Abstract
We assessed prevalent HIV cases in Atlanta to examine case distribution trends and population characteristics at the census tract level that may be associated with clustering effects. We calculated cluster characteristics (area and internal HIV prevalence) via Kuldorff's spatial scan method. Subsequent logistic regression analyses were performed to analyze sociodemographics associated with inclusion in a cluster. Organizations offering voluntary HIV testing and counseling services were identified and we assessed average travel time to access these services. One large cluster centralized in downtown Atlanta was identified that contains 60% of prevalent HIV cases. The prevalence rate within the cluster was 1.34% compared to 0.32% outside the cluster. Clustered tracts were associated with higher levels of poverty (OR = 1.19), lower density of multi-racial residents (OR = 1.85), injection drug use (OR = 1.99), men having sex with men (OR = 3.01), and men having sex with men and IV drug use (OR = 1.6). Forty-two percent (N = 11) of identified HIV service providers in Atlanta are located in the cluster with an average travel time of 13 minutes via car to access these services (SD = 9.24). The HIV epidemic in Atlanta is concentrated in one large cluster characterized by poverty, men who have sex with men (MSM), and IV drug usage. Prevention efforts targeted to the population living in this area as well as efforts to address the specific needs of these populations may be most beneficial in curtailing the epidemic within the identified cluster.
Keywords: HIV/AIDS, HIV prevalence, Spatial cluster detection, Geographic mapping
Introduction
With over 50,000 new HIV infections estimated to occur annually in the USA, the HIV/AIDS epidemic continues to be an important public health problem.1 Specifically, the Southeastern USA has emerged as a center of HIV/AIDS infection as the number of HIV/AIDS cases are increasing faster in the South compared to other regions of the country.2 It has been suggested that factors such as poverty, unemployment, inadequate access to healthcare, and sociocultural issues may contribute to this phenomenon.3,4
In 2006, the state of Georgia was ranked eighth in the nation for its reported rate of AIDS cases per 100,000 population, and 1,691 persons were newly diagnosed with HIV infection in 2007.5,6 Most of those newly diagnosed with HIV were African-American men in the 20–24 age category.5 Between 2000 and 2006 the rate of people who had ever been tested for HIV decreased in the metro area (and across the entire state).7 A recent prospective HIV incidence study at voluntary testing and counseling sites in Atlanta found that among 2,202 participants (81% black), the annual incidence of HIV infection was between 1.1–1.3% and the HIV prevalence was 3.2%.8 A 2008 report by the Georgia Division of Public Health found that, despite increased funding for prevention and care in recent years, gaps in knowledge, care, prevention, and intervention services related to HIV still existed. The report specifically found gaps in medical services for HIV-positive clients, transportation, comprehensive risk counseling and services, and peer counseling.9
The metropolitan Atlanta area is comprised of 31 counties with an estimated population of 5,376,285 persons as of July 1, 2008.10 The city of Atlanta is a smaller geographic area centrally located in Fulton and DeKalb counties with a diverse population of 445,709 persons including a majority of African Americans (55.8%).11 While the estimated mean household income is $84,675 (median: $47,464) in the city of Atlanta, a substantial number of persons (21.8%) living within the city of Atlanta have income below the poverty level.12,13
HIV service provision for many Atlantans is offered via the Grady Health System’s Ponce de Leon Center, serving over 4,000 HIV-infected patients. Federal funding, mostly through the Ryan White Care Act, enables the clinic to assist many lower-income persons with medical and dental care, mental health services, infusion therapies, housing, food, legal services, and community-based organizational support. The Ponce de Leon Center is one of the largest, most comprehensive centers for the care of the HIV-infected patients in the USA. The Georgia Department of Human Resources estimates that over 70% of HIV-infected patients who live in Atlanta reside within 2 miles of the clinic. An extensive network of referring agencies, including those representing AIDS service, faith-based, and community-based organizations, provide frontline HIV testing and counseling services throughout the metropolitan area to identify those eligible for the clinic's treatment services.
Although the risk factors of HIV have been extensively studied, the spatial distribution is largely unexplored. Prevailing assumptions seem to be that HIV prevalence and incidence are clustered in localized geographic areas characterized by poverty and race.14–18 Community-level or structural variables (such as poverty) are increasingly being recognized as factors which may place persons at increased risk for HIV.19–21 Recent CDC findings, drawn from surveys gathered from heterosexual persons living in diverse census tracts, revealed that HIV was detected in about one out of 42 persons (2.4%) living below the federal poverty level.22 It is theorized that community-level characteristics such as neighborhood household income reflect social and economic activity, which could be contributing to HIV transmission. Residents in impoverished areas may have a greater chance of encountering others who participate in illegal drug use, engage in prostitution, or have other experiences which put them at higher risk for HIV acquisition. Correspondingly, there may be a relationship to race and ethnicity as Blacks and Hispanics are more likely to live in areas with greater poverty. Therefore, it is reasonable to draw upon ecological systems models to examine the interaction of individual and community-level influences differentiating neighborhood HIV/AIDS rates.
