Summary
Polymeric networks and the ensuing hydrogels of methacrylic acid and N-vinyl pyrrolidone were successfully synthesized using a UV-initiated free radical polymerization and characterized to assess their applicability as carriers for directed drug delivery. FT-IR spectroscopy revealed shifts in peak absorbances that indicated the presence of hydrogen bonding complexes between functional groups, while SEM imaging showed that the different comonomers affect the surface morphology of the microparticles. Dynamic pH swelling studies demonstrated the pH responsiveness of the carriers in gastric and intestinal conditions and revealed that systems containing higher concentrations of methacrylic acid experienced the highest degree of hydrogen bonding complexation in gastric conditions. The presence of NVP in the systems enhanced swelling. Equilibrium swelling studies revealed that the mesh size was sufficiently large to allow drug diffusion across the networks.
Keywords: diffusion, hydrogels, mesh size, networks
Introduction
N-vinyl pyrrolidone (NVP), the monomer used to prepare the homopolymer poly(vinyl pyrrolidone) (PVP), has properties that make it an ideal candidate for inclusion in a carrier for the oral delivery of therapeutic proteins. NVP can be polymerized by a variety of techniques such as free radical and solution polymerizations that are relevant to drug delivery and other applications.[1] In its linear form, the polymer PVP is highly soluble in water; as a crosslinked network, it is capable of imbibing very large amounts of water and is therefore quite effective as a hydrogel. A downside of the hydrophilicity of PVP is that the high absorbency of the material leads to fragile structures.[2, 3]
The material integrity problems are often solved by copolymerizing NVP with 2-hydroxyethyl methacrylate (HEMA),[4, 5] carboxyvinyl polymer,[6] methyl methacrylate,[5, 7] acrylic acid,[8, 9] or methacrylic acid.[10–12] Applications of these copolymers have been in industrial settings for water treatment[13] and pervaporation[14] purposes, pharmaceutical settings as protein mimics,[8] and drug delivery purposes in the ocular,[15] oral and colonic[12] delivery of small molecular weight peptides.
The copolymerization of MAA and NVP was of great interest in this research. The inclusion of PVP in biological applications has led to mucoadhesive and bioadhesive properties[16, 17] that may increase the total residence time in the small intestine. Furthermore, as with the oft-used hydrogel system of methacrylic acid and grafted poly(ethylene glycol) chains – P(MAA-g-PEG) – a system of MAA and NVP will exhibit anionic pH-sensitivity as NVP is neutral. The amide group present on NVP in the form of a lactam ring has no cationic activity[3] and therefore the pKa will remain near that of PMAA.[18] NVP can form complexes with MAA in the form of hydrogen bonds.[19] Several researchers have reported on the formation of these complexes and the resultant conformational and structural changes. However these papers refer to complexes found between linear PMAA and linear PVP, not complexation occurring within a chemically crosslinked network.[13, 17, 20]
The aim of this work was to synthesize and characterize hydrogels of methacrylic acid and N-vinyl pyrrolidone in order to investigate the effects of monomeric ratios, crosslinking, and tethered structures on the overall dynamic behavior of the system. Using short crosslinkers will result in complexation behavior in a chemically crosslinked network similar to that existing between linear homopolymers of the materials due to the close proximity of the functional groups in the collapsed state. Thus, this is a rare contribution to the analysis of the complexation between MAA and NVP in a chemically crosslinked network.
Experimental Section
Hydrogel Synthesis
Hydrogels of methacrylic acid and N-vinyl pyrrolidone, henceforth denoted as P(MAA-co-NVP), were synthesized using a UV-initiated free radical polymerization. In the synthesis of these materials, the monomer content is defined as the monomers used to form the polymeric backbone and the crosslinking agent that yields the covalently bonded network. For P(MAA-co-NVP), the monomer mixture included methacrylic acid (MAA) (Sigma-Aldrich, St. Louis, MO) and N-vinyl pyrrolidone (NVP) (Sigma-Aldrich). Two separate crosslinking agents were used: ethylene glycol dimethacrylate (EGDMA) (Acros Organics, Morris Plains, NJ) and tetra(ethylene glycol) dimethacrylate (TEGDMA) (Fluka/Sigma-Aldrich). Irgacure® 184 (1-hydroxycyclohexyl phenyl ketone) (Sigma-Aldrich) was used as the photo-initiator and a combination of deionized water and ethanol was used as a solvent. All components were used as received.
The molar feed ratios of MAA:NVP synthesized were 1:1, 2:1, 4:1, 1:2 and 1:4. The crosslinking percentage, whether EGDMA or TEGDMA, was 1.0 mol-% of the total monomer content. The photo-initiator was added in the amount of 1.0 wt.-% of the total monomer content. The solvent makeup consisted of a 50:50 w/w water and ethanol mixture and was added to yield a 50:50 w/w total monomer to solvent ratio.
