Abstract
Nerve cuff electrodes are a principle tool of basic and applied electro-neurophysiology studies and are championed for their ability to achieve good nerve recruitment with low thresholds. We describe the design and method of fabrication for a novel circumpolar peripheral nerve electrode for acute experimental use. This cylindrical cuff-style electrode provides approximately 270 degrees of radial electrode contact with a nerve for each of an arbitrary number of contacts, has a profile that allows for simple placement and removal in an acute nerve preparation, and is designed for adjustment of the cylindrical diameter to ensure a close fit on the nerve. For each electrode, the electrical contacts were cut from 25 µm platinum foil as an array so as to maintain their positions relative to each other within the cuff. Lead wires were welded to each intended contact. The structure was then molded in silicone elastomer, after which the individual contacts were electrically isolated. The final electrode was curved into a cylindrical shape with an inner diameter corresponding to that of the intended target nerve. The positions of these contacts were well maintained during the molding and shaping process and failure rates during fabrication due to contact displacements were very low. Established electrochemical measurements were made on one electrode to confirm expected behavior for a platinum electrode and to measure the electrode impedance to applied voltages at different frequencies. These electrodes have been successfully used for nerve stimulation, recording, and conduction block in a number of different acute animal experiments by several investigators.
Keywords: Nerve cuff electrode, electrode fabrication, electrical stimulation, peripheral nerve
1. INTRODUCTION
Consistent and predictable electrical peripheral nerve stimulation requires a robust and stable physical interface between an electrode and a nerve. Many cuff-type electrode designs have proven to provide this by using a cylindrical geometry to conform to the shape of the nerve, encircling the nerve trunk (McCarty, 1965; Gardiner, 1967; Dubkin, 1970; Haugland, 1996; Rushton, 1997; Hoffer and Kallesoe, 1999; Rutten, 2002). This positions the electrical interface close to the neural tissue, resulting in lower stimulus threshold currents and a subsequent decrease in the power demands on a stimulator system relative to other types of electrodes, such as epimysial or intramuscular electrodes (Navarro et al, 2005). Other advantages of nerve cuff electrodes include the selective activation of a target nerve (e.g. not surrounding muscle tissue), and an established safety record for some cuff designs (Naples et al, 1988; Agnew et al, 1989).
There have been many successful nerve cuff electrode designs which have contributed important innovations such as a low activation threshold (Navarro et al, 2005), chronic safety (Naples et al, 1988; Agnew et al, 1989), fascicular selectivity (Veraart et al, 1993; Tyler and Durand, 2002) and manipulation of longitudinal current flow (Sweeney and Mortimer, 1968). The electrode described here is designed to be particularly useful for acute in-vivo experiments and includes three major features. First, the cross-section of the electrode allows for simple placement and removal from a nerve (similar to the placement of a hook electrode). This is particularly useful when placing an electrode in an anatomical region with difficult surgical access where placement of a spiral cuff (Naples et al, 1988) or helical electrode (Agnew et al, 1989) can be difficult. Second, the electrode cuff diameter can easily be modified to ensure a close-fitting electrode-nerve interface at the time of placement on the nerve by reforming the electrode around a mandrel with the desired diameter. Third, the electrode fabrication process allows for the design and fabrication of custom electrode configurations with an arbitrary diameter, number of longitudinal contacts (as many as six in some studies), contact configuration (monopolar or multipolar) and longitudinal dimensions with very low failure rates during fabrication.
This electrode design has been successfully used in multiple whole nerve neurostimulation studies to date, and has been shown to produce robust and repeatable recruitment of nerve fibers during stimulation (Bhadra et al, 2006; Miles et al, 2007; Bruns et al, 2008; Ackermann et al, 2009; Lewandowski et al, 2009; Mariano et al, 2009; Ackermann et al, 2010; Ackermann et al, in press) and conduction block (Bhadra and Kilgore, 2005; Bhadra et al, 2006; Miles et al, 2007; Boger et al, 2008; Ackermann et al, 2009; Ackermann et al, in press). It has also been used for recording compound action potentials (Lahowetz et al, 2007).