Assumptions regarding the spatial distribution of HIV have not been tested in much of the South, including in Atlanta. This analysis examines the clustering of HIV prevalent cases in four heavily populated counties that are part of the metropolitan Atlanta region (Clayton, DeKalb, Fulton, and Gwinnett) as well as examining socio-demographic factors that potentially characterize the clusters.
In high prevalence areas, such as Atlanta, understanding the spatial distribution of HIV is critical in determining how services should be allocated. Thus, this analysis may help improve public health policy and programmatic action for HIV prevention by informing local planning efforts for service provision within identified areas.
Methods
Study Population
The study population consisted of all persons diagnosed with HIV through 2007 residing in census tracts in Clayton, DeKalb, Fulton, and Gwinnett counties (n = 16,600), which comprise the central Atlanta metro region (population = 2,864,969).23 Census tracts are small, relatively homogeneous geographic units whose boundaries are determined by local residents.24 As census tracts boundaries are somewhat artificial and can be subject to the modifiable area unit problem, having the exact location of cases would have been preferable; however, due to confidentiality concerns around HIV this was not possible.25,26 Therefore, census tracts were used as they were the smallest geographic unit available. They are designed to be stable over time and are considered a preferred unit for geographic analyses when compared to non-population based measures, such as zip codes.27
Data on race, ethnicity, educational attainment, employment, and poverty were obtained from the US Census Bureau (2000 data) for each census tract in the four county area. HIV prevalence data (as of October 2007) was obtained from the Georgia Division of Public Health. The prevalent HIV cases were geocoded to census tract and listed with sex and exposure status (exposure status represents how the infection was acquired—through heterosexual contact, homosexual contact, IV drug use, or a combination of these).
The list of local HIV prevention service organizations was compiled from three published sources of HIV services, including a resource guide by the Southeast AIDS Training and Education Center, the African American Outreach Initiative, and the Kennesaw State University’s Georgia Statewide Resource Inventory (n = 26). These organizations were geocoded by address for the spatial analysis. Geographic coordinates of the census tract polygon centroids represented the 391 census tracts in the spatial analysis.
Cluster Identification
Clusters are defined geographic areas in which the prevalence of disease is disproportionately higher compared to neighboring areas. While many statistical methods have been designed to indentify clustering, many of them have limitations.28 Tests for global clustering detect the existence of at least one cluster, but not the specific location of the cluster(s).29 In contrast, cluster detection tests can provide the likely location of these clusters without a priori assumptions about the number, location, or size of the clusters.30 For this analysis, Kulldorff's spatial scan method, which has been widely used to detect clustering in a variety of health-related fields, was used to determine which census tracts fell in clusters of high HIV prevalence.27–29,31–36 Kulldorff's spatial scan method provides the number and location of spatial clusters.
We used the Poisson distribution to analyze the HIV prevalence data as the number of cases was significantly smaller than the population at risk. Random datasets of cases following the Poisson distribution were generated using 999 Monte Carlo simulations under the null hypothesis (i.e., that the expected HIV case counts in each census tract are proportional to the population of the tract and are randomly distributed). Potential clusters were identified by a circular window that scans each census tract centroid, maximizing the disproportion in HIV prevalence between the inside of the window and the outside of the window. Potential cluster size was defined by the percent of the population used in the analysis, with the lower limit automatically set to 0% and the upper limit set to 50%. We chose 50% as the upper limit because it allowed for the largest applicable cluster size (higher percentage would identify areas with unusually low rates outside the window rather than areas with high rates inside the window). Setting the upper limit to 50% allowed the program to search for both small and large clusters without pre-selection bias.
A maximum likelihood ratio is calculated for each potential cluster and the likelihood ratios of the observed events are tested against the distribution values from the simulated events. This method accounts for uneven geographic population distribution between the census tracts and does not assume an a priori spatial cluster size.28 The cluster identification was done using the SaTScan Program (version 8.0, freeware, http://www.satscan.org).
