Abstract
Fibroblasts are heterogeneous mesenchymal cells that play important roles in the production and maintenance of extracellular matrix. Although their heterogeneity is recognized, progenitor progeny relationships among fibroblasts and the factors that control fibroblast differentiation are poorly defined. The current study was designed to develop a reliable method that would permit in vitro differentiation of fibroblast-like cells from human and murine embryonic stem cells (ESCs). Undifferentiated ESCs were differentiated into embryoid bodies (EBs) with differentiation media. EBs were then cast into type I collagen gels and cultured for 21 d with basal media. The spindle-shaped cells that subsequently grew from the EBs were released from the gels and subsequently cultured as monolayers in basal media supplemented with serum. Differentiated cells showed a characteristic spindle-shaped morphology and had ultrastructural features consistent with fibroblasts. Immunocytochemistry showed positive staining for vimentin and alpha-smooth muscle actin but was negative for stage-specific embryonic antigens and cytokeratins. Assays of fibroblast function, including proliferation, chemotaxis, and contraction of collagen gels demonstrated that the differentiated cells, derived from both human and murine ESCs, responded to transforming growth factor-β1 and prostaglandin E2 as would be expected of fibroblasts, functions not expected of endothelial or epithelial cells. The current study demonstrates that cells with the morphologic and functional features of fibroblasts can be reliably derived from human and murine ESCs. This methodology provides a means to investigate and define the mechanisms that regulate fibroblast differentiation.
Keywords: Embryonic stem cell differentiation, Fibroblasts, Three-dimensional collagen gel cultures
Introduction
Fibroblasts represent a heterogeneous population of mesenchymal cells that play important roles in the production and maintenance of extracellular matrix (Raghow 1994; Ohnishi et al. 1998; Phan 2008). In addition, fibroblasts have important regulatory roles modulating the function of many other cell types (Knight 2001; Nanki et al. 2001; Rennard 2001; Hay 2005). While fibroblast heterogeneity is clearly recognized, progenitor progeny relationships among fibroblasts and the factors that control fibroblast differentiation are poorly defined. This is, to a significant degree, complicated by a lack of surface markers to define differentiated fibroblast phenotypes. Fibroblasts, therefore, are, at the present time, best characterized by morphology, ultrastructure, and function supported by molecular marker expression (Powell et al. 1999a; Powell et al. 1999b; Fireman et al. 2001; Eyden 2004; Eyden 2005).
The ultimate progenitor cell is the embryonic stem cell (ESC), which in in vitro culture systems, ECSs can differentiate in to cells of many lineages. Interestingly, the culture of ESCs is most commonly accomplished by co-culture with fibroblast feeder layers, which provide undefined but necessary cofactors. Because xenogenic fibroblasts present a number of theoretical and technical problems, several investigators have described methods to prepare autogenic and syngenic fibroblasts from ESCs to use as feeder layers for ESCs (Xu et al. 2004; Stojkovic et al. 2005; Yoo et al. 2005; Choo et al. 2008; Chen et al. 2009). However, these studies have not demonstrated that the fibroblast-like cells function like fibroblasts, which would be a necessary step toward an experimental system to delineate the differentiation pathways leading to heterogeneous populations of fibroblasts. The current study, therefore, was designed to develop a reliable methodology that would permit in vitro differentiation of fibroblasts from human and murine ESCs. The development of this methodology provides a means for delineating the mechanisms that control fibroblast differentiation and that lead to functionally heterogeneous mature cell populations.
Materials and Methods
Materials.
Native type I collagen (rat tail tendon collagen [RTTC]) was extracted from rat-tail tendons by a previously published method (Elsdale and Bard 1972). Commercially available reagents were obtained as follows: transforming growth factor (TGF)-β1 was from R&D Systems (Minneapolis, MN); prostaglandin E2 (PGE2), monoclonal anti-α-smooth muscle actin (SMA), anti-pan cytokeratin monoclonal, anti-vimentin monoclonal antibodies, anti-mouse IgG FITC (fluorescein isothiocyanate stain-green immunofluorescence) conjugate, propidium iodide and 2-mercaptoethanol were from Sigma (St. Louis, MO); ESGRO® (leukemia inhibitory factor; LIF), anti-stage specific embryonic antigen (SSEA)-1 and 4 monoclonal antibodies were from Chemicon International (Temecula, CA); Dulbecco’s modified eagle’s medium (DMEM), fetal calf serum (FCS), DMEM/F12 [1:1 mixture], KnockOut™ serum replacement, KnockOut™ DMEM, non-essential amino acids, l-glutamine, basic fibroblast growth factor (bFGF), collagenase type IV, and 0.05% Trypsin-EDTA were from Invitrogen (Carlsbad, CA).
Cell culture and differentiation.
Human ESCs Culture. The National Institutes of Health-approved human embryonic stem cell line H9.2 (passages 45–65; WiCell Research Institute, Madison, WI) was used in this study with the approval of the Institutional Review Board and Embryonic Stem Cell Research Oversight committee of the University of Nebraska Medical Center. Undifferentiated human ESCs were cultured on irradiated mouse embryonic fibroblasts (MEF) in six-well plates with human ESC culture medium containing 80% DMEM/F12, 20% KnockOut™ serum replacement, 1% non-essential amino acids, 1 mmol/l l-glutamine, 0.1 mmol/l 2-mercaptoethanol, and 4 ng/ml bFGF. Colonies were mechanically dissected with finely pulled glass micropipettes (1.0 mm OD; Clark Electromedical Instruments, Reading, UK) every 7 d and transferred to a freshly prepared MEF layer.