2. MATERIALS AND METHODS
2.1 Design
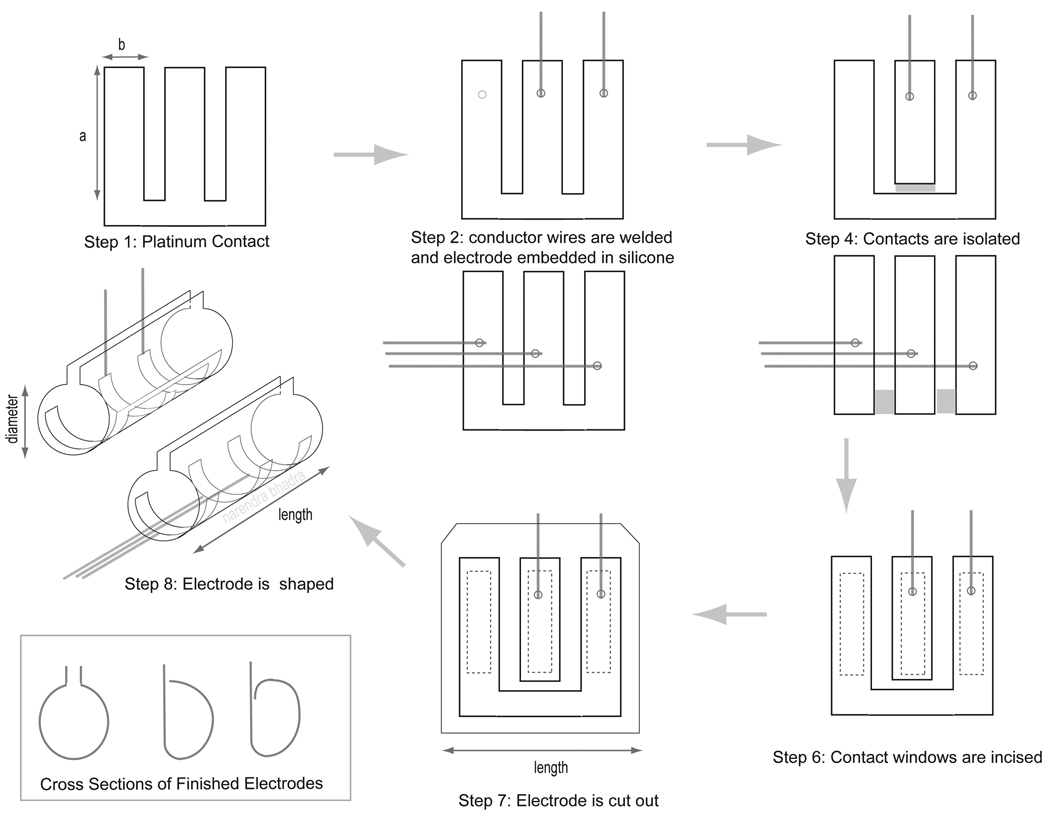
We developed a fabrication method which uses an array of platinum contacts to produce a circumpolar peripheral nerve electrode with precise electrode dimensions and reliably maintained contact positioning as outlined in Figure 1. The geometric dimensions of the cuff electrode were determined by the target peripheral nerve and the anatomical implantation site. The internal diameter of the cuff was determined by the diameter of the nerve trunk, allowing for a cuff to nerve diameter ratio between 0.9 and 1.1 as has been shown to result in safe and robust recruitment (Naples et al, 1988). The length of the cuff was based on the desired contact size and multipolar spacing and was limited by the anatomical implantation site. The thickness of the cuff wall typically ranged from 125 to 200 µm and was determined by the thickness of the silicone sheeting used to make the cuff.
Figure 1.
Illustration of the electrode fabrication steps using a typical tripolar electrode design as an example. The step numbers correspond to the steps outlined in the Fabrication sub-section of the Materials and Methods section. Two configurations are illustrated, one with three electrically isolated contacts with lead wires exiting axially along the nerve and one with the two outer contacts shorted together with lead wires exiting radially away from the nerve.
The dimensions and spacing of the electrode contacts were generally dictated by the electric field or recording needs of a particular application. The electrode contacts were made from 25 µm thick, 99.99% pure pore-free platinum foil (Alfa Aesar, Word Hill, MA, USA). The circumferential dimension of the electrode contact conforming around the circumference of the nerve trunk (drawn as ‘a’ in Step 1 of Figure 1), was typically designed to be between π and 2π times the diameter of the target peripheral nerve. A large radial electrical contact around the circumference of the target nerve was chosen to maximize recruitment of nerve fibers within the whole nerve (Loeb, 1996). Using a circumferential dimension that was larger than the nerve’s estimated circumference allowed flexibility for the electrode to provide circumferential contact for a range of actual nerve trunk diameters. The longitudinal dimension of the electrode contact along the axis of the nerve (drawn as ‘b’ in Step 1 of Figure 1) was typically selected to achieve the desired total contact area based on current density limitations for safety (Merrill et al, 2005) and on the maximum estimated stimulus currents required for the intended application. The spacing between the contacts is defined as the inter-polar distance (IPD), where the ‘poles’ are the geometric centers of each electrode contact. The IPD was often chosen to be the most efficient configuration for stimulation (Holsheimer and Wesselink, 1997) or blocking (Ackermann et al, 2009) given the nerve diameter, expected fiber diameter distribution within the nerve, and the intended purpose of the electrical stimulation (i.e. activation or conduction block). In some cases the IPD was limited by nerve geometry (e.g. exposed nerve length) and surgical access.
2.2 Fabrication
The following eight steps comprise the electrode fabrication process. A tripolar electrode will be used as an example in this section, however a variety of different configurations have been fabricated.
Step 1: Platinum Contact Array
The electrode contacts were cut from 25 µm platinum foil (Alfa Aesar, Word Hill, MA, USA) as a continuous array to maintain their position relative to each other in the cuff during the remaining fabrication steps. The array is illustrated in Step One of Figure 1. The dimensions of the platinum array were cut to include an additional 0.25 to 0.5 mm of platinum along each contact edge to provide a surface by which the platinum contacts could be anchored into the cuff.
Step 2: Weld Lead Wire to Platinum Contacts
Teflon insulated stainless steel 250 µm diameter lead wire (Fort Wayne Metal, Fort Wayne, Indiana, USA and Cooner Fine wire Co., Chatsworth, CA, USA) was used to form electrical connections to each intended electrode contact. The de-insulated tip of a lead wire was spot welded (Arc Welder, Unitek Co. Monrovia, CA, USA) to its intended contact with all welds made to the same side of the platinum array. The orientation of the lead wire and the platinum array in the weld were determined by the desired exit direction of the lead wire from the cuff, which was determined by access to the nerve at the implant site. Two possible orientations are illustrated in Step Two of Figure 1. The weld orientation for a radial exit direction with respect to the nerve is shown on the top and an axial direction is shown on the bottom.