Analysis of Census Tract Level Demographic Variables
After cluster tracts were determined, unadjusted and adjusted logistic regression models were used to analyze socio-demographic variables associated with a tract being in a cluster. Poverty, measured as the percent of people living below the poverty line in each census tract, was treated as the main exposure with the other covariates being potential confounders. Potential cofactors included in the analysis were the proportion of HIV cases by each exposure category; the proportion of African American, Caucasian, Hispanic/Latino, and multiracial residents; and the proportion of high school graduates and unemployed adults. In addition, we examined spatial variation by gender within the detected cluster area. All cofactors were measured at the census tract level and expressed as percent per tract, eg, percent of census tract that is African American, percent of census tract that is multi-racial. The adjusted model was tested for multicollinearity and assessed for confounding. Cofactors were classified as significant and remained in model when the P-value was less than 0.05. Additionally, we tested and adjusted for spatial autocorrelation using a spatial weight matrix in the model.
Spatial Analysis
The census tracts in the identified cluster were mapped onto choropleth maps of HIV prevalence. The number of census tracts in the cluster and its approximate area were calculated. The HIV prevalence ratio between the cluster and the surrounding area was calculated, as well as the percent of overall cases contained in the cluster. Travel time was calculated from each census tract centroid, which is used to represent the geographic location of each tract, to the nearest local HIV prevention service organizations. Finally, the number of service providers located inside the cluster was determined. All spatial analysis was done in ArcGIS 9.3 (ESRI, 2008).
Results
Characteristics of Study Population
The population living in the 391 census tracts included in this study had the following median demographic characteristics: age, 32.7 years (interquartile range (IQR), 29.9–35.7); family size, 2.7 (IQR, 2.4–3.0); 36% were African American; and 45% Caucasian; 25.1% have completed high school. The median percent of Hispanics living in the census tracts was less than 0.001%.
HIV cases in the metro area were predominately male (78%, n = 13,028) and African-American (72%, n = 12,023) with Caucasians being the largest racial minority (22%, n = 3,689). Only 4% (n = 697) of cases from all races were ethnically Hispanic. Men who have sex with men were the largest exposure category (42%, n = 6,998) followed by IV drug users (10%, n = 1,691). A large number of cases had an unreported exposure category (33%, n = 5,433) (Table 1).
Table 1.
Demographic characteristics of the HIV epidemic in Atlanta, GA
| HIV+ individuals n = 16,600 (%) | Entire study region n = 2,306,836 | |
|---|---|---|
| Exposure | ||
| IV drug user | 1,691 (10.2%) | – |
| MSM | 6,998 (42.2%) | – |
| MSM and IDU | 755 (4.6%) | – |
| Heterosexual transmission | 1,656 (10.0%) | – |
| Not reported | 5,433 (32.7%) | – |
| Other | 68 (0.4%) | – |
| Gender | ||
| Male | 13,028 (78.5%) | 1,136,147 (49.3%) |
| Female | 3,567 (21.5%) | 1,170,689 (50.7%) |
| Race | ||
| Black/African American | 12,023 (72.4%) | 917,052 (39.8%) |
| Caucasian | 3,689 (22.2%) | 1,061,483 (46.0%) |
| Hispanic (all races) | 697 (4.2%) | 182,463 (7.9%) |
| Other | 191 (1.2%) | 145,838 (6.3%) |
Cluster Location and Characteristics
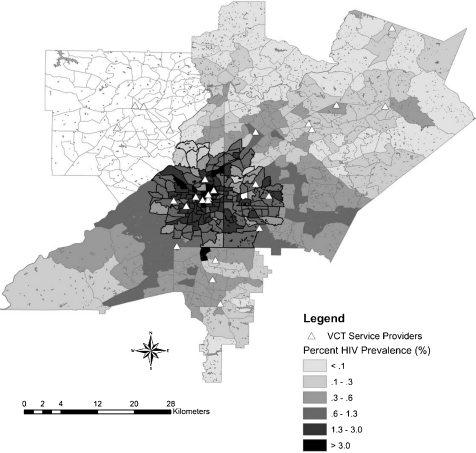
The cluster identification process revealed one large cluster, consisting of 157 tracts. The cluster was centralized in downtown Atlanta, spreading out toward the city edges (Figure 1). It consisted of 105 census tracts from Fulton County, 52 tracts from DeKalb County, and no census tracts from either Clayton or Gwinnett counties. The area of the cluster was 180 square miles with a diameter of approximately 15 miles. The census tracts contained within the cluster were smaller than those not in the cluster, indicating a higher population density. Tracts within the cluster had an average area of 1.19 square miles (range: 0.035–11.57) and tracts outside the cluster had an average area of 5.06 square miles (range: 0.46–70.32). Over the entire study area, average tract size was 3.50 square miles (range: 0.35–70.32).