The components were prepared in an amber bottle to eliminate light interference with the photo-initiator. The mixture was briefly sonicated (Bransonic® 8510, Branson Ultrasonics Corp., Danbury, CT) to yield homogeneity. The mixture was placed in a sealed glove box and bubbled with nitrogen for 20 minutes to remove oxygen, a free radical scavenger, from the environment. The mixture was then pipetted between two glass plates (150 × 150 × 3 mm3) separated by a Teflon spacer (0.7 mm thick) and exposed to UV light (Dymax 2000 Light Curing System, Torrington, CT) at an intensity of 16–17 mW/cm2 for 30 min. to yield the polymer film. Following synthesis, the film was removed from the glass plates and washed with deionized water (changed daily) for 7 days to remove unreacted components. The washed film was vacuum dried at 30°C for 48 hours and crushed using a mortar and pestle. Sieves were used to yield two size ranges of particles – 90–150 μm and <75 μm. The particles were stored in a desiccator until use. Because hydrogel disks were needed for certain experiments, a 9 mm punch was used to cut disks from the polymer film prior to vacuum drying. The selection of this disk diameter allowed for the assumption of one-dimensional penetrant diffusion as the aspect ratio of the disks was more than 10.[21]
One disk was cut from the film immediately following polymerization but prior to introduction to water for the washing procedure. The weights of this disk in air and hexanes, in accordance with the method detailed later utilizing the hanging basket apparatus, were recorded to yield the volume of the gel in the “relaxed” state. An explanation of this state may be found in the Results and Discussion section of this article.
Fourier Transform Infrared Spectroscopic Analysis
Fourier transform infrared spectroscopy (FT-IR) was utilized to ascertain the presence of certain functional groups to gain insight on the structure of the polymer. The spectra for the different copolymers were obtained using an FT-IR spectrophotometer (ThermoNicolet Nexus 470, Thermo Fisher Scientific, Waltham, MA) equipped with a deuterated triglycine sulfate (DTGS) detector and potassium bromide (KBr) beamsplitter. An average of 64 scans was taken in the wavenumber range of 400–4000 cm−1 at a resolution of 1.928 cm−1. Pellets containing 10 mg of sample and 200 mg spectroscopy grade KBr (Acros Organics) were prepared using a Carver laboratory press with vacuum line attachment at 15,000 psi compression force.
Scanning Electron Microscopy
The effect of comonomer composition on the surface properties and morphology of polymer microparticles was examined through scanning electron microscopy (SEM). The micrographs were obtained through use of a LEO 1530 FE-SEM (Oberkochen, Germany). Double-sided conductive carbon tape was applied to aluminum SEM stages prior to addition of dried P(MAA-co-NVP) microparticles to the adhesive surface. Microparticles crushed and sieved to the <75 μm range were used in these studies. The microparticles were sputter-coated with gold for 40 seconds (Model 3, Pelco International, Redding, CA) in an argon atmosphere prior to imaging.
Dynamic pH Swelling Studies
Swelling studies of the hydrogels under dynamic pH conditions were conducted to evaluate the responsiveness of the gel in an environment representative of the transition between the stomach and upper small intestine. The swelling medium for these studies was 3,3-dimethylglutaric acid (Acros Organics, Morris Plains, New Jersey). The pH of the media was controlled by the addition of sodium hydroxide (NaOH 0.2 N) and the ionic strength of the media was controlled to 0.1 M by the addition of sodium chloride (NaCl). pH values of 3.2, 3.6, 4.2, 4.8, 5.4, 5.8, 6.2, 6.6, 7.2 and 7.6 were utilized.
Samples of 50 mL of the buffer at each pH were added to glass jars and heated to 37°C to mimic physiological conditions. A hydrogel disk was placed in the buffer at pH 3.2 and allowed to swell for 5 min. After 5 min, the disk was removed from the buffer, blotted dry with filter paper, and weighed on a traditional laboratory scale. The disk was then placed in the buffer at pH 3.6 for 5 additional minutes and then dried and weighed as before. This procedure was repeated until the weight of the disk had been recorded at pH 7.6. Prior to swelling, the weight of the disk in the dry state was recorded.
Constant pH Swelling Studies
Swelling studies were also carried out at constant pH levels in order to determine structural parameters of the gels in gastric and intestinal conditions. In these studies, gastric conditions were simulated by 0.1 N hydrochloric acid (HCl) and intestinal conditions were simulated by 1X phosphate buffered saline (diluted from 10X; Fisher Scientific, Fair Lawn, NJ). The swelling media did not contain enzymes that would be present in an in vivo environment.
To evaluate equilibrium swelling of these swollen polymeric materials, 50 mL of the desired media were added to a glass jar and heated to 37°C. A hydrogel disk was added to the medium and allowed to swell until the gel reached equilibrium; the sample was swollen for a minimum of 72 hours. In addition to the weights in the dry and swollen states, the weight of the disk was also measured in hexanes, a non-solvent to the polymer, using a hanging basket apparatus.