Culture of embryoid bodies in type I collagen gels.
To prepare embryoid bodies (EBs), human ESCs from four- to five-wells of a six-well plate were treated with 1 mg/ml collagenase and cells were collected by centrifugation at 200×g for 2 min. The pellet was resuspended in differentiation medium containing 90% DMEM/F12, 10% Knockout serum replacement, 1% non-essential amino acids, and 1 mmol/l l-glutamine without 2-mercaptoethanol and bFGF (Schuldiner et al. 2000). Cells were then placed into a Petri dish (Sarstedt, Nümbrecht, Germany) and cultured for 4–5 d. Floating EBs from the Petri dish were collected into a 50 ml polypropylene conical tube (Falcon; Becton-Dickinson Labware, Franklin Lakes, NJ) and precipitated without centrifugation.
Collagen gels were prepared as described previously (Mio et al. 1996). Briefly, RTTC, distilled water and 4× concentrated DMEM were combined so that the final mixture resulted in 0.75 mg/ml collagen, with a physiologic ionic strength of 1× DMEM at pH 7.4. EBs from a Petri dish were then suspended in the neutralized collagen solution. Aliquots (1.0 ml/well) of the mixture of EBs in collagen were then cast into each well of a 12-well tissue culture plate (Falcon) and allowed to polymerize. After polymerization was completed, normally within 20 min at room temperature, basal medium (1:1 mixture of differentiation medium and DMEM/F12) was added on the top of the gels in a 12-well plate (1.0 ml/well). The basal medium was changed every 2–3 d and EBs were cultured for 21 d in type I collagen gels.
Murine ESCs and EBs culture.
The murine embryonic stem cell line (CRL-11632) was obtained from the American Type Culture Collection (Rockville, MD). KnockOut™ DMEM with 20% KnockOut serum replacement, 1% non-essential amino acid, 1 mmol/l l-glutamine, 0.1 mmol/l 2-mercaptoethanol and 103 units/ml LIF was used for culture medium, and KnockOut™ DMEM with 2% FCS for basal medium. Murine ESCs and EBs were cultured using the same methods as human cells.
Differentiated fibroblast culture.
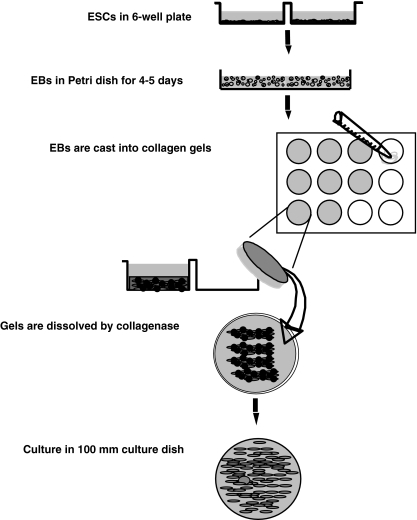
The gels in a 12-well culture plate were dissolved with 1 mg/ml collagenase at 37°C in a 5% CO2 atmosphere for 1 h. The resulting cells were resuspended with DMEM containing 10% FCS (10% FCS-DMEM) and centrifuged at 200×g for 5 min. The cells, containing EBs, were cultured in a 100 mm tissue culture dish (Falcon) with DMEM containing 10% FCS, 45 units/ml penicillin, 45 μg/ml streptomycin, and 1 μg/ml amphotericin B. When near confluent, the cells were trypsinized gently to prevent EBs from detaching and the cells were passaged in 10% FCS-DMEM (Fig. 9). Cultures were routinely inspected using phase contrast microscopy and cells were assessed after 4–5 passages.
Figure 9.
Schematic illustration of the method for differentiation of ESCs into fibroblasts in three-dimensional type I collagen gel culture. Undifferentiated ESCs are cultured on MEF feeder layer in six-well plate. ESCs are detached with collagenase and re-suspended with differentiation medium. Cells are then placed into a Petri dish and cultured for 4–5 d to allow formation of EBs. EBs are cast into type I collagen gels in a 12-well plate (1.0 ml/well) and cultured for 21 d three-dimensionally in collagen gels with basal medium. Gels are dissolved by collagenase and the cells are suspended in 10% FCS-DMEM. Differentiated fibroblasts are cultured in 100 mm culture dishes. EBs are lost with serial feeding and passaging.
Collagen gel contraction assay.
Collagen gels were prepared as described previously (Mio et al. 1996). Differentiated fibroblasts were trypsinized and mixed with the neutralized collagen solution so that the final cell density in the collagen solution was 3 × 105 cells/ml. Aliquots (0.5 ml/well) of the mixture of cells in collagen were cast into each well of 24-well tissue culture plates (Falcon) and the mixture was allowed to polymerize. After polymerization was completed, the gels were gently released from the 24-well tissue culture plates and transferred into 60-mm tissue culture dishes (three gels in each dish) which contained 5 ml of freshly prepared serum-free DMEM (SF-DMEM) with or without 10−10 mol/l TGF-β1 or 10−7 mol/l PGE2. The gels were then incubated at 37°C in a 5% CO2 atmosphere for 5 d. Gel contraction was quantified using an Optomax V image analyzer (Optomax, Burlington, MA) daily. Data were expressed as percentage of the initial gel size.
Chemotaxis assay.