FIGURE 1.
Overlay of prevalent HIV clustering and VCT provision in a four-county area.
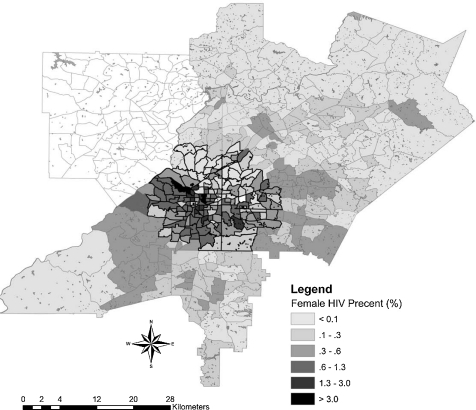
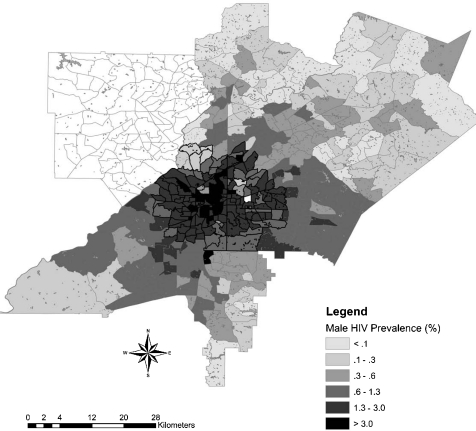
The cluster contained 60% of prevalent HIV cases in the metro area. The HIV prevalence within the cluster was 1.34%, compared to 0.32% outside the cluster. Tracts included in the cluster were associated with higher levels of poverty (OR = 1.19), lower density of multi-racial individuals in the population (OR = 1.85), and higher prevalence of behaviors that increase the risk of HIV exposure such as injection drug use (OR = 1.99), men having sex with men (OR = 3.01), and men having sex with men and IV drug use (OR = 1.6) (Table 2). In addition, examination of spatial variation by gender revealed important differences within the geographic cluster. HIV-positive men were more likely to live in the cluster than HIV-positive women (OR = 1.45, CI [1.35, 1.56]) (Figures 2 and 3).
Table 2.
Covariate associations with being in an HIV cluster
| Factor | Beta | SE | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | Mean (%) | SD | Minimum (%) | Maximum (%) |
|---|---|---|---|---|---|---|---|---|
| Poverty | 0.17 | 0.026 | 8.51 (5.30, 13.66) | 1.19 (1.13, 1.25) | 14.67 | 14.10 | 0 | 75.65 |
| Exposure | ||||||||
| IV drug user | 0.69 | 0.19 | 1.46 (1.33, 1.59) | 1.99 (1.36, 2.89) | 9.03 | 8.00 | 0 | 50.00 |
| MSM | 1.10 | 0.18 | 1.093 (0.73, 1.64) | 3.01 (2.10, 4.32) | 42.74 | 17.24 | 0 | 100.00 |
| MSM and IDU | 0.47 | 0.13 | 1.27 (1.18, 1.37) | 1.59 (1.23, 2.07) | 3.90 | 4.64 | 0 | 33.33 |
| Multi-racial | −0.62 | 0.15 | 1.64 (1.35, 1.96) | 1.85 (1.39, 2.50) | 1.79 | 1.10 | 0 | 7.99 |
FIGURE 2.
Overlay of prevalent HIV cases among females in a four-county area.
FIGURE 3.
Overlay of prevalent HIV cases among males in a four-county area.
Forty-two percent (N = 11) of community-based or AIDS service providers who offer voluntary testing and counseling in Atlanta are located in the cluster. The average travel time for Atlanta residents to these providers was calculated to be 13 minutes by car (SD = 9.24). Accessing these services on public transportation would likely take longer, depending on the public transit resources in the specific area.
Discussion
This study provides a unique visual description of HIV in Atlanta, Georgia. In addition, it considers the complex interplay of socioenvironmental characteristics at the neighborhood level that impact HIV prevalence in this area beyond reported individual risk factors. Using census data, we examined socioeconomic variables such as poverty, educational attainment, and employment status of residents within the defined census tracts. This approach builds upon other cluster analytic approaches by employing a community-level analysis to identify salient factors for structural interventions in this geographic area.2,37,38 As a major aim of the study was to improve public health practice by informing local planning efforts for service provision within identified areas, we also examined access to voluntary HIV testing and counseling sites within the four counties.