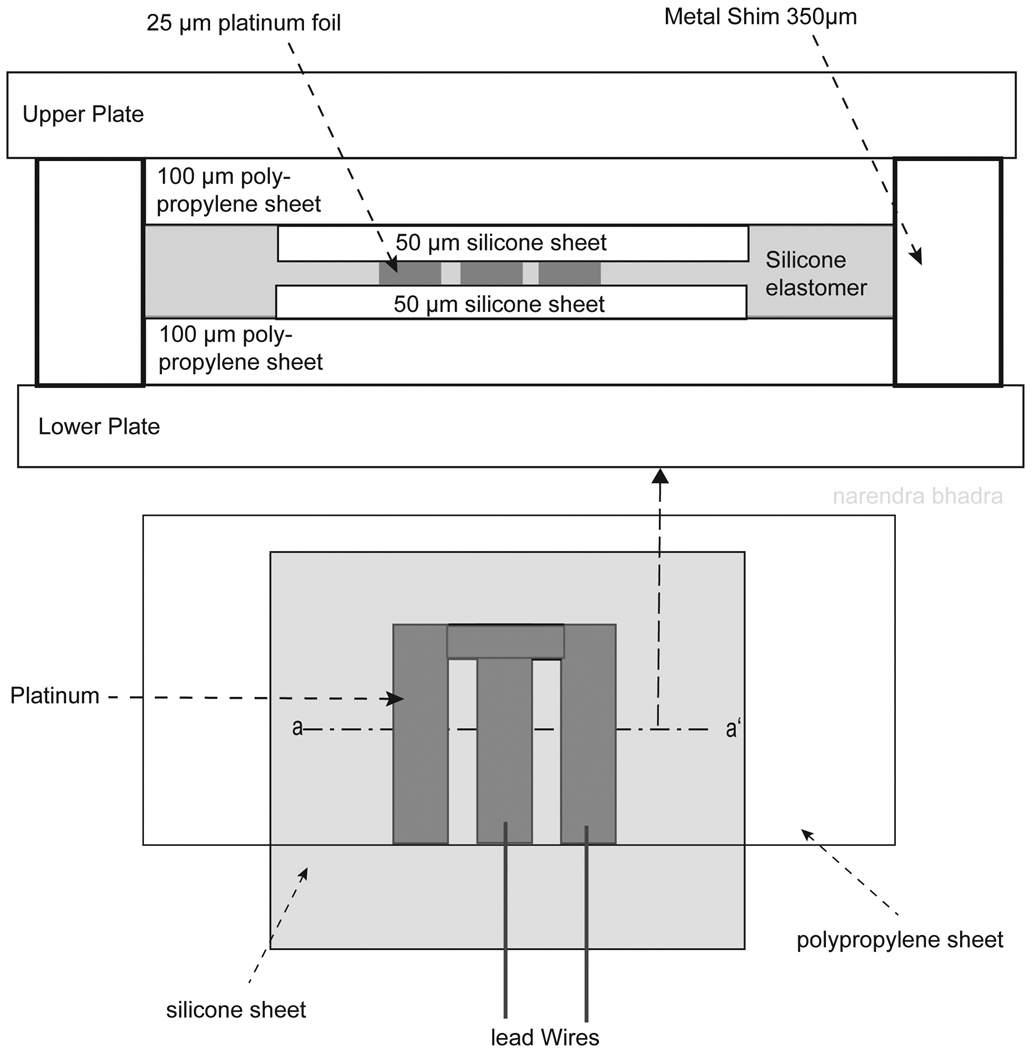
Step 3: Mold Platinum Contacts in Silicone
Two flat, interlocking metal molding plates were used to laminate the platinum foil between two sheets of insulating silicone. First, a piece of 100 µm thick polypropylene (PP 2500. 3M Visual Systems Division. Austin, TX, USA) was positioned over the bottom molding plate as shown in Figure 2. Next, the bottom sheet of 50 µm insulating silicone (NuSil Technology. Carpinteria, CA, USA) was placed over the polypropylene such that approximately 0.5 cm of silicone sheeting extended beyond the edge of the polypropylene as illustrated in the bottom portion of Figure 2. This additional length of silicone sheeting was used to encase the lead wires and the weld at their exit from the electrode cuff to limit moisture ingress. The polypropylene was used to add thickness to the electrode materials (thickness of 125 µm) to ensure uniform pressure was applied across the entire surface of the electrode while in the laminating press without damaging the thicker insulated lead wires (thickness of 250 µm), which were also between the plates. A small amount of adhesive silicone (MED-1137 adhesive silicone type A. NuSil Technology. Carpinteria, CA, USA) was applied to the welded side of the platinum array before positioning the platinum over the silicon sheeting with the welded side facing down to ensure good adhesion of the silicone and platinum. The platinum array was placed onto the silicone sheeting such that the edge of the array from which the lead wires exited was aligned with the edge of the polypropylene as shown in the bottom of Figure 2.
Figure 2.
Cross sectional illustration of the layers of electrode materials in the molding plates. The first layer on the lower plate was a piece of 100 µm polypropylene, followed by a layer of 50 µm silicone sheeting. The platinum array was placed between the two layers of silicone sheeting, followed by a second piece of polypropylene. The polypropylene was used to add thickness to the electrode materials (thickness of 125 µm) so equal pressure could be applied across the surface of the electrode while in the laminating press without damaging the thicker insulated lead wire (thickness of 250 – 350 µm). Metal shims were placed in the corners of the metal plates to maintain the final desired electrode thickness and limit compression of the electrode materials while in the laminating press.