Cell migration was assessed using the Boyden blindwell chamber (Neuroprobe Inc., Gaithersburg, MD) as previously described (Boyden 1962). Briefly, 26 μl of SF-DMEM containing human fibronectin (20 μg/ml) was placed into the bottom wells. Eight-micrometer pore polycarbonate membranes (Neuroprobe Inc.), which were precoated with 5 μg/ml gelatin in 0.1% acetic acid, were employed. Cells were trypsinized and suspended with 10%FCS-DMEM to stop the trypsin. Cells were then pelletted and re-suspended in SF-DMEM at a density of 1 × 106/ml. Fifty microliters of the cell suspension supplemented with or without TGF-β1 (10−10 mol/l) or PGE2 (10−7 mol/l) were then added into each top well. Cells were allowed to migrate at 37°C in a 5% CO2 atmosphere for 12 h. Cells that had not migrated were scraped off the upper surface of the membrane, and the membranes were air-dried. Cells were then stained with PROTOCOL (Fisher Scientific, Swedesboro, NJ) and mounted on a glass microscope slide. Chemotaxis was assessed by counting the number of cells in five high-power fields.
Proliferation assay.
Cells were plated into 12-well plates (105 cells per each well) in 10% FCS-DMEM with or without 10−10 mol/l TGF-β1 or 10−7 mol/l PGE2. Cells were fed with fresh 10% FCS-DMEM every 2 d. Cell numbers from three separate wells were determined after 24, 72, and 120 h using a Coulter electronic cell counter (Beckman Coulter Inc., Fullerton, CA).
Immunohistochemistry.
Differentiated EBs in type I collagen gels were fixed in 4% paraformaldehyde for 30 min. Differentiated fibroblasts were cultured until sub-confluent in eight chamber slides (Nunc Inc, Naperville, IL) in 10% FCS-DMEM and fixed in 4% paraformaldehyde for 30 min at passage 4. Cells were washed briefly with phosphate buffered saline followed by permeabilization with 0.1% Triton in sodium citrate buffer at 4°C for 5 min. After blocking with horse serum, the cells were incubated with monoclonal anti-α-SMA (1:200 dilution), anti-pan cytokeratin (1:200), anti-vimentin (1:200), anti-SSEA-1 (1:100), or anti-SSEA-4 (1:100) antibodies at 4°C overnight. After washing, cells were then incubated with FITC-conjugated anti-mouse IgG antibody followed by nuclear staining with propidium iodide. Stained cells were visualized and photographed using a Nikon Eclipse TE300 microscope (Nikon, Tokyo, Japan) equipped with a DP71 digital camera (Olympus, Tokyo, Japan).
Electron microscopic examination.
Cells were cultured on Thermanox™ coverslips (Thermo Fisher Scientific, Rochester, NY) and fixed in 2% glutaraldehyde, 2% paraformaldehyde, and 0.5% acrolein. After washing with 0.1 mol/l Sorenson’s phosphate buffer, samples were post-fixed in 1% osmium tetroxide. Samples were then washed with buffer and dehydrated in a graded ethanol series. After dehydration samples were embedded in Araldite. Thin sections were stained with 2% uranyl acetate and Reynolds lead citrate, and examined using a Philips 410LS transmission electron microscope (Philips Electronics, Eindhoven, The Netherlands) operated at 60Kv. Images were acquired with an Advanced Microscopy Techniques digital imaging system (Danvers, MA).
Statistical analysis.
Data were expressed as means ± standard error of the mean (SEM). Experiments with multiple comparisons were evaluated using one-way analysis of variance followed by Bonferroni’s test. For all comparisons, significance was determined using separate experiments performed on different occasions. Probability values of <0.05 were considered significant.
Results
Differentiation of human ESCs into fibroblast-like cells in three-dimensional type I collagen gel culture.
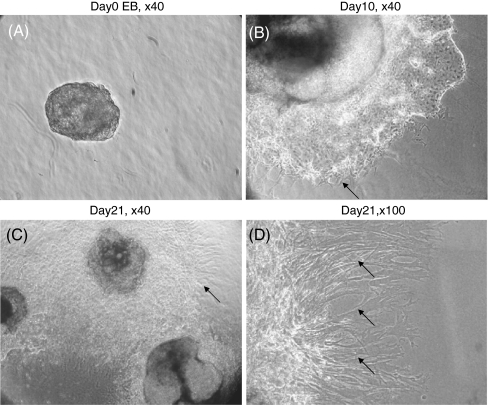
Generally, spindle-shaped cells appeared surrounding human EBs after 7–10 d of culture in three-dimensional type I collagen gels, and were increasingly prominent with further culture to day 21 (Fig. 1). The spindle-shaped cells were collected following collagenase treatment and re-plated into tissue culture plates in 10% FCS-DMEM. The EBs were also released from the collagen gel, but remained floating and were lost with serial feeding and passaging. The fibroblast-like cells were used at passage 4–6 for subsequent characterization.
Figure 1.
Morphology of human EBs differentiating in type I collagen gel culture. EBs were cast into type I collagen gels and allowed to differentiate for 21 d. (A) Day 0, (B) day 10, (C) day 21 (original magnification, ×40), (D) higher magnification (×100) demonstrating spindle-shaped cells surrounding differentiated EBs (arrows).