Previous studies have found that proximity is a major factor in social relationships in urban neighborhoods and that neighborhood social contexts influence social networks as well as individual actions.39,40 In a more specific finding, a 2001 study found that the experience of living in neighborhoods characterized by high poverty and drug use affected risk-taking behaviors.41 Likewise, researchers have found IV drug user risk of HIV is tied into the structural and social composition of their social networks.42 Therefore, increased risk of HIV inside the cluster may be a product of the structural and social environment in which the residents of this area live. In addition to targeted public health interventions, these communities may need structural interventions in order to disrupt the spread of HIV.
The findings reveal a unique picture of HIV prevalence in Atlanta centralized in a region within census tracts that include parts of the two largest counties in the Atlanta metropolitan area (MSA). These counties, in which excess cases of HIV were observed, include DeKalb and Fulton. In the cluster, the HIV prevalence was 1.34% compared to 0.32% outside the cluster. With an HIV prevalence of 1.34%, this cluster can be considered to be an area compatible with a “generalized epidemic,” as defined as an area in which the HIV prevalence is ≥1.0% in the general population.43 We did not find evidence of spatial clustering in Gwinnett and Clayton counties. Our data suggests that the HIV epidemic in Georgia is a concentrated one. As of 2007, the metropolitan area has reported 21,354 cases of HIV/AIDS, representing 64% of all reported cases statewide.5 Furthermore, within Atlanta, 157 contiguous census tracts form an area that contains 60% of prevalent cases in the city.
The geographic spread of HIV in the Atlanta area differed from other urban HIV distributions in US cities. A study of the geographic distribution of AIDS performed in New York City found that cases were clustered in specific neighborhoods of Manhattan and the Bronx.37 San Francisco exhibited a similar clustering pattern with larger numbers of HIV cases concentrated to specific neighborhoods.44 Likewise, Seattle's high HIV/AIDS diagnosis rates were concentrated in downtown neighborhoods, with lower rates found in areas farther from the center of the city.45 In contrast, our results indicate the prevalence of HIV cases is more generalized within the central metro-Atlanta area. Despite the dissimilarity in Atlanta’s spatial distribution, factors associated with clusters of HIV were similar among all of these cities (larger populations of MSM and areas of low socioeconomic status).
The likelihood of a census tract being included in the cluster was also associated with higher levels of poverty, and HIV behavioral risk factors such as injection drug use, men having sex with men (MSM), and injection drug usage by MSM. Much like the recent CDC study, we found HIV was more associated with poverty than race.22 These findings similarly compare to other reports indicating the dynamic interplay of individual and community-level predictive risk factors.37,38 Thus, our study demonstrates socioeconomic and cultural characteristics of particular communities may also be important associative risk factors. Future structural interventions such as poverty alleviation, educational attainment, and employment opportunities may ameliorate the conditions within the cluster that foster greater HIV transmission.
Racial data for HIV cases was unavailable by census tract for this study and was only provided for the aggregate four county area. However, we examined spatial variation by gender. The findings reveal spatial heterogeneity with a higher prevalence of HIV-positive men than women in the cluster. This may, in part, be explained by a greater density of MSM living in Atlanta’s midtown area who may be selecting local partners. Thus the demographic and risk characteristics are associated with greater male prevalence in this region. Such behavioral phenomena have also been observed in a spatial study of sexually transmitted infections and HIV in Wake County, North Carolina.38
Alternatively, most of the women in this study likely acquired HIV via heterosexual contact.5 In these cases, there is a lesser probability of local partner selection given more diffuse residential patterns. This finding suggests that HIV service provision for women should be more widespread in affected communities, whereas for men the services may be more concentrated in specific geographic zones.
Our study offers some insights on approaches that may optimize the ability to reach populations in the cluster area for voluntary HIV testing and counseling (VCT) and subsequent linkage to care. With 42% of HIV VCT providers located within the detected cluster, there is opportunity to expand service provision and education to communities in need. The majority of the community-based (CBOs) and AIDS service organizations (ASOs) that offer VCT are within the central downtown area or western portion of the cluster area. Communities located to the north and southeast of the downtown area have fewer HIV prevention resources despite the presence of several census tracts with ≥2.0% prevalence. Enhancing services within these areas where need for HIV prevention is apparent may contribute to future reduction of HIV incidence.