Silicone Elastomer Parts A (MED-4211 part A silicone elastomer. NuSil Technology. Carpinteria, CA, USA) and B (MED-4211 part B silicone elastomer. NuSil Technology, Carpinteria, CA, USA) were mixed in a 10 to 1 ratio by weight. The mixture was placed in a vacuum chamber (Welch dry vacuum pump, Welch Rietschle Thomas, Skokie, IL, USA) for a minimum of 10 minutes to extract air bubbles from the mixture. A small amount of this mixture was spread over the electrode contacts and around the perimeter of the platinum array to ensure adhesion of the layers of electrode material.
A second piece of 50 µm thick silicone sheeting was then carefully aligned with the first piece of silicone sheeting, sandwiching the platinum array between the two pieces of silicone sheeting as seen in the cross section in Figure 2. Lastly, a second piece of 100 µm thick polypropylene was aligned with the bottom piece of polypropylene such that the polypropylene did not cover the insulation of the lead wire.
Metal shims (thickness of 350 µm) were placed in the corners of the molding plates to maintain the final desired electrode thickness and limit compression of the electrode materials while in the laminating press. The laminating press (Carver laboratory press 2699. Fred S. Carver Inc. Wabash, IN, USA) was preheated to 100 degrees C and the molding plate assembly was placed in the press at 5,000 psi of pressure for 1 hour.
Step 4: Separation of Electrode Contacts
After cooling the molding plates, the tip of a single edge blade was used to electrically isolate the electrode contacts by physically cutting out the platinum connecting the contacts as shown in Step Four of Figure 1. The electrode array shown in Figure 1 could be cut into two possible electrode configurations, one maintaining connectivity between the outer two electrode contacts (shown in the top illustration in Step Four of Figure 1) or one providing individual control of the current flowing to each of the three contacts (shown in the bottom illustration). A small amount of the silicone elastomer mixture was then used to fill in the gap between the electrode contacts which was created by the razor.
Step 5: Re-molding the Electrode
The electrode materials were laminated a second time between the interlocking molding plates so the silicone elastomer insulating the electrode contacts would cure uniformly.
Step 6: Creation of Contact Windows
The contact windows were carefully cut out of the silicone sheet to the desired window spacing and dimensions using a sharp scalpel blade as illustrated in Step Six of Figure 1. The small free piece of silicone covering the contact was carefully removed with forceps, exposing the platinum contact.
Step 7: Final Incision of Cuff Electrode from Excess Sheeting
The silicone sheath surrounding the platinum electrode contacts was then cut to the desired cuff dimensions as illustrated in Step Seven of Figure 1. In most cases, a minimum overlap of 0.5 mm between the silicone and the excess platinum surrounding the electrode contact was required to anchor the contact in the silicone sheath for a robust and durable electrode (see Step Seven in Figure 1). The silicone sheath was extended around the lead wires with the edges tapered to slope in toward the lead wires (as seen in the bottom illustration of Step Seven in Figure 1). Cutting the cuff in this manner simplified the implant procedure by providing a surface to manipulate with forceps, provided strain relief for the lead wires, and helped prevent moisture ingress into the weld area.
Step 8: Forming Cuff Shape
The electrode was wrapped around a rod to give it a cylindrical shape with known diameter as illustrated in Step Six in Figure 1 (e.g. a 1.0 mm diameter rod was typically used for an electrode designed for use in a rat sciatic preparation).
2.3 Electrode Implant Procedure
The flexibility of the design and the cross section of electrode allowed for easy implantation of the electrode on exposed peripheral nerves. Once the target nerve was carefully dissected free from the adjoining tissue, the nerve cuff was placed below the nerve and pushed under and behind the nerve trunk. The final cuff placement was executed by sliding the cuff around the nerve, and enclosing the nerve in the cuff using a pair of blunt forceps. The final diameter of the cuff could be adjusted during implantation ensuring a conforming fit to the nerve.
2.4 Electrode Characterization
To confirm that the electrode design was capable of safely delivering the stimulation currents required for the desired applications without damage, the electrochemical nature of the electrodes was characterized using established cyclic voltammogram (CV), and electrochemical impedance spectroscopy (EIS) techniques (Cogan, 2008). The CV experiment was performed to identify the presence of electrochemical reactions occurring at the electrode surface during the potential sweep (Robblee and Rose, 1990; Mailley, 2004; Cogan, 2008). The EIS experiment was performed to characterize the electrical impedance of the electrode as a function of frequency (de Boer and van Ooserom, 1978; Franks at al, 2005; Cogan, 2008). A monopolar electrode was fabricated using the methods described in the previous section with a contact geometric surface area of 4 mm2, in the π to 2π range typically used for the 1 mm diameter rat sciatic nerve. The electrode was placed in an electrochemical cell (EuroCell. Gamry Instruments, Warminster, PA, USA) along with a coiled stainless steel wire counter electrode and a saturated calomel reference electrode (SCE) (Gamry Instruments, Warminster, PA, USA). Room temperature Lactated Ringers solution was used for the electrolyte in the cell. Hydrated air was gently bubbled through the cell while the electrochemical measurements were conducted to stir the electrolyte at the electrode surface. A potentiostat (Model 1287. Solartron Analytical, Hampshire, UK), frequency analyzer (Model 1252. Solartron Analytical, Hampshire, UK), and accompanying software were used to collect the measurements. The CV measurements were collected on three different days using a scan rate of 100 mV/s while sweeping the electrode potential between −1 V and +1 V with respect to the saturated calomel electrode (SCE) reference for 5 complete cycles. The EIS measurements were also collected on three different days and were conducted using a 10 mVrms sinusoidal voltage, sweeping the frequency from 100,000 Hz to 0.1 Hz.