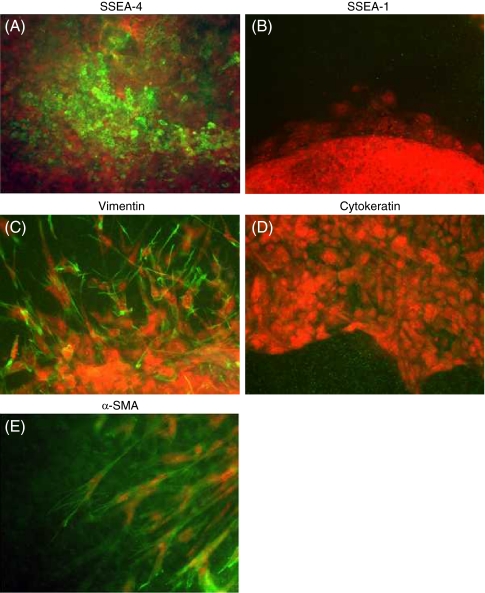
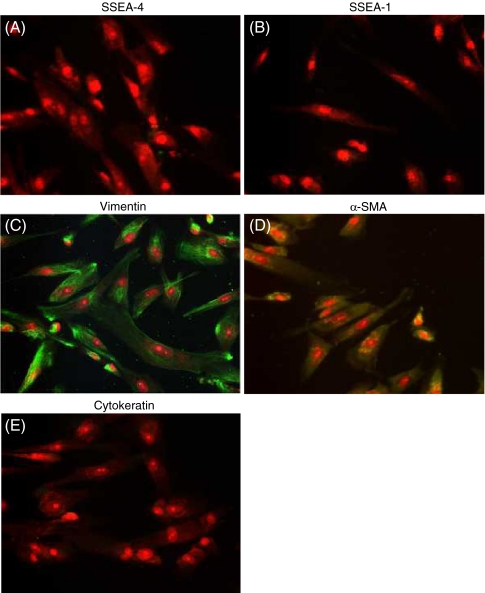
To evaluate the differentiation of human EBs in three-dimensional type I collagen gels, we first assessed several markers of cell differentiation by immunocytochemistry. EBs in collagen gels were SSEA-4 positive, a marker for undifferentiated embryonic stem cells, but SSEA-1 negative (Fig. 2A, B). Spindle-shaped cells surrounding the EBs were positive for vimentin and negative for cytokeratin (Fig. 2C, D). The leading edge of the spindle-shaped cells showed positive staining for α-SMA (Fig. 2E). We also stained fibroblast-like cells in monolayer culture. These cells were negative for both SSEA-4 and SSEA-1 (Fig. 3A, B). In contrast, fibroblast-like cells in monolayer culture showed positive staining for vimentin in 96% of cells (Fig. 3C) and for α-SMA in 92% of cells (Fig. 3D). They were entirely negative for cytokeratin (Fig. 3E). We further assessed the ultra-structural feature of the fibroblast-like cells derived from human ESCs by transmission electron microscopy. Cells showed a characteristic spindle-shaped morphology with prominent rough endoplasmic reticulum and stress fibers, which were consistent with fibroblasts and myofibroblasts (Figs. 4A, B; Dell’Orbo et al. 1992).
Figure 2.
Immunocytochemistry of differentiating human EBs in type I collagen gel culture. EBs were cast into three-dimensional collagen gels and allowed to differentiate. On day 21, cellular biomarkers were evaluated by immunocytochemistry. (A) SSEA-4, (B) SSEA-1, (C) vimentin, (D) cytokeratin, (E) α-SMA (original magnification, ×200).
Figure 3.
Immunocytochemistry of differentiated fibroblasts in monolayer culture. Collagen gels in which EBs had been cultured for 21 d were dissolved with collagenase and the cells were passaged into monolayer culture. At passage 4, cellular biomarkers were assessed by immunocytochemistry. (A) SSEA-4, (B) SSEA-1, (C) vimentin, (D) cytokeratin, (E) α-SMA (original magnification, ×200).
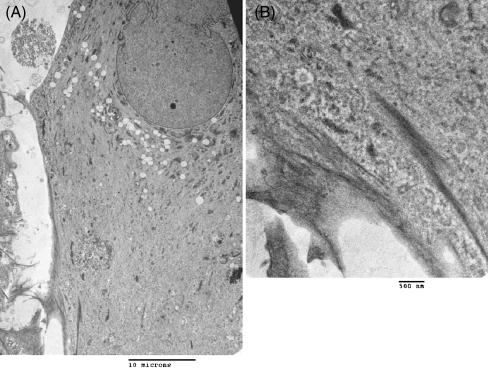
Figure 4.
Ultra-structure of fibroblasts derived from human ESCs. Cells were cultured on Araldite coverslips for transmission electron microscopy. Cells derived from human ESCs showed a characteristic spindle-shaped morphology with a prominent rough endoplasmic reticulum and stress fibers (A: magnification, ×2,400; (B) magnification ×14,000).
Characteristics of differentiated fibroblast-like cells.
To assess the functional features of the fibroblast-like cells, we evaluated cell proliferation, chemotaxis, and contraction of three-dimensional type I collagen gels mediated by the differentiated fibroblasts. In addition, we investigated the ability of exogenous TGF-β1 or PGE2 to modulate each function.