While HIV incidence rates have remained relatively stable in the past 5 years, the Georgia Division of Public Health found gaps in knowledge, care, prevention, and intervention services related to HIV in the state of Georgia.5,9 Even if these gaps were breached, within at-risk populations denial of risk factors and fear of being HIV-positive are barriers to accepting testing. Prevention education needs to address these issues in order to be effective.46
Moreover, although our study estimated a 13-minute travel time to ASOs and CBOs that offer VCT, accessibility may be an issue for those living in poverty (i.e., those without access to vehicles, etc.). The implementation of mobile HIV prevention units or establishment of physical space in these neighborhoods to serve lower-income residents may be a useful strategy in curtailing the spread of HIV.
Limitations
The present study has several limitations that need to be taken into consideration. The State of Georgia mandated HIV reporting as of December 31, 2003, which limited our ability to detect temporal trends in the MSA. The sensitive nature of HIV infection requires a level of confidentiality which prevented us from using a more precise measure of geographic location. The results of analysis may have been influenced by the aggregation of the data. With respect to behavioral risk criteria, the “no identified risk factor” (NIR) category may have resulted in significant underreporting of transmission type with 83% of female and 52% of male cases classified in this manner. Therefore, our study is unable to fully describe these individual risk characteristics in relation to other cofactors for which missing data are not a constraint. In addition, the data did not contain age as a factor for analysis. Racial data was similarly not provided at the census tract level and was considered in the analysis at the county level. Finally, travel time estimates are probably low, given the use of census tract as proxy for true residential address and that the time did not account for traffic, which is a consideration in this MSA.
Conclusion
The HIV epidemic in Atlanta is concentrated primarily in one large cluster characterized by a large prevalence of poverty, MSM populations, and IV drug usage. Prevention efforts targeted to the population living in this area, including structural interventions and the promotion of VCT by agencies situated in or near the affected census tracts, may be most beneficial in curtailing the epidemic within the identified cluster.
Acknowledgements
We thank Jennifer Taussig, Richard Dunville, and the HIV Epidemiology Unit at the Georgia Department of Community Health for assistance with data preparation. We also thank Emily McCollum for her valuable assistance reviewing the manuscript. Special thanks to our partner agencies for their support of this study including AID Atlanta, National AIDS Education and Services for Minorities (NAESM), SisterLove, Someone Cares, and Stand, Inc.
Sources of Support This study was supported by the Emory Center for AIDS Research (P30 AI050409), Global Health Institute, the Emory Vaccine Center (U19 AI057266), and the Emory HIV/AIDS Clinical Trials Unit (U01 AI069418).
References
- 1.Centers for Disease Control and Prevention. New HIV Incidence Estimates: CDC Responds. CDC HIV/AIDS Facts. 2008. http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/pdf/response.pdf.
- 2.Bautista CT, Sateren WB, Sanchez JL, Singer DE, Scott P. Geographic mapping of HIV infection among civilian applicants for United States military service. Health Place. 2008;14(3):608–615. doi: 10.1016/j.healthplace.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Southern States AIDS Directors Work Group. Southern States Manifesto: Update 2008. HIV/AIDS and Sexually Transmitted Diseases in the South. http://www.nmac.org/index/southern-states-manifesto. Accessed February 16, 2010.
- 4.Reif S, Geonnotti K, Whetten K. HIV infection and AIDS in the Deep South. Am J Public Health. 2006;96(6):970–973. doi: 10.2105/AJPH.2005.063149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georgia Department of Human Resources. 2007 Georgia HIV/AIDS Surveillance Summary. http://www.health.state.ga.us/epi/hivaids. Accessed on November 29, 2009.
- 6.Kaiser State Health Facts. Georgia: HIV Infection Cases Reported among States with Confidential Name-Based Reporting. 2007. http://www.statehealthfacts.org/profileind.jsp?cmprgn=1&cat=11&rgn=12&ind=521&sub=122.
- 7.Georgia Community Planning Group and Georgia Division of Health. Comprehensive HIV Prevention Plan 2009–2013, 2008. http://health.state.ga.us/pdfs/hivaids/2009HIVPreventionPlan.pdf.
- 8.Priddy FH, Pilcher CD, Moore RH, et al. Detection of acute HIV infections in an urban HIV counseling and testing population in the United States. J Acquir Immune Defic Syndr. 2007;44(2):196–202. doi: 10.1097/01.qai.0000254323.86897.36. [DOI] [PubMed] [Google Scholar]
- 9.State of Georgia Department of Human Resources. The Georgia HIV/AIDS Epidemic. Atlanta, GA; 2008.
- 10.US Census Bureau—Population Division. Annual Estimates of the Population of Metropolitan and Micropolitan Statistical Areas: April 1, 2000 to July 1, 2008 (CBSA-EST2008-01). http://www.census.gov/popest/metro/CBSA-est2008-annual.html. Published in 2009. Accessed on July 25, 2010.
