Abstract
We report on a 10.5-year-old girl with a mild form of campomelic dysplasia. She presented with short stature of prenatal onset, dysmorphic facial features, limitation of supination and pronation of the forearms, dysplastic nails, and bone abnormalities consisting especially of cone-shaped epiphyses of the middle phalanx of the 2nd fingers, brachydactyly and clinodactyly of the middle phalanx of both 5th fingers, short 4th metacarpals, radial and femoral head subluxation, hypoplastic scapulae, humeral and ulnar epiphyseal abnormalities, unossified symphysis pubis, and a significant delay in bone age. Molecular analysis of the SOX9 gene revealed the presence of a de novo missense mutation: p.P170L (c.509C>T). Mild and surviving cases of campomelic dysplasia are reviewed.
Key Words: Bone dysplasia, Dysmorphology, Malformations, Mental retardation, SOX9
Introduction
Campomelic dysplasia (CD) (derived from the Greek for ‘bent limb’) is a rare, often lethal, autosomal dominant osseous malformation syndrome (OMIM 114290). Its incidence is population sensitive. In Norway, it has been estimated to 1.6 per 10,000 [Normann et al., 1993] while in other populations a birth prevalence of 1 in 200,000 has been estimated [Mansour et al., 1995]. Most cases are due to mutations in the SOX9 gene – a member of the SOX (SRY-related HMG box) gene family – located on 17q23–qter, which plays a role in chondrogenesis and sex determination [Foster et al., 1994; Wagner et al., 1994]. In few patients, chromosomal rearrangements involving the cis-regulatory elements upstream of the gene and deletions upstream of SOX9 have also been reported [Wirth et al., 1996; Pop et al., 2005; Leipoldt et al., 2007; Lecointre et al., 2009].
This skeletal dysplasia is characterized by bowing of the femur and tibia, short 1st metacarpals, hypoplastic scapulae, non-mineralization of the pedicles of the thoracic vertebra, presence of 11 pairs of ribs, narrow iliac wings, and poor ossification of the pubis. The main facial features are a large head, flat nasal bridge, low-set and malformed ears, small jaw, and a cleft palate. A narrow chest, congenital dislocation of the hip, and bilateral talipes equinovarus are often common. A male-to-female autosomal sex reversal characterizes this syndrome in two-thirds of the affected karyotypic males.
Expression can be very variable. For instance, campomelia is present in 90% of the cases. Cardiac defects and hydronephrosis have been reported in one-third of the patients. Moreover, in nearly 95% of the cases, death occurs in the neonatal period due to breathing problems related to small chest size [Mansour et al., 1995].
This is a report on a Lebanese girl with skeletal abnormalities and dysmorphic facial features reminiscent of CD with mild course. Similar cases and differential diagnoses are discussed.
Clinical Report
The girl is the youngest child of a healthy G5P4A1 Lebanese woman. The parents are second cousins. Her pregnancy course was normal. No known toxic, medical exposures or unusual events were reported during the gestation. There was no history of birth defects, or increased miscarriage rate in relatives. The 3 older sibs were normal. Delivery was at term by spontaneous vaginal delivery. According to the parents, she had a low-pitched voice and was admitted for 5 days to the pediatric neonatal care. The baby girl's birth weight was 1,800 g (<3rd centile). The other neonatal parameters were not recorded but were recalled as very low by the parents. At that time, the mother was 31 years old and the father 32.
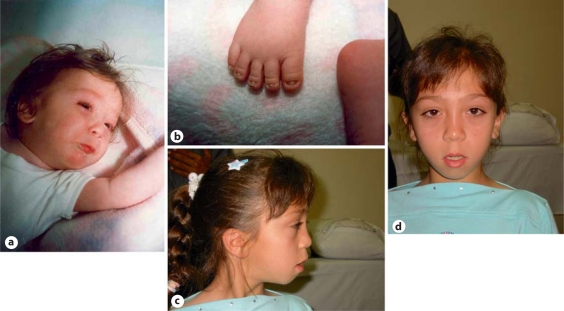
This girl was first evaluated at the age of 13 months. Her weight was 5,500 g (<3rd centile), length 60 cm (<3rd centile) and OFC 43.2 cm (3rd centile). Developmental milestones were delayed, as she could only sit but not stand. Physical examination showed a small and receding forehead, tense anterior fontanel, flat face, sparse hair, short nose with upturned nares, low-set ears, microstomia, short neck, short hands and feet, and brachydactyly and clinodactyly of the 5th fingers (fig. 1a). A single palmar crease was noted on both hands. Nails were hypoplastic especially on the feet (fig. 1b). External genitalia were normal. Neurological examination revealed a hypotonic girl.