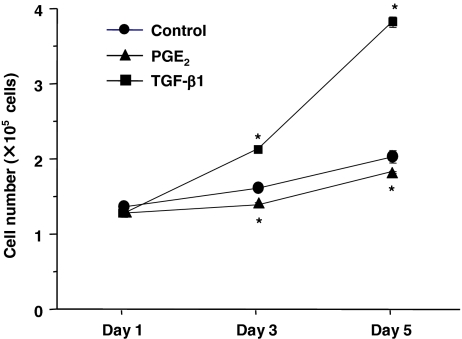
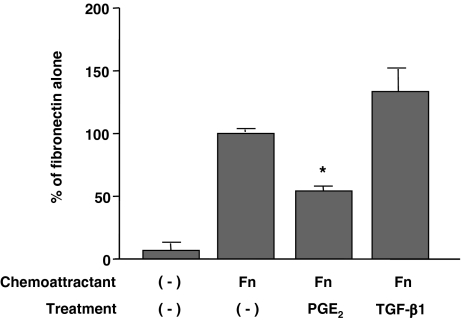
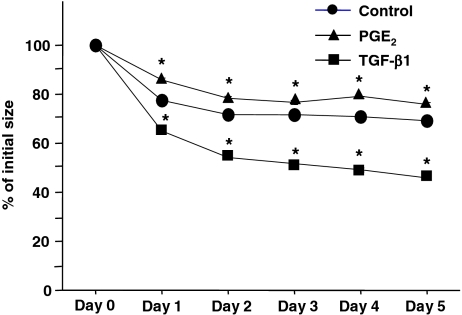
Differentiated fibroblasts slowly grew in 10% FCS-DMEM with longer than 48 h of doubling time (Fig. 5). Exogenous TGF-β1 significantly stimulated cell proliferation, whereas PGE2 significantly suppressed proliferation (Fig. 5). Ability of cell migration was assessed by the chemotaxis assay using fibronectin as a chemoattractant. Consistent with our previous reports on lung fibroblast chemotaxis (Kohyama et al. 2002), ESC-differentiated fibroblasts migrated towards fibronectin. Furthermore, exogenous PGE2 significantly inhibited, while TGF-β1 slightly but (not significant) augmented chemotaxis of these cells towards fibronectin (Fig. 6). These differentiated fibroblasts could also contract type I collagen gels when they were cast into the gels and cultured in SF-DMEM, which is considered as an in vitro model of tissue repair. Collagen gel contraction was significantly augmented by TGF-β1 but inhibited by PGE2 at all time points assessed (Fig. 7).
Figure 5.
Effects of TGF-β1 and PGE2 on proliferation of fibroblasts derived from human ESCs. Fibroblasts were cultured in monolayers in 10% FCS-DMEM with or without PGE2 (10−7 mol/l) or TGF-β1 (10−10 mol/l). Cells were detached with trypsin/EDTA and cell numbers were determined using a Coulter electronic cell counter. Vertical axis cell number (×105 cells/ml); horizontal axis, time (d). Each point shows mean ± SEM of three separate experiments, each of which included triplicated wells. Circles control, triangles PGE2, squares TGF-β1; *p < 0.05, compared with control group. SEMs are not evident as they are within the plot symbols.
Figure 6.
Chemotaxis of fibroblasts derived from human ESCs. Fibroblasts derived from differentiated EBs were trypsinized and chemotaxis toward fibronectin (10 μg/ml) was assessed in the presence or absence of either 10−7 mol/l TGF-β1 or 10−7 mol/l PGE2. Vertical axis percentage of fibronectin alone, horizontal axis conditions. Each point represents the mean ± SEM of three replicates in four separate experiments; *p < 0.05, compared to fibronectin alone (control group).
Figure 7.
Contraction of three-dimensional collagen gels by fibroblasts derived from human ESCs. Fibroblasts were cast into three-dimensional collagen gels. The gels were released into SF-DMEM supplemented with or without either 10−10 mol/l TGF-β1 or 10−7 mol/l PGE2. Gel size was measured daily by an image analyzer. Vertical axis gel size (percentage of initial area), horizontal axis time (d). Each point represents mean ± SEM of five separate experiments, each performed in triplicate gels. Circles control, triangles PGE2, squares TGF-β1; *p < 0.01, compared with control group. SEMs are not evident as they are within the plot symbols.
Differentiation of murine ESCs into fibroblast-like cells.
Having demonstrated that culture of human ESCs in three-dimensional type I collagen gels led to differentiation of fibroblast-like cells, we next sought to determine if similar results would be obtained with murine ESCs.
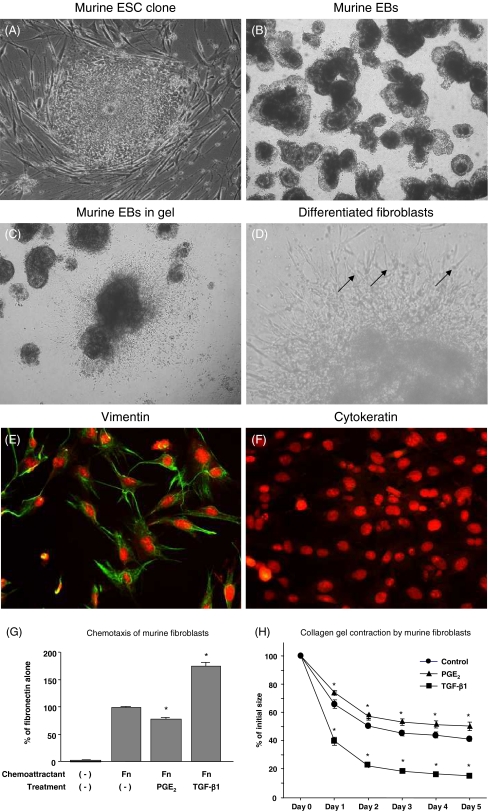
Spindle-shaped cells appeared after 10–14 d of culture of murine EBs in three-dimensional collagen gels (Fig. 8A–D). To evaluate the phenotype of these fibroblast-like cells, we performed immunocytochemical staining for vimentin and cytokeratin in monolayer culture. As expected, these cells showed positive staining for vimentin, but were negative for cytokeratin (Fig. 8E, F). It was also demonstrated that exogenous TGF-β1 significantly augmented chemotactic activity toward fibronectin and collagen gel contraction of the fibroblast-like cells derived from murine ESCs, whereas PGE2 significantly inhibited both chemotaxis and gel contraction (Fig. 8G, H).