3. RESULTS
3.1 Design and Fabrication
The electrode could be reliably made at a low cost and was well suited for acute animal experimentation. Cutting the platinum contacts into an array and electrically isolating them after laminating them in the silicone sheath reliably maintained the position of electrode contacts relative to each other and resulted in low failure rates of less than 10% for electrodes with an established design. The conforming circumpolar peripheral nerve electrode was easily adapted to a variety of nerve diameters and contact configurations for different peripheral nerve stimulation, recording and conduction block applications. The stimulation thresholds and electrode impedances showed acceptable tolerances measured during experimentation. If handled carefully, the electrodes could be used in multiple experiments before they failed. Failure typically resulted from a separation of silicone and platinum around the edges of the contact window.
3.2 Electrochemical Measurements
The CV and EIS measurements were conducted using a monopolar electrode with a 4 mm2 contact geometric surface area. This electrode showed performance characteristics typical of platinum foil electrodes, as shown in Figure 3. While cycling the electrode potential between −1 V and +1 V, the electrode current stayed within −165 and 65 µA for all three repeat measurements. The voltammogram depicts the electrochemical behavior of the electrode interface. The oxidation of platinum is visible at potentials greater than 0.7 V/SCE. Oxygen desorption and reduction occur near 0 V/SCE. Successive steps of hydrogen adsorption take place at potentials between −1 and −0.6 V/SCE and the distinct negative peak at −1 V/SCE is associated with hydrogen formation from water reduction (Robblee and Rose, 1990; Mailley, 2004; Cogan, 2008).
Figure 3.
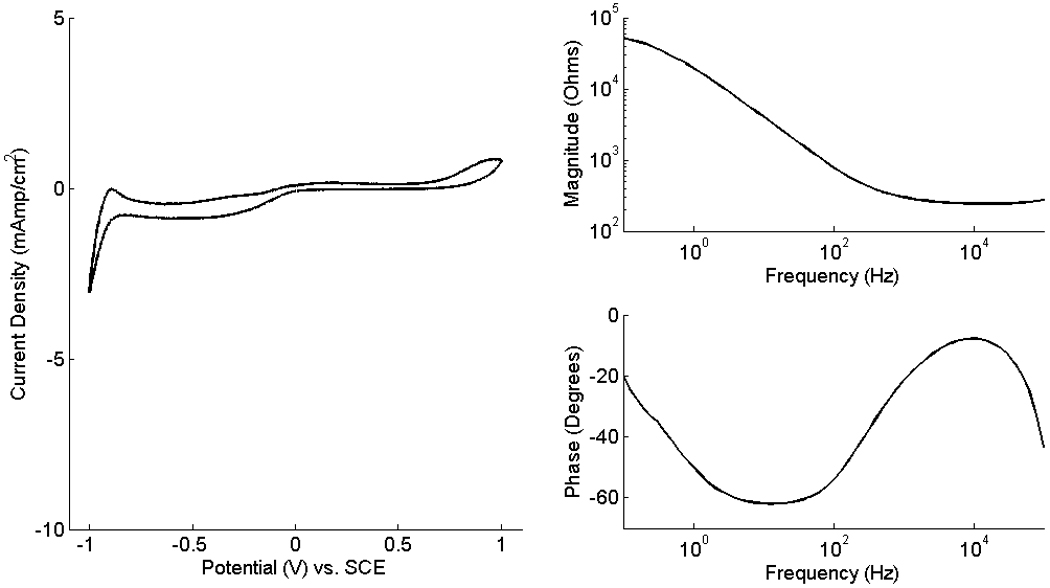
(left) CV of a monopolar electrodes with contact surface area of 4 mm2. The electrode current was measured while the potential was swept cyclically at a constant rate of 100 mV/s between −1 V and 1 V with respect to the SCE reference. The 5th cycle of the potential sweep is shown. (right) The impedance magnitude and phase angle of the EIS measurements were conducted while sweeping the 10 mVrms sinusoidal voltage of the electrode frequency from 100,000 Hz to 0.1 Hz.
The electrode impedance (magnitude and phase) calculated from one of the three EIS measurements is shown in Figure 3 (right). Per typical practice (de Boer and van Oosterom, 1978; Schuettler, 2007; Cogan, 2008), the impedance magnitude measurements were used to estimate the ohmic resistance of the solution between the test and reference electrodes, RΩ, and the charge transfer resistance due to transfer of charges at the electrode-electrolyte interface, Rt. The impedances measured at very high frequencies (100,000 Hz) were used to estimate RΩ and were calculated to be 373.61 +/− 159.11 (mean +/− SD) Ω. At very low frequencies (0.1 Hz), the electrode impedance was used to estimate the sum of RΩ and Rt and ranged from 35.47 +/− 17.93 (mean +/− SD) kΩ.
4. DISCUSSION
The electrode design and fabrication method presented offers a low-cost and reliable means to manufacture electrodes for acute in-vivo experimentation. The materials are relatively inexpensive and readily available and the fabrication method has shown low failure rates. A batch of five electrodes can be made within eight man-hours, producing multipolar cuff electrodes with sufficiently precise tolerances for stimulation and recording applications.