Fig. 1.
Photograph of the patient at age 13 months (a, b) and 10.5 years (c, d). Note in a the sparse hair, short nose microstomia, short neck, and in b the hypoplastic nails, and in c and d the triangular face, slightly down-turned palpebral fissures, low-set and posteriorly rotated ears, long philtrum, thin upper lips, everted lower lip, small chin, retrognathism, and short neck.
Abdominal ultrasonography, echocardiography and magnetic resonance imaging of the brain did not show any abnormalities. The chromosome study of lymphocytes with high resolution G-and R-banding was normal, 46,XX.
She reached autonomous walking at the age of 2.5 years. At the age of 6, she enrolled in a specialized school for disabled children. She had a mild mental retardation.
We saw her once again at the age of 10.5 years. At that time, her height was 117 cm (<3rd centile) and her OFC 51 cm (25th centile). She had a triangular face, slightly down-turned palpebral fissures, low-set and posteriorly rotated ears, long philtrum, thin upper lips, mild limitation in opening of the mouth, everted lower lip, arched palate, small chin, retrognathism, short neck, limitation of supination and pronation of the forearms and dysplastic nails especially on the feet (fig. 1c, d). Osteotendinous reflexes, sensory and cranial nerve examinations were normal. Ophthalmological findings were unremarkable.
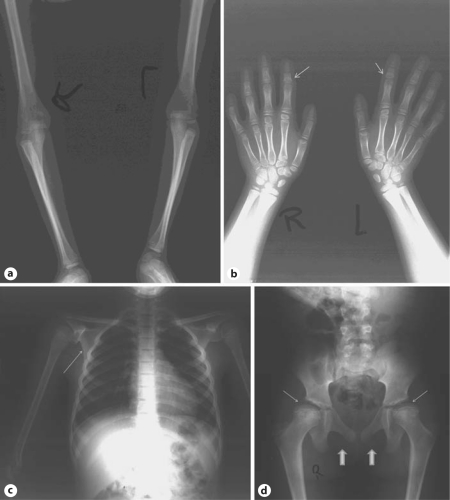
Abdominal CT scan was normal. A total body X-ray examination showed radial head subluxation, humeral and ulnar epiphyseal abnormalities, short 4th metacarpals, cone-shaped epiphyses of the middle phalanx of the 2nd fingers, brachydactyly and clinodactyly of the middle phalanx of both 5th fingers, hypoplastic scapulae, femoral head subluxation, and small and flattened proximal femoral epiphyses. The symphysis pubis was only ossified in the upper part. The cranium, vertebrae, sternum and ribs showed no specific abnormalities. There was a significant delay in bone age (fig. 2). Routine blood and urine examinations were unremarkable.
Fig. 2.
X-ray images at age 10.5 years showing a radial head sub-luxation, humeral and ulnar epiphyseal abnormalities, b short 4th metacarpals, cone-shaped epiphyses of the middle phalanx of the 2nd fingers (arrows), brachydactyly and clinodactyly of the middle phalanx of both 5th fingers, delay in bone age, c hypoplastic scapulae (arrow), and d femoral head subluxation, small and flattened proximal femoral epiphyses (thin arrows), and unossified upper part of the symphysis pubis (large arrows).
Menstruations started at the age of 13. At the age of 15, she was in very good health except for mild hearing impairment noted a few months earlier. In general, she was a little bit shy, saying simple sentences with some distortions. She was able to read and write simple sentences.
Molecular Analysis
EDTA blood samples were collected from both parents and the patient after informed consent had been obtained. DNA was extracted from leukocytes by standard salt-precipitation methods [Grimberg et al., 1989].