Figure 8.
Differentiation of murine ESCs into fibroblasts in three-dimensional collagen gel culture. (A) Murine ESCs in monolayer culture with feeder layer (original magnification, ×400). (B) Murine EBs were ready to cast into gels (original magnification, ×200). (C) Murine EBs in three-dimensional collagen gels (original magnification, ×200). (D) Spindle-shaped cells surrounding murine EBs as indicated by the arrows (original magnification, ×400). (E and F) Immunocytochemistry of differentiated murine fibroblasts in monolayer culture stained for vimentin (E) and cytokeratin (F) (original magnification, ×200). (G) Chemotaxis toward fibronectin. Chemotaxis of murine fibroblasts toward fibronectin was assessed in the presence or absence of either 5 × 10−11 mol/l TGF-β1 or 10−7 mol/l PGE2. (H) Contraction of three-dimensional collagen gels by fibroblasts derived from murine ESCs. Collagen gels containing fibroblasts were floated in the media with or without 10−10 mol/TGF-β1 or 10−7 mol/l PGE2. Circles control, triangles PGE2, squares TGF-β1; *p < 0.05, compared with control group.
Discussion
Fibroblasts represent a heterogeneous population of cells present in mesenchymal connective tissues (Fries et al. 1994; Powell et al. 1999a; Powell et al. 1999b; Phan 2008). These cells are thought to be the major cells responsible for the production and maintenance of extracellular matrix (Raghow 1994; Ohnishi et al. 1998). In addition, fibroblasts produce mediators that regulate epithelial and endothelial cell proliferation and functions (Roberts and Sporn 1989; Vignola et al. 1997). Fibroblasts, moreover, can also regulate inflammatory cell recruitment and activation (Glaros et al. 2009).
Cytokines can modulate fibroblast functions (Scotton and Chambers 2007). TGF-β, for example, induces the expression of α-SMA and increases fibroblast production of extracellular matrix (Ronnov-Jessen and Petersen 1993; Desmouliere and Gabbiani 1994). Cells with these features are sometimes termed “myofibroblasts” as the increased expression of α-SMA containing fibers resembles the fibers present in smooth muscle cells (Hinz et al. 2007). In addition to mediator-induced modulation of structure and function, which may be transient, heterogeneous populations of fibroblasts with stable phenotypes have been described in many tissues (Fries et al. 1994; Cassiman et al. 2002). Alterations in populations of differentiated fibroblasts, moreover, have been described in a number of disease states (Kahari 1993; Fireman et al. 2001; Holz et al. 2004; Sugiura et al. 2007; Togo et al. 2008; Sato et al. in press).
The origin of differentiated fibroblasts is poorly understood. Recent evidence suggests that circulating cells may contribute as progenitors of tissue fibroblasts (Lama and Phan 2006; Hinz et al. 2007; Hong et al. 2007; Phan 2008). Little is known, however, about the mechanisms that control differentiation of stem/progenitor cells into stable populations of fibroblasts. The current study provides a method for the evaluation of fibroblast differentiation from embryonic stem cells as illustrated in Fig. 9.
Several other investigators have reported the derivation of fibroblast-like cells from ESCs (Xu et al. 2004; Stojkovic et al. 2005; Yoo et al. 2005; Choo et al. 2008; Chen et al. 2009). Both differentiation from embryoid bodies (Xu et al. 2004; Choo et al. 2008), as described in the current report, and direct differentiation from ECS were used. All these studies were designed to prepare non-xenogenic fibroblast-like cells to use as feeder layers. The utility of the various types of derived cells for this purpose was well established. Much less attention has been given to demonstrating that the derived cells function as fibroblasts. Yoo et al. (2005) described their cells as being keratin negative and positive for the enzyme prolyl hydroxylase, which plays a role in collagen biosynthesis. Stojkovic et al. assessed a number of histochemical markers, but the derived cells differed from foreskin fibroblasts, which are a specific type of differentiated cell.
Several lines of evidence support describing the fibroblast-like cells prepared by the method described in the current report as fibroblasts. First, these cells have the characteristic spindle-shaped morphology of fibroblasts. In addition, the ultrastructure of the cells is consistent with that of fibroblasts/myofibroblasts. Finally, cells cultured in the present study expressed the cytoskeletal protein vimentin, which is characteristically present in fibroblasts, and lacked the cytoskeletal protein cytokeratin, which is characteristically present in epithelial cells.