This electrode has been implanted during acute experiments on rat sciatic nerves (Bhadra and Kilgore, 2005; Miles et al, 2007; Ackermann et al, 2009; Ackermann et al, 2010; Ackermann et al, in press), feline sacral roots (Boger et al, 2008; Mariano et al, 2009), sural nerves (Lahowetz et al, 2007), and pudendal nerves (Bhadra et al, 2006; Boger et al, 2008; Bruns et al, 2009), and rabbit femoral nerves (Lewandowski et al, 2009). The electrode configuration chosen depended on the intended application (ie. stimulation, conduction block, or recording) and the surgical access. We have used this design and fabrication method to create cuff electrodes with 1 to 6 contacts with equal success. Monopolar electrodes have been used during conduction block experiments in rats, involving a single circumferential electrical contact wrapped around the nerve, as photographed in Figure 4a, with a large diameter return electrode placed subcutaneously on the dorsum of the animal (Kilgore et al, 2009; Ackermann, 2010). Bipolar electrodes with two identical circumferential electrical contacts spaced longitudinally along the nerve have been used for both neural stimulation and conduction block in the rat (Ackermann et al, 2009; Ackermann et al, 2010; Ackermann et al, in press). Tripolar electrodes, having three identical contacts spaced evenly along the nerve, have been used for stimulation, recording, and conduction block (Bhadra and Kilgore, 2005; Bhadra et al, 2006; Lahowetz et al, 2007; Miles et al, 2007; Boger et al, 2008; Bruns et al, 2009; Lewandowski et al, 2009; Mariano et al, 2009; Ackermann, in press).
Figure 4.
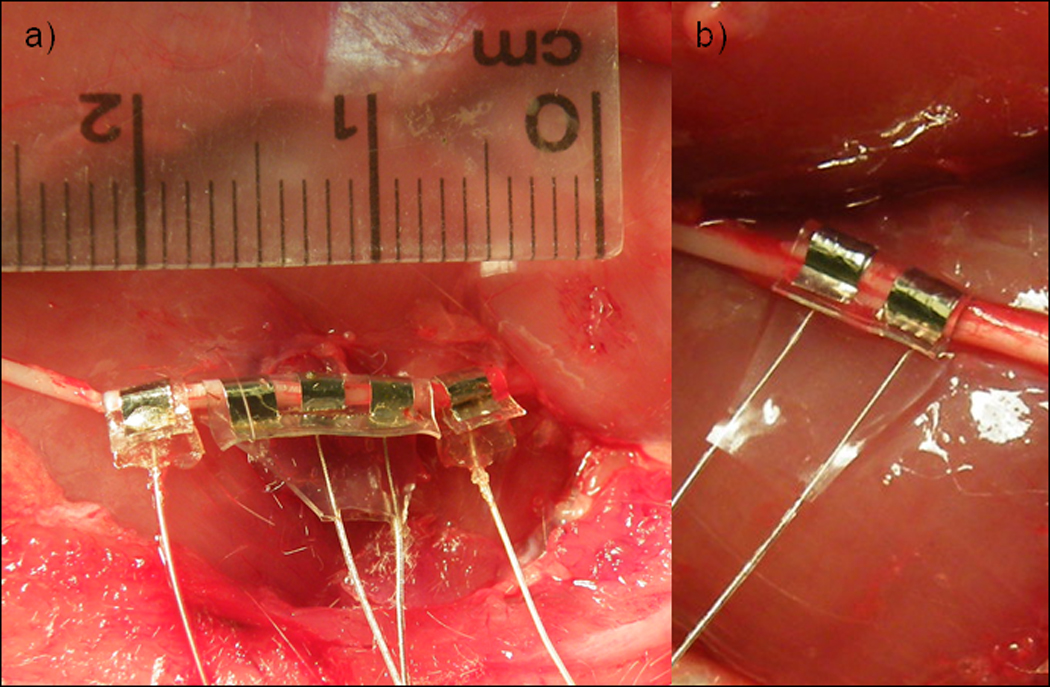
Photographs of monopolar (a), bipolar (b), and tripolar (a) nerve cuffs implanted on rat sciatic nerves for acute experimentation. The cross section of these cuffs provides for easy implantation and approximately 270 of circumferential contact around the nerve.
This electrode has three major design strengths. First, the cross section allows for very convenient acute placement while maintaining the insulating nature of a cuff. Second, the cuff diameter can be easily modified at the time of an experiment by reforming the cuff over a mandrel. Third, the fabrication process is extraordinarily flexible and allows for a wide range of design configurations that can be achieved using the same equipment and materials for all desired contact and cuff dimensions. We have successfully modified the design to manipulate the IPD, the longitudinal contact dimension, and the distance from the outer edge of the flanking contacts to the cuff edge. Ackermann et al, have used IPDs ranging from 0.5 mm to 4 mm (Ackermann et al, 2009; Ackermann et al, in press). We found that an IPD of 4 mm is likely a rough maximum IPD because larger IPDs caused an undesirable bowing in the silicone sheath between the contacts, dilating the cuff diameter in that location. In another study by Ackermann, electrodes with longitudinal contact dimensions in the range of 0.5 mm to 4 mm were manufactured with equal success (Ackermann, 2010). For electrodes with wide longitudinal dimensions, particular care was required to ensure good adherence of the platinum to the top silicone sheet (particularly at the edges of the contact window). This was achieved by spreading a generous amount of the uncured silicone elastomer over the platinum foil (Step Three in the fabrication process). This prevented the edges of the silicon anchoring the contact into the electrode from peeling off of the platinum once the window was cut. Lastly, the distance from the outer edge of the flanking contacts to the cuff edge has been varied from 0.5 mm to 4 mm. For lengths greater than 2 mm, the ends of the silicone sheath did not maintain a constant diameter cylindrical shape, opening at the ends of the electrode sheath and thus decreasing the insulating effect of the cuff. Sutures inserted around the ends of the cuff once implanted helped to maintain cuff closure in these cases.