- 11.US Census Bureau. Atlanta city, Georgia: ACS Demographic and Housing Estimates: 2006–2008. http://factfinder.census.gov/servlet/ADPTable?_bm=y&-geo_id=16000US1304000&-qr_name=ACS_2008_3YR_G00_DP3YR5&-ds_name=ACS_2008_3YR_G00_&-_lang=en&-redoLog=false&-_sse=on. Published in 2009. Accessed on July 25, 2010.
- 12.US Census Bureau. Atlanta city, Georgia: Selected Economic Characteristics: 2006-2008. http://factfinder.census.gov/servlet/ADPTable?_bm=y&-geo_id=16000US1304000&-qr_name=ACS_2008_3YR_G00_DP3YR3&-ds_name=&-_lang=en&-redoLog=false. Published in 2009. Accessed on July 25, 2010.
- 13.US Census Bureau. Atlanta city, Georgia: Selected Social Characteristics in the United States: 2006-2008. http://factfinder.census.gov/servlet/ADPTable?_bm=y&-geo_id=16000US1304000&-qr_name=ACS_2008_3YR_G00_DP3YR2&-ds_name=&-_lang=en&-redoLog=false. Published in 2009. Accessed on July 25, 2010.
- 14.Vermund SH, Hodder SL, Justman JE, et al. Addressing research priorities for prevention of HIV infection in the United States. Clin Infect Dis. 2010;50(Suppl 3):S149–S155. doi: 10.1086/651485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geanuracos CG, Cunningham SD, Weiss G, Forte D, Reid LM, Ellen JM. Use of geographic information systems for planning HIV prevention interventions for high-risk youths. Am J Public Health. 2007;97(11):1974–1981. doi: 10.2105/AJPH.2005.076851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chutuape KS, Ziff M, Auerswald C, Castillo M, McFadden A, Ellen J. Examining differences in types and location of recruitment venues for young males and females from urban neighborhoods: findings from a multi-site HIV prevention study. J Urban Health. 2009;86(1):31–42. doi: 10.1007/s11524-008-9329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weir SS, Pailman C, Mahlalela X, Coetzee N, Meidany F, Boerma JT. From people to places: focusing AIDS prevention efforts where it matters most. AIDS. 2003;17(6):895–903. doi: 10.1097/00002030-200304110-00015. [DOI] [PubMed] [Google Scholar]
- 18.Wohl DA, Khan MR, Tisdale C, et al. Locating the places people meet new sexual partners in a southern US City to inform HIV/STI prevention and testing efforts. [published online ahead of print July 8, 2010]. AIDS Behav. Jul 8 2010. doi:10.1007/s10461-010-9746-4. [DOI] [PMC free article] [PubMed]
- 19.Haan M, Kaplan GA, Camacho T. Poverty and health. Prospective evidence from the Alameda County Study. Am J Epidemiol. 1987;125(6):989–998. doi: 10.1093/oxfordjournals.aje.a114637. [DOI] [PubMed] [Google Scholar]
- 20.Waitzman NJ, Smith KR. Phantom of the area: poverty-area residence and mortality in the United States. Am J Public Health. 1998;88(6):973–976. doi: 10.2105/AJPH.88.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forna FM, Fitzpatrick L, Adimora AA, et al. A case-control study of factors associated with HIV infection among black women. J Natl Med Assoc. 2006;98(11):1798–1804. [PMC free article] [PubMed] [Google Scholar]
- 22.Denning P, DiNenno, E. Communities in crisis: is there a generalized HIV epidemic in improverished urban areas of the United States? Paper presented at: International AIDS Conference; 21 July 2010; Vienna.
- 23.US Census Bureau. USA State and County QuickFacts. http://quickfacts.census.gov/qfd/states/13/1304000.html. Accessed on April 21, 2010.