A major distinction between fibroblasts on the one hand and epithelial cells and endothelial cells on the other are their responses to PGE2 and TGF-β (Liu et al. 2000; Umino et al. 2000; Zhu et al. 2000). In general, TGF-β inhibits epithelial cell proliferation and migration (Vignola et al. 1997). In contrast, TGF-β augments proliferation and migration of fibroblasts (Kurosaka et al. 1998; Togo et al. 2008). Similarly, PGE2 characteristically stimulates proliferation and migration of epithelial cells while it inhibits proliferation and migration of fibroblasts (Zhu et al. 2000; Huang et al. 2007; Stenson 2007; Togo et al. 2008). Cells cultured in the current study responded to PGE2 and TGF-β in a manner characteristic of fibroblasts. In addition, fibroblasts cultured in three-dimensional collagen gels attached to collagen and exerted mechanical tension, resulting in gel contraction. This property is thought to be a model of tissue reorganization (Mio et al. 1996). Characteristically, TGF-β augments and PGE2 inhibits this process in fibroblasts (Campbell et al. 2004; Togo et al. 2008) as was observed in the cells prepared in the current study. In contrast, the ability of endothelial and epithelial cells to contract collagen gels differs markedly. Endothelial cells are unaffected by TGFß or PGE (Liu et al. 2000) and epithelial cells contract gels, but only when plated on the surface and PGE has no effect (Liu et al. 1998; Umino et al. 2000).
In summary, the current study demonstrates that cells with the morphologic and functional features of fibroblasts can be reliably derived from human and murine ESCs. The development of this methodology provides a means to define the mechanisms that regulate fibroblast differentiation.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J. Exp. Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BH, Agarwal C, Wang JH. Tgf-beta1, tgf-beta3, and pge(2) regulate contraction of human patellar tendon fibroblasts. Biomech. Model. Mechanobiol. 2004;2:239–245. doi: 10.1007/s10237-004-0041-z. [DOI] [PubMed] [Google Scholar]
- Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J. Hepatol. 2002;36:200–209. doi: 10.1016/S0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- Chen HF, Chuang CY, Shieh YK, Chang HW, Ho HN, Kuo HC. Novel autogenic feeders derived from human embryonic stem cells (hescs) support an undifferentiated status of hescs in xeno-free culture conditions. Hum. Reprod. 2009;24:1114–1125. doi: 10.1093/humrep/dep003. [DOI] [PubMed] [Google Scholar]
- Choo A, Ngo AS, Ding V, Oh S, Kiang LS. Autogeneic feeders for the culture of undifferentiated human embryonic stem cells in feeder and feeder-free conditions. Methods Cell Biol. 2008;86:15–28. doi: 10.1016/S0091-679X(08)00002-2. [DOI] [PubMed] [Google Scholar]
- Dell’Orbo C, Gioglio L, Quacci D, Ruggeri A. The dependency of collagen fibrillogenesis in vitro on fibroblast culture conditions. Fibroblasts in mono- and multi-layers. Arch. Histol. Cytol. 1992;55:235–241. doi: 10.1679/aohc.55.235. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Gabbiani G. Modulation of fibroblastic cytoskeletal features during pathological situations: the role of extracellular matrix and cytokines. Cell Motil. Cytoskeleton. 1994;29:195–203. doi: 10.1002/cm.970290302. [DOI] [PubMed] [Google Scholar]
- Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyden B. Fibroblast phenotype plasticity: relevance for understanding heterogeneity in “Fibroblastic” tumors. Ultrastruct. Pathol. 2004;28:307–319. doi: 10.1080/019131290882204. [DOI] [PubMed] [Google Scholar]
- Eyden B. The myofibroblast: a study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructure. Part 1—normal and reactive cells. J. Submicrosc. Cytol. Pathol. 2005;37:109–204. [PubMed] [Google Scholar]
- Fireman E, Shahar I, Shoval S, Messer G, Dvash S, Grief J. Morphological and biochemical properties of alveolar fibroblasts in interstitial lung diseases. Lung. 2001;179:105–117. doi: 10.1007/s004080000051. [DOI] [PubMed] [Google Scholar]
- Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, Phipps RP. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin. Immunol. Immunopathol. 1994;72:283–292. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- Glaros T, Larsen M, Li L. Macrophages and fibroblasts during inflammation, tissue damage and organ injury. Front. Biosci. 2009;14:3988–3993. doi: 10.2741/3506. [DOI] [PubMed] [Google Scholar]
- Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev. Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am. J. Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz O, Zuhlke I, Jaksztat E, Muller KC, Welker L, Nakashima M, Diemel KD, Branscheid D, Magnussen H, Jorres RA. Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur. Respir. J. 2004;24:575–579. doi: 10.1183/09031936.04.00143703. [DOI] [PubMed] [Google Scholar]
- Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin e(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via e prostanoid 2 receptor and camp signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L405–L413. doi: 10.1152/ajplung.00232.2006. [DOI] [PubMed] [Google Scholar]
- Kahari VM. Activation of dermal connective tissue in scleroderma. Ann. Med. 1993;25:511–518. [PubMed] [Google Scholar]
- Knight D. Epithelium-fibroblast interactions in response to airway inflammation. Immunol. Cell Biol. 2001;79:160–164. doi: 10.1046/j.1440-1711.2001.00988.x. [DOI] [PubMed] [Google Scholar]