Cyclic voltammograms and electrochemical impedance spectroscopy measurements were conducted to confirm that this type of electrode exhibits electrochemical behavior that is typical of platinum electrodes and the design was capable of safely delivering the currents necessary for the desired stimulation applications. The electrochemical technique of cyclic voltammetry is a three electrode measurement that measures the current flowing between the test electrode and a counter electrode while the potential of the test electrode with respect to a noncurrent-carrying reference electrode is swept cyclically between two potential limits at a constant rate (Cogan, 2008). This measurement provides information about the electrochemical reactions taking place at the electrode surface. The CV can identify the water electrolysis window, a desirable operational potential window between hydrogen and oxygen evolution (Rozman et al, 2000). The performance and electrical impedance were found to be characteristic of published results of platinum foil electrodes (de Boer and Oosterom, 1978; Rozman et al, 2000; Mailley, 2004; Schuettler, 2007; Cogan, 2008). Although we cycled the electrode potential between −1 V and 1 V with respect to an SCE reference electrode, the optimal operating range was revealed to be between −0.6 V and 0.7 V. The typical operating range for platinum is between −0.6 V and 0.8 V versus an Ag|AgCl reference electrode.
EIS measurements determine the electrical impedance and phase angle using a low amplitude sinusoidal voltage excitation of the electrode across a broad frequency range (Cogan, 2008). In addition, EIS measurements provide information on the recording capabilities of the electrode because the electrode impedance influences signal strength (Schuettler, 2007; Cogan, 2008). The ohmic resistance, RΩ, and the charge transfer resistance, Rt, were determined for the 4 mm2 monopolar electrode and were found to be consistent with published values for electrode contacts with surface areas ranging from 0.05 mm2 to 1 cm2 (de Boer and Oosterom, 1978; Rozman et al, 2000; Mailley, 2004; Franks et al, 2005; Schuettler, 2007). These estimated resistance values can be used to create an equivalent circuit model of the electrode interface for theoretical evaluation the electrode (de Boer and Oosterom, 1978; Mailley, 2004; Franks et al, 2005; Schuettler, 2007).
This novel electrode design exhibits substantial flexibility for use with a variety of nerve diameters and stimulation applications. The electrochemical characteristics were typical of platinum electrodes and suggested safe delivery of the current amplitudes tested for stimulation applications. The simple and inexpensive fabrication method offers researchers a reliable means to produce electrodes for acute in-vivo experimentation.
Research Highlights
Peripheral nerve cuff electrode design and fabrication methods for use in acute animal experimentation
Design is easily adapted to a variety of nerve diameters and contact configurations
Materials are relatively inexpensive and readily available and the fabrication method has shown low failure rates
Successfully used for nerve stimulation, recording, and conduction block applications in a number of acute animal experiments by several investigators
ACKNOWLEDGMENTS
This work was supported by the National Institute of Biomedical Imaging and Bioengineering Grant R01-EB-002091. The last author was partially funded by the National Institute of Diabetes and Digestive and Kidney Diseases R01-DK077089.
ABBREVIATIONS
The circumferential dimension of the electrode contact around the circumference of the nerve trunk
The longitudinal dimension of the electrode contact along the axis of the nerve trunk
- IPD
Inter-Polar Distance, or spacing between the electrode contacts, where ‘pole’ refers to the geometric center of the electrode contact
- CV
Cyclic Voltammogram
- EIS
Electrode Impedance Spectroscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ackermann DM, Jr., Foldes EL, Bhadra N, Kilgore KL. Effect of Bipolar Cuff Electrode Design on Block Thresholds in High-Frequency Electrical Neural Conduction Block. IEEE Trans Neural Sys Rehab Eng. 2009;17:469–477. doi: 10.1109/TNSRE.2009.2034069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann DM, Jr., Foldes EL, Bhadra N, Kilgore KL. Conduction block of peripheral nerve using high-frequency alternating currents delivered through an intrafascicular electrode. Muscle Nerve. 2010;41:117–119. doi: 10.1002/mus.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann DM., Jr. Doctoral Dissertation. Cleveland: Case Western Reserve University; 2010. Reduction of the Onset Response in High Frequency Nerve Block. [Google Scholar]
- Ackermann DM, Jr., Foldes EL, Bhadra N, Kilgore KL. Effect of Nerve Cuff Electrode Geometry on Onset Response Firing in Conduction Block of Whole Nerve Using High Frequency Alternating Currents. IEEE Trans Neural Sys Rehab Eng. doi: 10.1109/TNSRE.2010.2071882. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew WF, McCreery DB, Yuen TGH, Bullara LA. Histologic and Physiologic Evaluation of Electrically Stimulated Peripheral Nerve: Considerations for the Selection of Parameters. Annals of Biomed Eng. 1989;17:39–60. doi: 10.1007/BF02364272. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Kilgore KL. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve. 2005;32:782–790. doi: 10.1002/mus.20428. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Bhadra N, Kilgore KL, Gustafson KJ. High frequency electrical conduction block of the pudendal nerve. J Neural Eng. 2006;3:180–187. doi: 10.1088/1741-2560/3/2/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger A, Bhadra N, Gustafson KJ. Bladder voiding by combined high frequency electrical pudendal nerve block and sacral root stimulation. Neurourol Urodyn. 2008;27:435–439. doi: 10.1002/nau.20538. [DOI] [PubMed] [Google Scholar]
- Bruns TM, Bhadra N, Gustafson KJ. Variable patterned pudendal nerve stimuli improves reflex bladder activation. IEEE Trans Neural Syst Rehabil Eng. 2008;16:140–148. doi: 10.1109/TNSRE.2007.914460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- de Boer RW, van Oosterom A. Electrical properties of platinum electrodes: impedance measurements and time-domain analysis. Med & Biol Eng & Comput. 1978;16:1–10. doi: 10.1007/BF02442925. [DOI] [PubMed] [Google Scholar]
- Dubkin C. A constant-contact stimulating electrode for nerves. J Appl Physiol. 1970;28:350. doi: 10.1152/jappl.1970.28.3.350. [DOI] [PubMed] [Google Scholar]
- Franks W, Schenker I, Schmutz P, Hierlemann A. Impedance characterization and modelling of electrodes for biomedical applications. IEEE Trans Biomed Eng. 2005;52:1295–1302. doi: 10.1109/TBME.2005.847523. [DOI] [PubMed] [Google Scholar]
- Gardiner AS. Peripheral nerve electrode applicator. Anaesthesia. 1967;22:492–493. doi: 10.1111/j.1365-2044.1967.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Haugland M. A flexible method for fabrication of nerve cuff electrodes. Conference Proceedings of IEEE Eng Med Biol Soc; Amsterdam, Netherlands: 1996. [Google Scholar]
- Hoffer JA, Kallesoe K. Nerve cuff electrodes for prosthetic and research applications. Conference Proceedings of the International FES Society; Sendai, Japan: 1999. [Google Scholar]
- Holsheimer J, Wesselink WA. Optimum electrode geometry for spinal cord stimulation: The narrow bipole and tripole. Med Biol Eng Comput. 1997;35:493–497. doi: 10.1007/BF02525529. [DOI] [PubMed] [Google Scholar]
- Kilgore KL, Foldes EA, Ackermann DM, Bhadra N. Combined direct current and high frequency nerve block for elimination of the onset response. Conference Proceedings of IEEE Eng Med Biol Soc; Minneapolis, MN: 2009. [DOI] [PubMed] [Google Scholar]
- Lahowetz EA, Bhadra N, Kilgore KL. High Frequency Conduction Block of Sensory Nerves. Conference Proceedings of the International FES Society; Philadelphia, PA: 2007. [Google Scholar]
- Lewandowski BE, Kilgore KL, Gustafson KJ. In Vivo Demonstration of a Self-Sustaining, Implantable, Stimulated-Muscle-Powered Piezoelectric Generator Prototype. Ann Biomed Eng. 2009;37:2390–2401. doi: 10.1007/s10439-009-9770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb GE, Peck RA. Cuff electrodes for chronic stimulation and recording of peripheral nerve activity. J of Neurosci Methods. 1996;64:95–103. doi: 10.1016/0165-0270(95)00123-9. [DOI] [PubMed] [Google Scholar]
- Mailley S, Hyland M, Mailley P, McLaughlin JA, McAdams E. Thin film platinum cuff electrodes for neurostimulation: in vitro approach of safe neurostimulation parameters. Bioelectrochemistry. 2004;63:359–364. doi: 10.1016/j.bioelechem.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Mariano TY, Bhadra N, Gustafson KJ. Suppression of Reflex Urethral Responses by Sacral Dermatome Stimulation in an Acute Spinalized Feline Model. Neurourol Urodyn. 2010;29:494–500. doi: 10.1002/nau.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty LP. A stimulating electrode for nerves. J Appl Physiol. 1965;20:542. doi: 10.1152/jappl.1965.20.3.542. [DOI] [PubMed] [Google Scholar]
- Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J of Neurosci Methods. 2005;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Miles JD, Kilgore KL, Bhadra N, Lahowetz EA. Effects of ramped amplitude waveforms on the onset response of high-frequency mammalian nerve block. J. Neural Eng. 2007;4:390–398. doi: 10.1088/1741-2560/4/4/005. [DOI] [PubMed] [Google Scholar]
- Naples GG, Mortimer JT, Scheiner A, Sweeney JD. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans Biomed Eng. 1988;35:905–916. doi: 10.1109/10.8670. [DOI] [PubMed] [Google Scholar]
- Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J Peripher Nerv Syst. 2005;10:229–258. doi: 10.1111/j.1085-9489.2005.10303.x. [DOI] [PubMed] [Google Scholar]
- Robblee LS, Rose TL. The Electrochemistry of Electrical Stimulation. Conference Proceedings of IEEE Eng Med Biol Soc; Philadelphia, PA: 1990. [Google Scholar]
- Rozman J, Milosev I, Jenko M. Platinum stimulating electrodes in physiological media. J of Med Eng and Tech. 2000;24:123–128. doi: 10.1080/03091900050135040. [DOI] [PubMed] [Google Scholar]
- Rushton DN. Functional electrical stimulation. Physiol Meas. 1997;18:241–275. doi: 10.1088/0967-3334/18/4/001. [DOI] [PubMed] [Google Scholar]
- Rutten WL. Selective electrical interfaces with the nervous system. Annu Rev Biomed Eng. 2002;4:407–452. doi: 10.1146/annurev.bioeng.4.020702.153427. [DOI] [PubMed] [Google Scholar]
- Schuettler M. Electrochemical Properties of Platinum Electrodes in Vitro: Comparison of Six Different Surface Qualities. Conference Proceedings of IEEE Eng Med Biol Soc; Lyon, France: 2007. [DOI] [PubMed] [Google Scholar]
- Sweeney JD, Mortimer JT. An Asymmetric Two Electrode Cuff for Generation of Unidirectionally Propagated Action Potentials. IEEE Trans Biomed Eng. 1986;33:541–549. doi: 10.1109/TBME.1986.325818. [DOI] [PubMed] [Google Scholar]
- Tyler DJ, Durand DM. Functionally Selective Peripheral Nerve electrode: Stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng. 2002;10:294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- Veraart C, Grill WM, Mortimer JT. Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans on Biomed Eng. 1993;40:640–653. doi: 10.1109/10.237694. [DOI] [PubMed] [Google Scholar]