Given that clinical presentation and radiological abnormalities of the patient were suggestive of a CD, the 3 coding exons of SOX9 (GenBank accession number: NM_000346.3) were sequenced. DNA was amplified by PCR. Primers were designed using Primer 3 (http://frodo.wi.mit.edu). PCR products were purified using the Ultra PCR Clean-Up Kit (ABgene, Surrey, UK), and both strands were sequenced using the BigDye® Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, Calif., USA). The labeled products were subjected to electrophoresis on an ABI 3130 (Applied Biosystems). Electropherograms were analyzed using Sequence Analysis Software version 5.2 (Applied Biosystems) and compared to the reference sequence using ChromasPro version 1.22 (Technelysium, Tewantin, Qld., Australia).
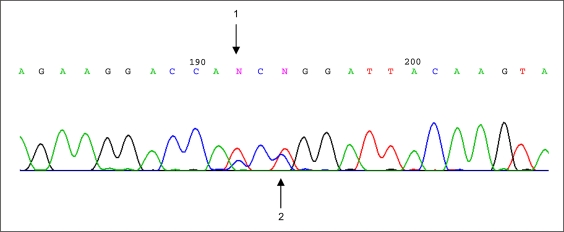
A novel missense mutation, p.P170L (c.509C>T), and an already reported polymorphism, H169H (c.507C>T), were detected at a heterozygous state in the patient (fig. 3). The c.509C>T mutation was not found in the parents, while the c.507C>T was present in the father.
Fig. 3.
Chromatogram picture of SOX9 sequencing showing (1) the polymorphism p.H169H (c.507C>T) and (2) the mutation p.P170L (c.509C>T) detected at heterozygous state in this study.
Discussion
This female patient reported here has developmental delay, short stature of prenatal onset, dysmorphic facial features, dysplastic nails, and bone abnormalities reminiscent of CD. SOX9 analysis revealed the presence of a missense mutation, p.P170L, confirming the former diagnosis.
Few long-term survivors with CD have been reported [Houston et al., 1983; Gillerot et al., 1989; Meyer et al., 1997; Pfeifer et al., 1999; Giordano et al., 2001; Moog et al., 2001; Mansour et al., 2002; Offiah et al., 2002; Savarirayan et al., 2003; Sock et al., 2003; Hill-Harfe et al., 2005; Velagaleti et al., 2005; Lekovic et al., 2006; Leipoldt et al., 2007; Wada et al., 2009; Staffler et al., 2010]. In general, they share common facial features such as relative macrocephaly, depressed nasal bridge, hypertelorism, high or cleft palate, long philtrum, low-set ears, and micrognathia [Mansour et al., 2002; Offiah et al., 2002; Wada et al., 2009] (table 1). On X-rays, hypoplastic scapulae, defective ischiopubic ossification, hypoplastic patellae, and spinal dysraphism can be seen. Recurrent apnea, upper respiratory infections, kyphoscoliosis, mild learning disabilities, conductive hearing loss, myopia, dislocation of the radial heads and of the hips, and short stature can complicate the course of the disease [Giordano et al., 2001; Moog et al., 2001; Mansour et al., 2002; Leipoldt et al., 2007; Staffler et al., 2010] (table 1). Our patient presented mainly a flat face, relative macrocephaly, long philtrum, micrognathia, limitation of supination and pronation of the forearms, slight developmental delay, and some hearing impairment that started at the age of 14. However, now at age 15, she does not complain about any other complications seen in surviving CD cases such as severe kyphoscoliosis, myopia, or dental caries [Mansour et al., 2002]. Her scapulae were hypoplastic but not as usually seen in CD (table 1). The dysplastic nails seen in our patient have been previously reported in 2 CD patients [Moog et al., 2001; Mansour et al., 2002].
Table 1.
Major clinical and radiological features of the surviving CD patients compared with the present report
| Main features | Present case | Review of published reports (%) |
|---|---|---|
| General features | ||
| Mental retardation | + | 16/19 (84) |
| Short stature, short limbs | + | 19/21 (90) |
| Facial features | ||
| Flat face | + | 17/21 (81) |
| Macrocephaly | + | 20/20 (100) |
| Depressed nasal bridge | + | 19/25 (76) |
| Hypertelorism | − | 6/15 (40) |
| Low-set ears | − | 7/17(41) |
| Long philtrum | + | 7/13 (54) |
| High/cleft palate | − | 20/28 (71) |
| Micrognathia | + | 20/27 (74) |
| Bone malformations | ||
| Spinal dysraphism | − | 13/19 (68) |
| Hypoplastic scapula | + | 16/21 (76) |
| Defective ischiopubic ossification | + | 12/19 (63) |
| Bowing of the bones | − | 12/22 (55) |
| Dislocation of radial head/hip | + | 5/17 (29) |
| Hypoplastic patellae | − | 3/15 (20) |
| Others | ||
| Apnea | − | 12/26 (46) |
| Infections | − | 15/22 (68) |
| Scoliosis | − | 17/20 (85) |
| Hearing defect | + | 7/14 (50) |
It has been postulated that surviving cases: (1) are usually secondary to somatic mosaicism of the SOX9 mutation, (2) depend on the type of the mutation abolishing the expression of the gene or leaving some residual activity of the mutant SOX9 protein, and (3) depend on the presence or absence of a chromosomal rearrangement spearing the coding SOX9 region but affecting the cis-regulatory elements upstream of the gene [Meyer et al., 1997; Pfeifer et al., 1999; Giordano et al., 2001; Mansour et al., 2002; Smyk et al., 2007; Staffler et al., 2010]. For instance, the long-term survivors of CD are significantly overrepresented in the group of translocation and inversion cases as compared to the group of CD cases with mutations in the SOX9 coding region [Pfeifer et al., 1999].
Our patient had a mutation at codon 170 affecting a highly conserved proline residue in the HMG domain proteins. Proline has a major effect upon protein structure because of the restrictions imposed by its ring-like shape. The presence of proline has a significant effect upon orientation of the polypeptide chain. Substitution of proline for an amino acid with more flexible side chain can lead to significant structural changes in the protein domain. The same mutation was reported recently on a boy aged 11.5 years with a mild CD [Wada et al., 2009], showing that this mutation leads to a mild phenotype. This patient had normal male external genitalia. Like our patient, he did not have bowing of the lower limbs but had a small thoracic cage and a cleft palate. Nevertheless, still, no clear phenotype-genotype correlation has been found in CD cases. For instance, a missense mutation (p.P170R) was previously identified at the same codon in a baby boy who died at 1 month [Meyer et al., 1997]. This mutation reduced DNA binding by forming a complex that reproducibly moves slower than the complex formed by the wild-type HMG domain indicating that this amino-acid complex results in an altered DNA bending angle. Thus, the p.P170R has been shown to retain some DNA binding ability [Meyer et al., 1997]. Our mutation p.P170L was supposed to be more severe than the p.P170R because the leucine R group is nonpolar and aliphatic, while the arginine R group is positively charged and hydrophilic. In fact, it was recently speculated about the presence of a threshold of overall SOX9 function below which severe cases occur [Staffler et al., 2010]. Our mutation does not seem to completely disrupt the affected allele.
Differential diagnosis must be considered among surviving CD patients. They might have features of ischio-pubic-patella syndrome (IPP; OMIM 147891) [Offiah et al., 2002]. However, in IPP, hypoplastic patellae, hypoplastic lesser trochanters and shortening of the 4th and 5th metacarpals and 2nd phalanges allow differentiation. Moreover, mutations in TBX4 (a gene encoding T-box protein 4) have now been reported [Bongers et al., 2004]. The Stüve-Wiedemann syndrome is a neonatally lethal osseous chondrodysplasia characterized by bowing of the limbs, particularly the femurs, tibiae and fibulae, and neonatal respiratory distress. Very few patients survive beyond 1 year. The latter are differentiated from surviving CD cases by the presence of short and bowed long bones with internal cortical thickening, wide metaphyses, abnormal trabecular pattern, normal scapulae, prominent joints, restricted joint mobility, osteoporosis, spontaneous fractures, and temperature instability, altered sweating, dental abnormalities, hyporeflexia and smooth tongue [Jung et al., 2010]. Kyphomelic dysplasia (OMIM 211350) is also characterized by bowing of the long bones. Furthermore, there is a major angulation of the femurs that improves with age, short and stubby long bones, tibial asymmetry, platyspondyly, and wide iliac wings. The mode of inheritance is autosomal recessive. Other syndromes presenting bowing of the femora such as cartilage-hair-hypoplasia (OMIM 250250), Schwartz-Jampel syndrome type 1 (OMIM 255800), and osteogenesis imperfecta (OMIM 166210) are at a variance with surviving CD patients. Finally, in Cumming syndrome (OMIM 211890) no surviving cases have been reported and besides the campomelia, there are short bones, polysplenia, polycystic dysplasia, and no sex reversal or tall and narrow pelvis [Watiker et al., 2005].
In conclusion, few patients with mild CD phenotype have been reported, most probably because many cases remain undiagnosed. Report on such cases and their related mutations might be very helpful allowing for better patient care and family counseling.
References
- Bongers EM, Duijf PH, van Beersum SE, Schoots J, Van Kampen A, et al. Mutations in the human TBX4 gene cause small patella syndrome. Am J Hum Genet. 2004;74:1239–1248. doi: 10.1086/421331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Gillerot Y, Vanheck CA, Foulon M, Podevain A, Koulischer L. Campomelic syndrome: manifestations in a 20 week fetus and case history of a 5 year old child. Am J Med Genet. 1989;34:589–592. doi: 10.1002/ajmg.1320340428. [DOI] [PubMed] [Google Scholar]
- Giordano J, Prior HM, Bamforth JS, Walter MA. Genetic study of SOX9 in a case of campomelic dysplasia. Am J Med Genet. 2001;98:176–181. doi: 10.1002/1096-8628(20010115)98:2<176::aid-ajmg1027>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Grimberg J, Nawoschik S, Belluscio L, McKee R, Turck A, Eisenberg A. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res. 1989;17:8390. doi: 10.1093/nar/17.20.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Harfe KL, Kaplan L, Stalker HJ, Zori RT, Pop R, et al. Fine mapping of chromosome 17 translocation breakpoints >900 kb upstream of SOX9 in acampomelic dysplasia and a mild, familial skeletal dysplasia. Am J Hum Genet. 2005;76:663–671. doi: 10.1086/429254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CS, Opitz JM, Spranger JW, Macpherson RI, Reed MH, et al. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al in 1971. Am J Med Genet. 1983;15:3–28. doi: 10.1002/ajmg.1320150103. [DOI] [PubMed] [Google Scholar]
- Jung C, Dagoneau N, Baujat G, Le Merrer M, David A, et al. Stüve-Wiedemann syndrome: long-term follow-up and genetic heterogeneity. Clin Genet. 2010;77:266–272. doi: 10.1111/j.1399-0004.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- Lecointre C, Pichon O, Hamel A, Heloury Y, Michel-Calemard L, et al. Familial acampomelic form of campomelic dysplasia caused by a 960 kb deletion upstream of SOX9. Am J Med Genet. 2009;149A:1183–1189. doi: 10.1002/ajmg.a.32830. [DOI] [PubMed] [Google Scholar]
- Leipoldt M, Erdel M, Bien-Willner GA, Smyk M, Theurl M, et al. Two novel translocation breakpoints upstream of SOX9 define borders of the proximal and distal breakpoint cluster region in campomelic dysplasia. Clin Genet. 2007;71:67–75. doi: 10.1111/j.1399-0004.2007.00736.x. [DOI] [PubMed] [Google Scholar]
- Lekovic GP, Rekate HL, Dickman CA, Pearson M. Congenital cervical instability in a patient with camptomelic dysplasia. Childs Nerv Syst. 2006;22:1212–1214. doi: 10.1007/s00381-006-0071-1. [DOI] [PubMed] [Google Scholar]
- Mansour S, Hall CM, Pembrey ME, Young ID. A clinical and genetic study of campomelic dysplasia. J Med Genet. 1995;32:415–420. doi: 10.1136/jmg.32.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S, Offiah AC, McDowall S, Sim P, Tolmie J, Hall C. The phenotype of survivors of campomelic dysplasia. J Med Genet. 2002;39:597–602. doi: 10.1136/jmg.39.8.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Sudbeck P, Held M, Wagner T, Schmitz ML, et al. Mutational analysis of the SOX9 gene in campomelic dysplasia and autosomal sex reversal: lack of genotype/phenotype correlations. Hum Mol Genet. 1997;6:91–98. doi: 10.1093/hmg/6.1.91. [DOI] [PubMed] [Google Scholar]
- Moog U, Jansen NJ, Scherer G, Schrander- Stumpel CT. Acampomelic campomelic syndrome. Am J Med Genet. 2001;104:239–245. [PubMed] [Google Scholar]
- Normann EK, Pedersen JC, Stiris G, van der Hagen CB. Campomelic dysplasia – an underdiagnosed condition? Eur J Pediatr. 1993;152:331–333. doi: 10.1007/BF01956747. [DOI] [PubMed] [Google Scholar]
- Offiah AC, Mansour S, McDowall S, Tolmie J, Sim P, Hall CM. Surviving campomelic dysplasia has the radiological features of the previously reported ischio-pubic-patella syndrome. J Med Genet. 2002;39:e50. doi: 10.1136/jmg.39.9.e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer D, Kist R, Dewar K, Devon K, Lander ES, et al. Campomelic dysplasia translocation breakpoints are scattered over 1 Mb proximal to SOX9: evidence for an extended control region. Am J Hum Genet. 1999;65:111–124. doi: 10.1086/302455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop R, Zaragoza MV, Gaudette M, Dohrmann U, Scherer G. A homozygous nonsense mutation in SOX9 in the dominant disorder campomelic dysplasia: a case of mitotic gene conversion. Hum Genet. 2005;117:43–53. doi: 10.1007/s00439-005-1295-y. [DOI] [PubMed] [Google Scholar]
- Savarirayan R, Robertson SP, Bankier A, Rogers JG. Variable expression of campomelic dysplasia in a father and his 46,XY daughter. Pediatr Pathol Mol Med. 2003;22:37–46. doi: 10.1080/pdp.22.1.37.46. [DOI] [PubMed] [Google Scholar]
- Smyk M, Obersztyn E, Nowakowska B, Bocian E, Cheung SW, et al. Recurrent SOX9 deletion campomelic dysplasia due to somatic mosaicism in the father. Am J Med Genet. 2007;143A:866–870. doi: 10.1002/ajmg.a.31631. [DOI] [PubMed] [Google Scholar]
- Sock E, Pagon RA, Keymolen K, Lissens W, Wegner M, Scherer G. Loss of DNA-dependent dimerization of the transcription factor SOX9 as a cause for campomelic dysplasia. Hum Mol Genet. 2003;12:1439–1447. doi: 10.1093/hmg/ddg158. [DOI] [PubMed] [Google Scholar]
- Staffler A, Hammel M, Wahlbuhl M, Bidlingmaier C, Flemmer AW, et al. Heterozygous SOX9 mutations allowing for residual DNA-binding and transcriptional activation lead to the acampomelic variant of campomelic dysplasia. Hum Mutat. 2010;31:E1436–E1444. doi: 10.1002/humu.21238. [DOI] [PubMed] [Google Scholar]
- Velagaleti GV, Bien-Willner GA, Northrup JK, Lockhart LH, Hawkins JC, et al. Position effects due to chromosome breakpoints that map approximately 900 kb upstream and approximately 1.3 Mb downstream of SOX9 in two patients with campomelic dysplasia. Am J Hum Genet. 2005;76:652–662. doi: 10.1086/429252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Nishimura G, Nagai T, Sawai H, Yoshikata M, et al. Mutation analysis of SOX9 and single copy number variant analysis of the upstream region in eight patients with campomelic dysplasia and acampomelic campomelic dysplasia. Am J Med Genet. 2009;149A:2882–2885. doi: 10.1002/ajmg.a.33107. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Watiker V, Lachman RS, Wilcox WR, Barroso I, Schafer AJ, Scherer G. Differentiating campomelic dysplasia from Cumming syndrome. Am J Med Genet. 2005;135A:110–112. doi: 10.1002/ajmg.a.30650. [DOI] [PubMed] [Google Scholar]
- Wirth J, Wagner T, Meyer J, Pfeiffer RA, Tietze HU, et al. Translocation breakpoints in three patients with campomelic dysplasia and autosomal sex reversal map more than 130 kb from SOX9. Hum Genet. 1996;97:186–193. doi: 10.1007/BF02265263. [DOI] [PubMed] [Google Scholar]