- Kohyama T, Liu X, Wen FQ, Kobayashi T, Abe S, Ertl R, Rennard SI. Nerve growth factor stimulates fibronectin-induced fibroblast migration. J. Lab. Clin. Med. 2002;140:329–335. doi: 10.1067/mlc.2002.128347. [DOI] [PubMed] [Google Scholar]
- Kurosaka H, Kurosaka D, Kato K, Mashima Y, Tanaka Y. Transforming growth factor-beta 1 promotes contraction of collagen gel by bovine corneal fibroblasts through differentiation of myofibroblasts. Invest. Ophthalmol. Vis. Sci. 1998;39:699–704. [PubMed] [Google Scholar]
- Lama VN, Phan SH. The extrapulmonary origin of fibroblasts: stem/progenitor cells and beyond. Proc. Am. Thorac. Soc. 2006;3:373–376. doi: 10.1513/pats.200512-133TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XD, Skold M, Umino T, Zhu YK, Romberger DJ, Spurzem JR, Rennard SI. Endothelial cell-mediated type I collagen gel contraction is regulated by hemin. J. Lab. Clin. Med. 2000;136:100–109. doi: 10.1067/mlc.2000.108153. [DOI] [PubMed] [Google Scholar]
- Liu X, Umino T, Cano M, Ertl R, Veys T, Spurzem J, Romberger D, Rennard SI. Human bronchial epithelial cells can contract type I collagen gels. Am. J. Phys. 1998;274:L58–L65. doi: 10.1152/ajplung.1998.274.1.L58. [DOI] [PubMed] [Google Scholar]
- Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three dimensional collagen gel matrix. In Vitro Cell. Dev. Biol. 1996;32:427–433. doi: 10.1007/BF02723005. [DOI] [PubMed] [Google Scholar]
- Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J. Immunol. 2001;167:5381–5385. doi: 10.4049/jimmunol.167.9.5381. [DOI] [PubMed] [Google Scholar]
- Ohnishi K, Takagi M, Yoshimochi K, Satomi S, Konttinen YT. Matrix metalloproteinase mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab. Invest. 1998;78:1077–1087. [PubMed] [Google Scholar]
- Phan SH. Biology of fibroblasts and myofibroblasts. Proc. Am. Thorac. Soc. 2008;5:334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am. J. Physiol. 1999;277:C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am. J. Physiol. 1999;277:C183–C201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 1994;8:823–831. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- Rennard SI. Epithelial cells and fibroblasts. In: Chadwick D, Goode JA, editors. Chronic obstructive pulmonary disease: pathogenesis to treatment. Chichester: Wiley; 2001. pp. 104–119. [Google Scholar]
- Roberts AB, Sporn MB. Regulation of endothelial cell growth, architecture, and matrix synthesis by TGF-beta. Am. Rev. Respir. Dis. 1989;140:1126–1128. doi: 10.1164/ajrccm/140.4.1126. [DOI] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW. Induction of a-smooth muscle actin by transforming growth factor-b1 in quiescent human breast gland fibroblasts. Lab. Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- Sato T.; Liu X.; Nelson A.; Nakanishi M.; Kanaji N.; Wang X.; Kim M.; Li Y.; Sun J.; Michalski J.; et al. Reduced mir-146a increases prostaglandin e2 in chronic obstructive pulmonary disease fibroblasts. Am. J. Respir. Crit. Care Med (in press). [DOI] [PMC free article] [PubMed]
- Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- Stenson WF. Prostaglandins and epithelial response to injury. Curr. Opin. Gastroenterol. 2007;23:107–110. doi: 10.1097/MOG.0b013e3280143cb6. [DOI] [PubMed] [Google Scholar]
- Stojkovic P, Lako M, Stewart R, Przyborski S, Armstrong L, Evans J, Murdoch A, Strachan T, Stojkovic M. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005;23:306–314. doi: 10.1634/stemcells.2004-0137. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Liu X, Duan F, Kawasaki S, Togo S, Kamio K, Wang XQ, Mao L, Ahn Y, Ertl RF, et al. Cultured lung fibroblasts from ovalbumin-challenged “Asthmatic” mice differ functionally from normal. Am. J. Respir. Cell Mol. Biol. 2007;37:424–430. doi: 10.1165/rcmb.2007-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo S, Holz O, Liu X, Sugiura H, Kamio K, Wang X, Kawasaki S, Ahn Y, Fredriksson K, Skold CM, et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am. J. Respir. Crit. Care Med. 2008;178:248–260. doi: 10.1164/rccm.200706-929OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umino T, Wang H, Zhu Y, Liu X, Manouilova SL, Spurzem JR, Leuschen MP, Rennard SI. Modification of type I collagenous gels by alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2000;22:702–707. doi: 10.1165/ajrcmb.22.6.3806. [DOI] [PubMed] [Google Scholar]
- Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, la-Rocca AM, Bellia V, Bonsignore G, Bousquet J. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am. J. Respir. Crit. Care Med. 1997;156:591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- Xu C, Jiang J, Sottile V, McWhir J, Lebkowski J, Carpenter MK. Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells. 2004;22:972–980. doi: 10.1634/stemcells.22-6-972. [DOI] [PubMed] [Google Scholar]
- Yoo SJ, Yoon BS, Kim JM, Song JM, Roh S, You S, Yoon HS. Efficient culture system for human embryonic stem cells using autologous human embryonic stem cell-derived feeder cells. Exp. Mol. Med. 2005;37:399–407. doi: 10.1038/emm.2005.50. [DOI] [PubMed] [Google Scholar]
- Zhu YK, Umino T, Liu XD, Wang H, Romberger DJ, Spurzem JR, Rennard SI. Effect of collagen concentration on fibroblast mediated contraction of collagen gels. Chest. 2000;117:234S–235S. doi: 10.1378/chest.117.5_suppl_1.234S. [DOI] [PubMed] [Google Scholar]