Results and Discussion
Hydrogel Synthesis
Selected mixtures were successfully polymerized into polymer films with one exception that will be discussed momentarily. The final makeup of these materials was governed more by the reactivity ratios of the components than the monomer molar feed ratios. It is a difficult endeavor to analyze the chemical makeup of chemically crosslinked networks; therefore, in this work, all systems will be referred to by their pre-polymerization monomer molar feed amounts.
The reactivity ratios of MAA and NVP indicate that the final chemical composition of the networks will be different than the monomer feed ratios. Bianco and Gehlen[22] report reactivity ratios for the materials as 0.56 for MAA, r1, and 0.04 for NVP, r2. As these values are both less than 1, the materials are prone to form an alternating structure, but the values of the reactivity ratios indicate that the copolymer should have a higher MAA content as MAA favors polymerizing with itself and NVP favors polymerizing with MAA.
To account for the effect of the reactivity ratios on the final polymer composition, the well-known copolymerization equation was utilized to determine F1 and F2, the mole fractions of the monomers in the copolymer, from f1 and f2, the mole fractions of the monomers in the feed, as shown in Equation (1):
| (1) |
The resulting mole fractions in the copolymers are shown in Table 1. Of course, the values of F1 and F2 calculated here prevail only in the first 40–50% of conversion of the reaction. Yet, they provide a reasonable indication of the copolymer structure.
Table 1.
Monomer mole fractions in the copolymer as calculated by the copolymerization equation (Equation (1))
| Molar Feed Ratio | f1 | f2 | F1 | F2 |
|---|---|---|---|---|
| 1:1 | 0.5 | 0.5 | 0.6 | 0.4 |
| 2:1 | 0.67 | 0.33 | 0.68 | 0.32 |
| 4:1 | 0.8 | 0.2 | 0.76 | 0.24 |
| 1:2 | 0.33 | 0.67 | 0.54 | 0.46 |
| 1:4 | 0.2 | 0.8 | 0.5 | 0.5 |
The P(MAA-co-NVP) 1:4 with 1% TEGDMA crosslinking copolymer could not be successfully synthesized into a film. The hydrophilic nature of NVP, coupled with the additional free volume associated with the longer crosslinker, led to the inability to form a stable film. While this material could be dried in its resultant form, no disks could be prepared. Between measurement at the relaxed state and measurement following drying, the 1:1, 2:1, and 4:1 copolymers lost approximately 40% of their initial weight. The 1:2 copolymer lost 53% of its initial weight and the 1:4 copolymer lost 70% of its initial weight.
Fourier Transform Infrared Spectroscopic Analysis
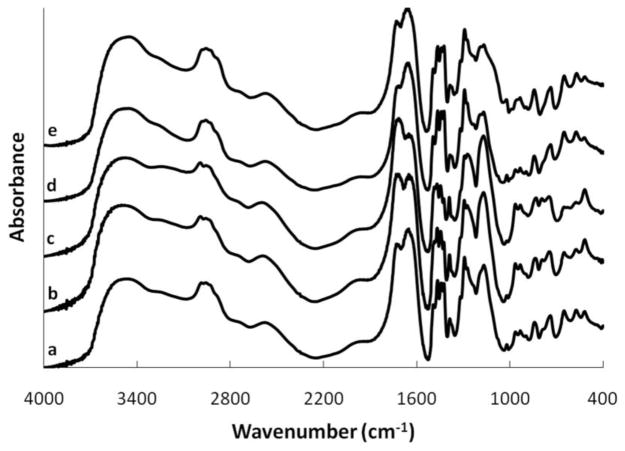
The structure of the microparticles and the effects of molar feed ratio and crosslinker choice were analyzed using FT-IR spectroscopy as shown in Figure 1. The broad area between 3100–3500 cm−1 is due to the presence of –OH groups with the area around 3200 cm−1 indicating bonded groups and the area around 3460 cm−1 indicating free hydroxyl groups.[23] The peak around 3500 cm−1 may be attributed to the C-N bond of N-vinyl pyrrolidone, explaining the relative decrease in height as the MAA concentration increases, and increase in height for higher quantities of NVP in the structure.[24] A stretching vibration due to the presence of the –CH3 group of methacrylic acid appears at approximately 2985 cm−1. The height of this peak increases with higher feed ratios of the MAA monomer. CH2 stretching vibrations are seen at about 2956 cm−1.
Figure 1.
FT-IR spectrum of P(MAA-co-NVP) copolymers with 1% EGDMA crosslinking. (a) 1:1, (b) 2:1, (c) 4:1, (d) 1:2, (e) 1:4
The carboxyl groups of the methacrylic acid units exhibit a vibrational band at 1727 cm−1 in the 1:1 MAA:NVP structure, and this band shifts slightly lower to 1715 cm−1 in the 2:1 and 4:1 MAA:NVP structures. Similar shifts have been noted in the literature with poly(acrylic acid) (PAA) and PVP structures with the decrease in wavenumber being attributed to an increase in hydrogen bonding.[25]
The peak at 1650 cm−1 is indicative of the free carbonyl group on the lactam ring of PVP and does not shift for any of the synthesized materials. This value is on the low end of reported values for this peak, though, which could be the result of a negative shift due to hydrogen bonding.[9, 26] The peaks around 1450 cm−1 are due to vibrational stretching bands in the pyrrolidone ring. The peak around 1170 cm−1 can be attributed to the C-N absorption band in the PVP structure,[10] but it is also possible that this peak, coupled with the peak around 1292 cm−1, is indicative of the stretching of C=O coupled with the bending of O-H in the methacrylic acid units.[23, 27]
Scanning Electron Microscopy
SEM imaging showed that the microparticles were of irregular geometries with a variety of microparticle sizes existing below 75 μm. Of primary interest in these studies was the determination of the effect of monomer molar feed ratio on the surface properties and morphology of the microparticles.
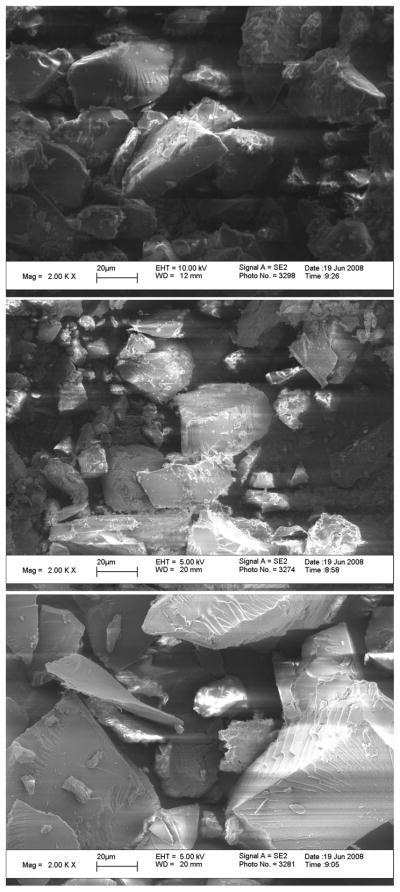
As shown in Figure 2, the surface features are affected by the different monomers. The image of the 1:1 MAA:NVP copolymer (top image) contains a mixture of smooth and rough areas. The image of the 4:1 MAA:NVP copolymer (middle) shows more surface defects than the equimolar ratio, suggesting that the presence of an excess of methacrylic acid leads to rougher areas. This is similar to previous SEM images of crosslinked PMAA microparticles that possess highly coarse surfaces in the absence of a comonomer.[23] The presence of rougher areas on a material signifies increased surface area, which may accelerate diffusion. The micrograph of the 1:4 MAA:NVP copolymer (bottom) reveals many smooth surfaces, indicating that an excess of N-vinyl pyrrolidone yields a softer material. The smooth surfaces of these materials are in agreement with images taken of hydrogels of N-vinyl pyrrolidone and itaconic acid where the gel contained an excess of NVP.[24] This may be due to the low glass transition temperature of PVP (54°C)[28] yielding material properties more similar to an amorphous liquid than a solid.
Figure 2.
SEM images of P(MAA-co-NVP) microparticles, <75 μm particle size, at various monomer molar feed ratios and 1% EGDMA crosslinking. (Top) 1:1, (Middle) 4:1, (Bottom) 1:4
Dynamic pH Swelling Studies
The primary goal of these studies was to evaluate the effects of different monomeric mixtures on the physicochemical behavior of the system in simulated physiological fluids. Swelling studies performed in dynamic pH conditions provided insight on the behavior of the materials in gastric conditions and the pH level at which the materials began decomplexing, allowing for swelling of the material and the release of loaded agents.
To evaluate the swelling behavior of the gels, the weight swelling ratio was determined. The weight swelling ratio, q, of the hydrogels was calculated by Equation (2) where Ws and Wd are the weights in the swollen and dry state.
| (2) |
The decision to use a 5 min swelling time at each pH was a result of an estimation of the diffusional time to reach equilibrium, t, from Equation (3), where lo is the initial diameter of a microparticle and D is a representative diffusional coefficient of water in a polymer.
| (3) |
For this estimation, lo was 100 μm and D was 10−6 cm2/s. The result of this estimation is that a microparticle would reach equilibrium in 100 s. Five min was therefore selected as it provided enough time to represent a microparticle swelling to equilibrium and allow for the appropriate experimental steps to be conducted. It is recognized that 5 min is not nearly enough time for a hydrogel disk to reach equilibrium, but early effects of the pH adjustment can be witnessed.
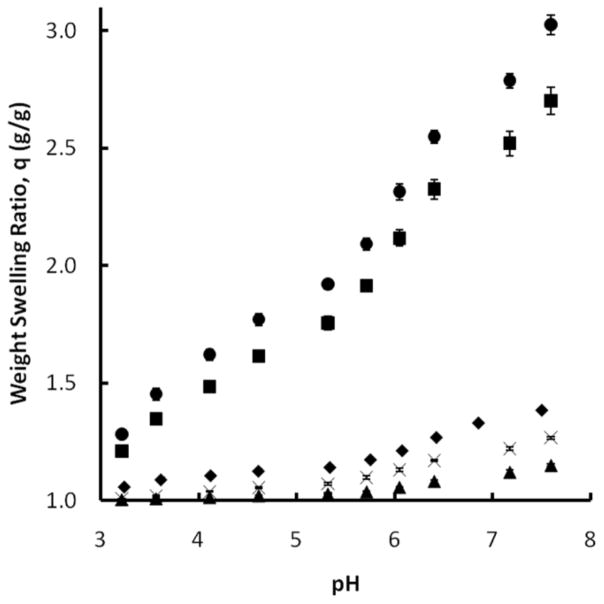
As shown in Figure 3, the addition of more MAA monomer decreased the swelling potential of the system. This can be attributed to the introduction of more hydrophobic groups to the polymer backbone which would repel the swelling media and hinder swelling. This notion suggests that the 4:1 MAA:NVP system would swell less than its 2:1 counterpart, however it can be seen that the 2:1 system does in fact swell less than the 4:1 system. The reversal of the expected trend suggests an additional mechanism is affecting the swelling of these copolymer mixtures. The results indicate a greater degree of hydrogen bonding complexation in the 2:1 system than the 4:1 system, evidenced by a more horizontal swelling trend at pH values up to approximately 5.6. Beyond this point, the two systems swell at similar rates. A highly complexed system would be less affected by pH increases below the pKa of the polymer as hydrogen bonds would work to maintain the system in a collapsed state.
Figure 3.
Dynamic weight swelling ratio, q, of P(MAA-co-NVP) disks, 1% EGDMA crosslinking, in DMGA/NaOH buffers. 1:1 (◆), 2:1 (▲), 4:1 (✕), 1:2 (■), 1:4 (●) (n=3 ± SD)
The addition of NVP monomer revealed a steady increase of the weight swelling ratio in low pH settings for the 1:2 and 1:4 MAA:NVP copolymers, a result of the highly hydrophilic nature of NVP. The trends, shown in Figure 3, suggest the loss of complexation sites in the 1:2 and 1:4 copolymers. The high degree of swelling at pH values below the pKa of MAA raises concern about the ability of the materials with elevated amounts of NVP to effectively protect therapeutic proteins in gastric conditions from release or degradation. Equilibrium swelling studies are required to make a more adequate conclusion on this matter prior to in vitro loading and release studies.
For the 1:1 system, the swelling trends suggest that the presence of the longer crosslinker has little effect on the weight swelling ratio of the system at short times. The presence of more ethylene glycol units in the polymer network should increase swelling because of the hydrophilicity of the material, but these effects were not pronounced in this system. The longer crosslinker did affect swelling at low pH in the 4:1 system, however. It is possible that the presence of the longer crosslinker disrupts the improved complexation within the 4:1 system as the compatible functional groups are farther apart from each other. This effect would be in addition to any increased swelling due to the presence of the hydrophilic ethylene glycol units.
Constant pH Swelling Studies: Swelling Theory and Governing Equations
In addition to determining the weight swelling ratio at equilibrium for the gels, it was also necessary to calculate the polymer volume fraction in the swollen state. The polymer volume fraction can be calculated by Equation (4) and is shown in a generalized form where the subscript x could be the swollen state, represented by the subscript s, or the relaxed state, which would be represented by the subscript r. The subscripts a and h are indicative of weighing in air or hexanes.
| (4) |
The molecular weight between crosslinks, , can be evaluated by the Peppas-Merrill equation, shown in Equation (5). The Peppas-Merrill equation is a modification of the Flory-Rehner equation, which was designed to be used for determining for a gel that was not prepared in the presence of a solvent. The Peppas-Merrill equation accounts for these preparation conditions by accounting for the volume of the gel in the relaxed state, υ2,r. The relaxed state is the state of the gel immediately following polymerization but prior to introduction of the gel to a solvent like water. The term “relaxed” is actually a misnomer in that the gel is actually in a state of tension following crosslinking; the introduction of the solvent to the gel helps it to reach a relaxed state. The Flory-Rehner equation may be achieved by setting υ2,r equal to one.
| (5) |
Within the Peppas-Merrill equation, is the number average molecular weight of a linear polymer, i.e. one formed without a crosslinking agent. If one knows the swelling properties of the gel, one may calculate by varying and holding all other parameters constant. At a critical molecular weight, the sensitivity of the equation becomes negligible and will plateau to a certain value. The at which this occurs can therefore be used under the assumption that the polymerization is going to high conversion, which should occur for a UV-initiated solution polymerization that is not limited by the same diffusional concerns faced in a bulk polymerization.
V1, the molar volume of water, was 18.0 cm3 mol−1. Even though the swelling media was not purely water, water makes up such a large portion of the dilute acids and bases used that assuming it as such allowed for the determination of values. ῡ is the specific volume of the polymer and was calculated by Equation (6), where ρh is the density of hexanes (0.668 g/mL):
| (6) |
The final term unaccounted for in the Peppas-Merrill equation is χ1, the Flory interaction parameter. The Flory interaction parameter provides an indication as to how good a solvent is for a particular polymer, with values below 0.5 indicating a good solvent and values greater than 0.5 indicative of a poor solvent. One of the requirements for the Flory-Rehner or Peppas-Merrill equations to hold is that χ1 must be less than 0.5. The value of χ1 was calculated by taking a weighted average of the χ values of the homopolymers PMAA and PVP with water and weighting them by their molar fractions in the feed. The χ for PVP is 0.48.[29] The χ for PMAA can be determined as a function of the polymer volume fraction in the swollen state,[30, 31] as shown in Equation (7).
| (7) |
This correlation was determined for non-ionized PMAA gels. An additional assumption for either the Flory-Rehner or Peppas-Merrill theories to be valid is that the gel must be neutral. The assumption in this work that the gel is neutral, although it contains an ionizable component in methacrylic acid, is based on the fact that PMAA is less ionizable than gels like poly(acrylic acid) (PAA). A more robust model to account for the ionizable component is the Brannon-Peppas model for ionic gels.[32]
Additional requirements that must be in place include a Gaussian distribution of chain lengths and that the gel must be swollen to equilibrium. In this work, we are assuming the Gaussian distribution without verification; the Peppas-Lucht model is one of several non-Gaussian models that could be utilized if evidence suggested that this assumption was not valid.[33] The necessity for the gel to be swollen to equilibrium is accounted for by swelling the hydrogel disks for several days as opposed to a few minutes, which would be sufficient for microparticles. The network structure of a disk and particles is identical in the equilibrium state and therefore parameters like will remain constant for either variety.
The mesh size, also referred to as the pore size, is the dimension available in a polymer network for a compound to diffuse in or out of the network. Knowing the mesh size of a drug delivery system is important for determining whether or not sufficient transfer can occur between the loaded drug and the desired target. The mesh size was calculated using Equation (8):
| (8) |
where α is the elongation ratio in any direction and is the r.m.s. end to end distance in the unperturbed state. If isotropic swelling is assumed, then Equation (9) is used:
| (9) |
as υ2,s is the reciprocal of the volume swelling ratio, Q.
Equation (10) introduces the r.m.s. end to end distance in the freely jointed state, :
| (10) |
Here, Cn is the characteristic ratio of the polymer. This is taken as a weighted average of the characteristic ratios of the homopolymers (PMAA = 14.6;[28] PVP = 12.3[34]). Equation (11) yields:
| (11) |
where l is the bond length of a carbon-carbon bond (1.54 Å) and n, the number of links per chain, is calculated from Equation (12):
| (12) |
Here, Mr is the molecular weight of the repeat unit, taken as a weighted average of the molecular weights of the monomers (MAA = 86.08; NVP = 111.14). Compiling Equation (8) – (12), the mesh size was calculated by Equation (13):
| (13) |
Constant pH Swelling Studies: Results
P(MAA-co-NVP) hydrogels were swollen to equilibrium in simulated gastric and intestinal conditions and evaluated through the use of the previously defined equations and relationships. Minimal swelling was expected in simulated gastric conditions as the hydrogels are of an anionic nature. Anionic hydrogels resist swelling at pH values below the pKa of the ionic constituent and will begin to swell once this threshold is passed. For methacrylic acid, the pKa is 4.8–4.9.[35, 36] Furthermore, hydrogen bonding complexes between the carboxylic acid group of MAA and the carbonyl group on the lactam ring of NVP exist below the pKa, providing more resistance to swelling. These complexes fail when the carboxylic acid group deprotonates, leading to a buildup of negative charges that repel each other, enhancing the swelling of the system.
In simulated gastric conditions, the copolymers with 1% EGDMA crosslinking that contained equimolar amounts of MAA and NVP and those with higher molar feed amounts of MAA swelled to a similar degree (q1:1 = 1.37, q2:1 = 1.23, q4:1=1.43). The lower swelling in the 2:1 feed ratio system confirms the hypothesis made with the dynamic pH studies that the 2:1 system has a higher degree of hydrogen bonding complexation than the other ratios. These materials have a high υ2,s as a majority of the swollen polymer is not fluid. These results are in contrast to previous studies conducted with the P(MAA-g-PEG) system. Lowman and Peppas determined that the highest degree of complexation occurred with the system of equimolar methacrylic acid to ethylene glycol units, citing the cooperative nature of the interactions and the stoichiometric ratio of the complexing groups.[37]
The copolymers with higher amounts of NVP monomer experienced more swelling than the other ratios due to the hydrophilic nature of NVP (q1:2 = 1.90, q1:4 = 2.81). The υ2,s for these materials was lower as well, with respective values of 0.47 and 0.32. A large amount of fluid uptake leads to limited complexation in gastric conditions which might not provide adequate protection to the loaded protein, resulting in premature degradation or release. It was determined that the length of the crosslinker does not affect swelling in these materials at a very low pH as the 1% EGDMA systems swell to a similar level as the 1% TEGDMA systems, with minimal variance seen in the polymer volume fractions of the different materials.
Equilibrium swelling in simulated intestinal fluid resulted in an order of magnitude increase in the weight swelling ratios and an order of magnitude decrease in the polymer volume fractions. It was determined that increasing the molar feed ratio of MAA yields an increase in the weight swelling ratio of the hydrogel (q1:1 = 12.53, q2:1 = 12.71, q4:1=13.97). This effect is due to the presence of an increasing number of negative charges along the polymer backbone, resulting in a greater amount of ionic repulsion. The respective polymer volume fractions for these systems were 0.066, 0.063 and 0.057. In gastric conditions, these values were 0.71, 0.77, and 0.68, clearly indicating the presence of a large volume of fluid within the material at intestinal pH levels. The increased fluid uptake for these materials should lead to rapid drug release in intestinal conditions.
Copolymers containing more N-vinyl pyrrolidone, as before, demonstrated the highest swelling ratios and lowest polymer volume fractions in intestinal conditions. The hydrogel disk of the 1:4 system was extremely fragile following swelling. This material property, coupled with the significantly decreased complexation potential in gastric conditions, leads to the conclusion that P(MAA-co-NVP) 1:4 is not a suitable choice for drug delivery applications.
The crosslinker length was shown to affect swelling in intestinal conditions. For the 1:1 system, the weight swelling ratio increased from 12.53 to 17.11 and the polymer volume fraction decreased from 0.066 to 0.047. In the 4:1 system, these changes were 13.97 to 17.37 and 0.057 to 0.045. While the swelling did increase for these materials, the structural integrity was not affected as with the NVP-laden systems.
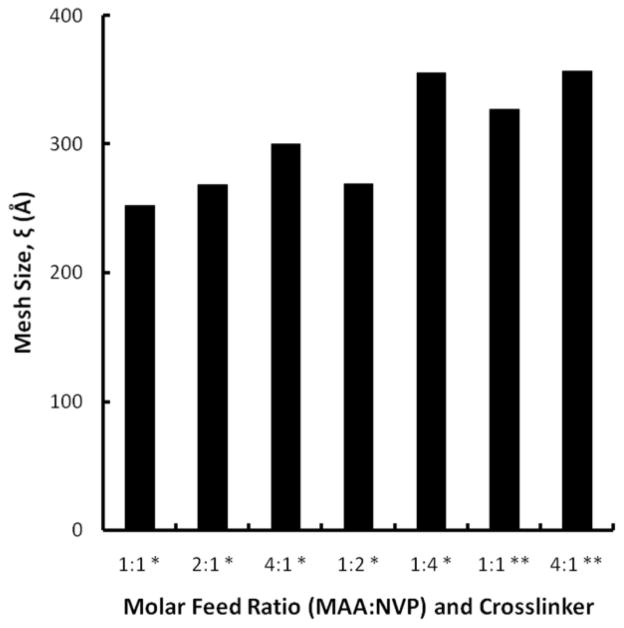
Analysis of the swelling behavior at equilibrium allowed for the determination of the mesh size for the hydrogel networks. The mesh size for the systems in intestinal conditions varied in the same manner as the weight swelling ratios; increasing the molar feed content of either MAA or NVP or utilizing TEGDMA instead of EGDMA led to an increase in the mesh size (Figure 4). The values ranged from 252 Å for the 1:1 1% EGDMA system to 357 Å for the 4:1 1% TEGDMA system. For oral drug delivery applications, the hydrodynamic radius of the therapeutic agent needs to be less than that of the mesh size of the delivery system in order for diffusion to occur. Insulin, a model protein, possesses a hydrodynamic radius of 12–13 Å in the monomeric form and 28 Å in the hexameric form.[38] The difference in size between the networks and this protein indicate that diffusion should readily occur.
Figure 4.
Equilibrium mesh size, ξ, of P(MAA-co-NVP) copolymers at various monomer molar feed amounts in simulated intestinal conditions. * 1% EGDMA, ** 1% TEGDMA
As previously stated, the Peppas-Merrill equation does not hold for χ1 values greater than 0.5. This limitation made it impossible to determine values for the polymers in gastric conditions due to the υ2,s dependency in Equation (7) for calculating the χ of PMAA. As shown in Table 2, the weighted average χ values for all P(MAA-co-NVP) copolymers are greater than 0.5 due to the high υ2,s values of the materials in low pH conditions. Without the ability to determine , no mesh sizes could be calculated for these materials in gastric conditions. Rubber elasticity studies provide a solution to this problem and have been utilized to determine the molecular weight between crosslinks by previous researchers.[39, 40] With rubber elasticity studies, may be calculated from Equation (14):
| (14) |
where τ is the tensile stress, α is the elongation of the sample, ρ2,r is the polymer density in the relaxed state; all other terms are identical to their previous definitions. These studies were not performed in this work but it is assumed that mesh sizes for the P(MAA-co-NVP) copolymers in gastric conditions would be of a similar magnitude to those determined for P(MAA-g-PEG) polymers. Tuesca et al. report network mesh sizes of approximately 90 Å for P(MAA-g-PEG) hydrogels in gastric pH levels.[40] It is anticipated that the P(MAA-co-NVP) 1:2 and 1:4 systems would have mesh sizes of a slightly higher value because of their increased swelling in low pH conditions relative to the remaining systems.
Table 2.
Calculation of χ1 for P(MAA-co-NVP) copolymers in simulated gastric conditions
| Crosslinker | Molar Feed Ratio (MAA:NVP) | υ2,s | χMAA | χwt. avg. |
|---|---|---|---|---|
| EGDMA | 1:1 | 0.707 | 0.864 | 0.711 |
| 2:1 | 0.772 | 0.903 | 0.768 | |
| 4:1 | 0.678 | 0.847 | 0.759 | |
| 1:2 | 0.473 | 0.724 | 0.612 | |
| 1:4 | 0.322 | 0.633 | 0.557 | |
| TEGDMA | 1:1 | 0.697 | 0.858 | 0.707 |
| 4:1 | 0.711 | 0.866 | 0.774 |
Conclusions
Hydrogels of methacrylic acid and N-vinyl pyrrolidone were successfully synthesized using a UV-initiated free radical polymerization and characterized by a variety of techniques. The presence of hydrogen bonding complexes was confirmed by FT-IR and dynamic pH swelling studies revealed that systems containing higher concentrations of MAA experienced the highest degree of complexation. SEM revealed the effects of the different comonomers on the surface morphology of the microparticles.
Constant pH swelling studies demonstrated that the materials have a suitable mesh size at equilibrium to allow for drug diffusion across the network. The materials possess pH responsiveness when transported from gastric to intestinal conditions that should provide protection to an encapsulated drug from premature degradation or release in oral delivery applications. The exception to this was the system containing the highest concentration of NVP because of its enhanced swelling at low pH levels and decreased material integrity in high pH conditions.
These studies have demonstrated that microparticles of many P(MAA-co-NVP) mixtures have the necessary properties to be utilized as carriers for the oral delivery of proteins. Drug loading and release studies are needed for these materials to truly ascertain their potential as drug delivery systems.
Acknowledgments
This work is funded by the National Institutes of Health (NIH EB-000246). D.A.C. acknowledges the National Science Foundation for a Graduate Research Fellowship. The authors would like to thank the laboratory of Dr. Benny Freeman at The University of Texas at Austin for experimental assistance.
References
- 1.Sandler SR, Karo W. Poly(N-Vinyl pyrrolidone) 2. Academic Press; Boston: 1992. p. 261. [Google Scholar]
- 2.Davis TP, Huglin MB, Yip DCF. Polymer. 1988;29:701. [Google Scholar]
- 3.Haaf F, Sanner A, Straub F. Polym J. 1985;17:143. [Google Scholar]
- 4.Atta AM, Arndt KF. Polym Int. 2004;53:1870. [Google Scholar]
- 5.Perera DI, Shanks RA. Polym Int. 1996;39:121. [Google Scholar]
- 6.Takayama K, Nagai T. Chem Pharm Bull. 1987;35:4921. doi: 10.1248/cpb.35.4921. [DOI] [PubMed] [Google Scholar]
- 7.Davis TP, Huglin MB. Polymer. 1990;31:513. [Google Scholar]
- 8.Hider RC, Lloyd JC, Wheeler P. J Colloid Interface Sci. 1978;65:1. [Google Scholar]
- 9.Devine DM, Higginbotham CL. Eur Polym J. 2005;41:1272. [Google Scholar]
- 10.Solpan D, Kolge Z, Torun M. J Macromol Sci, Pure Appl Chem. 2005;A42:705. [Google Scholar]
- 11.Solpan D, Kolge Z, Torun M. J Macromol Sci, Pure Appl Chem. 2006;A43:129. [Google Scholar]
- 12.Mahkam M, Mohammadi R, Siadat SOR. J Chin Chem Soc-Taip. 2006;53:727. [Google Scholar]
- 13.Liu SY, