mice was increased by about 3-fold after S-NFLX (3.75 nmol/0.2 μl). The allopregnanolone content in the hippocampus was also increased by 80%. However, allopregnanolone levels did not change in the olfactory bulb or the frontal cortex.
Figure 2.
The decrease of allopregnanolone content in selected corticolimbic structures of socially isolated mice is reversed by microinfusing S-NFLX (3.75 nmol/0.2 μl) bilaterally into the BLA. Each value is the mean ± SEM of five mice. *, P < 0.05 and **, P < 0.01 allopregnanolone content of socially isolated mice treated with S-NFLX compared with vehicle-treated socially isolated mice (Student’s t test). OB, olfactory bulb; FC, frontal cortex; BLA, basolateral amygdala; HIP, hippocampus; STR, striatum. # Striatal allopregnanolone levels determined following a bilateral microinfusion of S-NFLX (3.75 nmol/0.2 μl) in the striatum.
Remarkably, in socially isolated mice that received S-NFLX (3.75 nmol/0.2 μl) infused bilaterally in the striatum, the levels of allopregnanolone failed to change in the striatum (Figure 2).
3.5 Locomotor activity in a novel cage
Bilateral microinfusion of S-NFLX (3.75 nmol/0.2μl) or pregnanolone (3.75 nmol/0.2 μl) into the BLA did not result in differences in locomotor activity (horizontal and vertical activity) among treated socially isolated mice or mice microinjected with vehicle [horizontal activity (counts): 3445±480 in vehicle-, 2924±254 in pregnanolone-, and 3657±429 in S-NFLX-treated mice; vertical activity (counts): 358±81 in vehicle-, 413±51 in pregnanolone-, and 345±62 in S-NFLX-treated mice; n=5].
4. Discussion
Our results support the hypothesis that allopregnanolone has an antiaggressive effect in the BLA. This effect is observed by microinfusion of the SBSS S-NFLX at neurosteroidogenic doses (Figure 1). However, direct application of S-NFLX to the striatum failed to change the social isolation-induced aggression of a resident male mouse to a same-sex intruder. Analyses of allopregnanolone levels following S-NFLX microinfusion showed that in this neurosteroid, levels increased about 300% in the BLA and 80% in the hippocampus when S-NFLX was infused into the BLA whereas allopregnanolone levels failed to change in the frontal cortex and olfactory bulb of the same mice (Figure 2). In accordance with the behavioral correlates, allopregnanolone levels failed to increase in socially isolated mice that received doses of S-NFLX directly into the striatum (Figure 2). When pregnanolone (an analog of allopregnanolone) or S-NFLX was injected mono-laterally, for instance only in the left or right BLA, aggression levels were not decreased, which suggests that bilateral microinfusion of these compounds into the BLA is necessary to decrease aggressive behavior. Lack of change in locomotor activity suggests that in socially isolated mice, the antiaggressive effect of pregnanolone or S-NFLX was not mediated by an impairment of locomotion. Our results are in accord with and extend a number of previous studies which have shown that allopregnanolone has anxiolytic or antidepressant effects when microinfused into the amygdala (Engin and Treit, 2007; Akwa et al., 1999; Shirayama et al., 2010). However, our results are not in agreement with previous investigations that have shown that administering allopregnanolone systemically has a bitonic effect on aggressive behavior with low doses amplifying and larger doses decreasing aggression of mice (Fish et al., 2002, see also Engel and Grant, 2001). It is conceivable that experimental conditions, including housing conditions, in pairs (Fish et al., 2002) versus individual caging, which consistently decreases corticolimbic allopregnanolone levels (present work), or administration route (systemic versus local microinfusion) play a role in the observed behavioral differences of the response to allopregnanolone.
Of note, the neuronal networks responsible for the regulation of aggression and fear responses involve hippocampal and cortical pyramidal neuron excitatory glutamatergic projections to the BLA (Nelson and Trainor, 2007; Milad et al., 2007; Rauch et al., 2006; LeDoux, 2000; Akirav and Maroun 2007; Sah and Westbrook, 2008; Sacco and Sacchetti, 2010). We have shown that socially isolated mice express a marked downregulation of allopregnanolone biosynthesis in the amygdala, hippocampus, olfactory bulb, and frontal cortex (Pibiri et al., 2008). In these brain areas, the reduction of allopregnanolone levels is most probably mediated by a downregulation of 5α-reductase type I expression in glutamatergic neurons (Agis-Balboa et al., 2007; Pibiri et al., 2008; reviewed in Pinna et al., 2009; Pinna, 2010).
It is conceivable that in socially isolated mice, a decrease in allopregnanolone biosynthesis in principal glutamatergic neurons of the amygdala, frontal cortex, and hippocampus may trigger increased excitability by reducing the inhibitory potency of GABA action at GABAA receptors located post- and extra-synaptically on the same glutamatergic neurons that synthesize allopregnanolone (Agis-Balboa et al., 2007; reviewed in Sah and Westbrook, 2008; Pinna et al., 2008; Pinna, 2010). It is also likely that this could be the underlying mechanism that leads to the increased aggression and exaggerated contextual fear conditioning seen in socially isolated mice (Pibiri et al., 2008; Pinna, 2010; see also Makkar et al., 2010).
The present study underscores the pivotal role of allopregnanolone not only on corticolimbic circuits but also on the amygdaloid complex in particular, which acts as a relay center in the regulation of several emotional behavioral responses to environmental stimuli.
The fact that microinfusions of S-NFLX into the BLA trigger an increase of allopregnanolone levels in the amygdala and hippocampus but not in the cortex and olfactory bulb suggests that the BLA neurosteroidogenic network may extend to the hippocampus. This possibility has been previously hypothesized in a study which demonstrated that a microinfusion of allopregnanolone in the ventral tegmental area elicits anti-anxiety and social and sexual behavioral changes by increasing hippocampal and cortical allopregnanolone levels (Frye and Rhodes, 2006; 2008). It remains to be further clarified whether the levels of allopregnanolone in the hippocampus may be increased simply because of a diffusion of S-NFLX or allopregnanolone from the BLA to other corticolimbic areas or because of an activity-dependent mechanism.
Notwithstanding the mechanism whereby allopregnanolone levels are increased in the hippocampus, it is likely that allopregnanolone levels in the hippocampus regulate aggressive behavior. Several other studies have shown that allopregnanolone infused into the hippocampus produces an antidepressant-like effect in rats subjected to the forced swim test through actions on GABAA receptors located on neurons of the lateral septum and dorsal hippocampus (Rodriguez-Landa et al., 2009). Further, activation of allopregnanolone biosynthesis by stimulating peripheral mitochondrial benzodiazepine receptors in the dorsal hippocampus has also proven to exert an anxiolytic effect in the elevated-plus maze and the shock-probe burying test (Bitran et al., 2000). Finally, in a previous study these authors reproduced this anxiolytic effect by microinfusion of the allopregnanolone precursor pregnanolone into the hippocampus (Bitran et al., 1999). However, another investigation reported that these anxiolytic effects were not observed when allopregnanolone was microinfused into the hippocampus (Engin and Treit, 2007).
S-NFLX microinjected into the striatum fails to modulate aggression and does not increase the levels of allopregnanolone in this brain region. Given the elevated density of medium spiny neurons that possess the enzymatic machinery to produce allopregnanolone from progesterone (Agis-Balboa et al., 2007) this finding remains a surprising result that requires further investigation at the enzymatic level. Of note, this brain region as well as the cerebellum fails to show a downregulation of neurosteroidogenic enzyme expression and allopregnanolone levels following a protracted period of social isolation (Agis-Balboa et al., 2007; Pibiri et al., 2008). This finding suggests that neurosteroidogenesis in GABAergic long projecting neurons may not be susceptible to environmental stressors such as social isolation, although it efficiently decreases allopregnanolone biosynthesis in glutamatergic neurons (Agis-Balboa et al., 2007). Likewise, GABAergic long projecting neurons also seem to fail to respond to agents that stimulate neurosteroidogenesis (Pinna et al., 2010). Interestingly, in agreement with our study, infusions of allopregnanolone into the striatum failed to exert behavioral effects (Shirayama et al., 2010). These authors showed that microinfusion of allopregnanolone into the cerebral ventricle, the CA3 region of hippocampus or the central region of amygdala exerted antidepressant-like effects; however, infusion of allopregnanolone into the nucleus accumbens and medial prefrontal cortex fails to produce antidepressant effects (Shirayama et al., 2010). Thus, the effects of SBSS drugs on neurosteroid biosynthesis are not expressed globally throughout the brain but appear to be region- and neuron-specific. This is an advantage for the treatment of psychiatric disorders associated with a downregulation of neurosteroid expression such as depression or PTSD because the selective action of SBSS offers a suitable alternative to using drugs with global action on the brain and severe side effects such as those induced by benzodiazepines (Pinna et al., 1997).
Our report demonstrates for the first time that the S-NFLX-mediated increase of allopregnanolone levels in the BLA and hippocampus exerts a potent antiaggressive action, suggesting that these areas play a pivotal role in the regulation of aggression. It remains to be clarified whether the microinfusion of neurosteroidogenic drugs upstream of the BLA, such as a direct application in the hippocampus or in the frontal cortex, also exerts a similar antiaggressive action by normalizing the glutamatergic excitatory outflow that reaches the amygdaloid complex from these brain regions.
Acknowledgments
This paper is dedicated to the memory of Professor Emeritus Erminio Costa in acknowledgment of his invaluable mentoring. This study was supported by National Institute of Mental Health Grant MH 085999 (to Graziano Pinna).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis-Balboa RC, Pinna G, Kadriu B, Costa E, Guidotti A. Downregulation of 5α-reductase type I mRNA expression in cortico-limbic glutamatergic circuits of mice socially isolated for four weeks. Proc Natl Acad Sci USA. 2007;104:18736–41. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–13. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–25. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–72. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML. Neurosteroidogenesis: relevance to neurosteroid actions in brain and modulation by psychotropic drugs. Crit Rev Neurobiol. 2004;16:67–74. doi: 10.1615/critrevneurobiol.v16.i12.70. [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5 alpha-pregnan-3 alpha-ol-20-one. Eur J Pharmacol. 1989;166:325–9. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3alpha-OH-5beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–24. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Bitran D, Foley M, Audette D, Leslie N, Frye CA. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology. 2000;151:64–71. doi: 10.1007/s002130000471. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Costa E, Guidotti A. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SR, Grant KA. Neurosteroids and behavior. International Review of Neurobiology. 2001;46:321–348. doi: 10.1016/s0074-7742(01)46067-3. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol. 2007;18:461–70. doi: 10.1097/FBP.0b013e3282d28f6f. [DOI] [PubMed] [Google Scholar]
- Fish EW, De Bold JF, Miczek KA. Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology (Berl) 2002;163:459–66. doi: 10.1007/s00213-002-1211-2. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; London, UK: 1997. www.hbuk.co.uk/ap/ [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviors and concomitantly increase 3alpha,5alpha-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomized estrogen-primed rats. J Neuroendocrinol. 2006;18:960–75. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 3alpha, 5alpha-THP to the VTA enhance exploratory, anti-anxiety, social, and sexual behavior and increase levels of 3alpha, 5alpha-THP in midbrain, hippocampus, diencephalon, and cortex of female rats. Behav Brain Res. 2008;187:88–99. doi: 10.1016/j.bbr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. Neurosteroids’ effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinology. 2009;34(Suppl 1):S143–61. doi: 10.1016/j.psyneuen.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5α-dihydroprogesterone in psychiatric disorders. Brain Res Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 2000;67:137–43. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Cooper MA, Simmons RD, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABAA receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S48–58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35:1625–52. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Uzunova V, Pinna G, Taki K, Uzunov DP, Watanabe H, Mienvielle JM, Guidotti A, Costa E. Permissive role of brain allopregnanolone content in the regulation of pentobarbital-induced righting reflex loss. Neuropharmacology. 1999;38:955–963. doi: 10.1016/s0028-3908(99)00018-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nomura H, Murakami Y, Taki K, Takahata H, Watanabe H. Long-term social isolation enhances picrotoxin seizure susceptibility in mice: up-regulatory role of endogenous brain allopregnanolone in GABAergic systems. Pharm Biochem Behav. 2003;75:831–835. doi: 10.1016/s0091-3057(03)00169-2. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci. 2007;1121:546–61. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- Mohler H. GABAA receptors in central nervous system disease: anxiety, epilepsy, and insomnia. J Recept Signal Transduct. 2006;26:731–40. doi: 10.1080/10799890600920035. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum “blues”. Obstet Gynecol. 2001;97:77–80. doi: 10.1016/s0029-7844(00)01112-1. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–46. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Norberg L, Wahlström G, Bäckström T. The anaesthetic potency of 3 alpha-hydroxy-5 alpha-pregnan-20-one and 3 alpha-hydroxy-5 beta-pregnan-20-one determined with an intravenous EEG-threshold method in male rats. Pharmacol Toxicol. 1987;61:42–7. doi: 10.1111/j.1600-0773.1987.tb01770.x. [DOI] [PubMed] [Google Scholar]
- Pearlstein T. Premenstrual dysphoric disorder: out of the appendix. Arch Womens Ment Health. 2010;13:21–3. doi: 10.1007/s00737-009-0111-4. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased allopregnanolone content during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci USA. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Galici R, Schneider HH, Stephens DN, Turski L. Alprazolam dependence prevented by substituting with the beta-carboline abecarnil. Proc Natl Acad Sci USA. 1997;94:2719–23. doi: 10.1073/pnas.94.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E, Guidotti A. Brain allopregnanolone regulates the potency of the GABAA receptor agonist muscimol. Neuropharmacology. 2000;39:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci USA. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, Doueiri MS, Guidotti A, Costa E. Brain neurosteroids in gender-related aggression induced by social isolation. Crit Rev Neurobiol. 2004;16:75–82. doi: 10.1615/critrevneurobiol.v16.i12.80. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior. Proc Natl Acad Sci USA. 2005;102:2135–40. doi: 10.1073/pnas.0409643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology. 2006a;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, Zhubi A, Matsumoto K, Grayson DR, Costa E, Guidotti A. Imidazenil and diazepam increase locomotor activity in mice exposed to protracted social isolation. Proc Natl Acad Sci USA. 2006b;103:4275–4280. doi: 10.1073/pnas.0600329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa R, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochemical Research. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9:24–30. doi: 10.1016/j.coph.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G. Behavioral Pharmacology. 2010. In a mouse model relevant for PTSD, selective brain steroidogenic stimulants (SBSSs) improve behavioral deficits by normalizing allopregnanolone biosynthesis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant gamma-aminobutyric acid — a receptor subunit composition on the action of allosteric modulators of gamma amino butyric acid-gated Cl-currents. Mol Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- Puia G, Mienville JM, Matsumoto K, Takahata H, Watanabe H, Costa E, Guidotti A. On the putative physiological role of allopregnanolone on GABAA receptor function. Neuropharmacology. 2003;44:49–55. doi: 10.1016/s0028-3908(02)00341-6. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–13. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Res. 1997;752:61–71. doi: 10.1016/s0006-8993(96)01447-3. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Landa JF, Contreras CM, García-Ríos RI. Allopregnanolone microinjected into the lateral septum or dorsal hippocampus reduces immobility in the forced swim test: participation of the GABAA receptor. Behav Pharmacol. 2009;20:614–622. doi: 10.1097/FBP.0b013e328331b9f2. [DOI] [PubMed] [Google Scholar]
- Sacco T, Sacchetti B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science. 2010;329:649–56. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
- Sah P, Westbrook RF. Behavioral Neuroscience: The circuit of fear. Nature. 2008;454:589–90. doi: 10.1038/454589a. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem. 2000;75:732–40. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Muneoka K, Fukumoto M, Tadokoro S, Fukami G, Hashimoto K, Iyo M. Infusions of allopregnanolone into the hippocampus and amygdala, but not into the nucleus accumbens and medial prefrontal cortex, produce antidepressant effects on the learned helplessness rats. Hippocampus. 2010 Jul 9; doi: 10.1002/hipo.20824. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ströhle A, Romeo E, di Michele F, Pasini A, Yassouridis A, Holsboer F, Rupprecht R. GABAA receptor-modulating neuroactive steroid composition in patients with panic disorder before and during paroxetine treatment. Am J Psychiatry. 2002;159:145–7. doi: 10.1176/appi.ajp.159.1.145. [DOI] [PubMed] [Google Scholar]
- Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci USA. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–44. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Ceci M, Kohler C, Uzunov DP, Wrynn AS. Region-specific dysregulation of allopregnanolone brain content in the olfactory bulbectomized rat model of depression. Brain Res. 2003;976:1–8. doi: 10.1016/s0006-8993(03)02577-0. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Wrynn AS, Kinnunen A, Ceci M, Kohler C, Uzunov DP. Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur J Pharmacol. 2004;486:31–4. doi: 10.1016/j.ejphar.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3α-reduced neurosteroids to depression and antidepressant action. Psychopharmacology. 2006;186:351–361. doi: 10.1007/s00213-005-0201-6. [DOI] [PubMed] [Google Scholar]
- Walf AA, Sumida K, Frye CA. Inhibiting 5α-reductase in the amygdala attenuates antianxiety and antidepressive behavior of naturally receptive and hormone-primed ovariectomized rats. Psychopharmacology. 2006;186:302–311. doi: 10.1007/s00213-005-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Kajunski EW. Sexually dimorphic effects of postnatal allopregnanolone on the development of anxiety behavior after early deprivation. Pharmacol Biochem Behav. 2004;78:465–71. doi: 10.1016/j.pbb.2004.03.021. [DOI] [PubMed] [Google Scholar]