- 24.Glossary of basic geographic and related terms—census 2000. Washington: Bureau of Census; 2001. [Google Scholar]
- 25.Coulton CJ, Korbin J, Chan T, Su M. Mapping residents’ perceptions of neighborhood boundaries: a methodological note. Am J Community Psychol. 2001;29(2):371–383. doi: 10.1023/A:1010303419034. [DOI] [PubMed] [Google Scholar]
- 26.Gehlke CE, Biel K. Certain effects of grouping upon the size of the correlation coefficient in census tract material. J Am Stat Assoc. 1934;29(185A):169–170. doi: 10.2307/2277827. [DOI] [Google Scholar]
- 27.Omer SB, Enger KS, Moulton LH, Halsey NA, Stokley S, Salmon DA. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol. 2008;168(12):1389–1396. doi: 10.1093/aje/kwn263. [DOI] [PubMed] [Google Scholar]
- 28.Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Stat Med. 1995;14(8):799–810. doi: 10.1002/sim.4780140809. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Pickle LW, Das B. Evaluating spatial methods for investigating global clustering and cluster detection of cancer cases. Stat Med. 2008;27(25):5111–5142. doi: 10.1002/sim.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu YW, Hsu CE, Wang MQ, Nkhoma ET. Examining geographic and temporal variations of AIDS mortality: evidence of racial disparities. J Natl Med Assoc. 2008;100(7):788–796. doi: 10.1016/s0027-9684(15)31372-9. [DOI] [PubMed] [Google Scholar]
- 31.Huang L, Stinchcomb DG, Pickle LW, Dill J, Berrigan D. Identifying clusters of active transportation using spatial scan statistics. Am J Prev Med. 2009;37(2):157–166. doi: 10.1016/j.amepre.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulldorff M, Feuer EJ, Miller BA, Freedman LS. Breast cancer clusters in the northeast United States: a geographic analysis. Am J Epidemiol. 1997;146(2):161–170. doi: 10.1093/oxfordjournals.aje.a009247. [DOI] [PubMed] [Google Scholar]
- 33.Kulldorff M, Mostashari F, Duczmal L, Katherine Yih W, Kleinman K, Platt R. Multivariate scan statistics for disease surveillance. Stat Med. 2007;26(8):1824–1833. doi: 10.1002/sim.2818. [DOI] [PubMed] [Google Scholar]
- 34.Mostashari F, Kulldorff M, Hartman JJ, Miller JR, Kulasekera V. Dead bird clusters as an early warning system for West Nile virus activity. Emerg Infect Dis. 2003;9(6):641–646. doi: 10.3201/eid0906.020794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heffernan R, Mostashari F, Das D, Karpati A, Kulldorff M, Weiss D. Syndromic surveillance in public health practice, New York City. Emerg Infect Dis. 2004;10(5):858–864. doi: 10.3201/eid1005.030646. [DOI] [PubMed] [Google Scholar]
- 36.Kulldorff M. A spatial scan statistic. Commun Stat., Theory Methods. 1997;26(6):1481–1496. doi: 10.1080/03610929708831995. [DOI] [Google Scholar]
- 37.Wallace RG. AIDS in the HAART era: New York’s heteroeneous geography. Soc Sci Med. 2003;56(6):1155–1171. doi: 10.1016/S0277-9536(02)00121-1. [DOI] [PubMed] [Google Scholar]
- 38.Law DC, Serre ML, Christakos G, Leone PA, Miller WC. Spatial analysis and mapping of sexually transmitted diseases to optimise intervention and prevention strategies. Sex Transm Infect. 2004;80(4):294–299. doi: 10.1136/sti.2003.006700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenbaum SD, Greenbaum PE. The ecology of social networks in four urban neighborhoods. Soc Netw. 1985;7(1):47–76. doi: 10.1016/0378-8733(85)90008-5. [DOI] [Google Scholar]
- 40.Huckfeldt RR. Social contexts, social networks, and urban neighborhoods: environmental constraints on friendship choice. Am J Sociol. 1983;89(3):651–669. doi: 10.1086/227908. [DOI] [Google Scholar]
- 41.Friedman SR, Flom PL, Kottiri BJ, et al. Consistent condom use in the heterosexual relationships of young adults who live in a high-HIV-risk neighbourhood and do not use “hard drugs”. AIDS Care. 2001;13(3):285–296. doi: 10.1080/09540120120043937. [DOI] [PubMed] [Google Scholar]
- 42.Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Soc Sci Med. 2005;61(5):1026–1044. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization and UNAIDS. Initiating Second Generation HIV Surveillance Systems: Practical Guidelines. (WHO/HIV/2002.17); 2002.
- 44.HIV/AIDS Epidemiology Annual Report: San Francisco Department of Public Health; 2008.
- 45.Geographic distribution of HIV/AIDS across King County. Seattle, WA: Public Health–Seattle & King County; 2008.
- 46.Kellerman SE, Lehman JS, Lansky A, et al. HIV testing within at-risk populations in the United States and the reasons for seeking or avoiding HIV testing. J Acquir Immune Defic Syndr. 2002;31(2):202–210. doi: 10.1097/00126334-200210010-00011. [DOI] [PubMed] [Google Scholar]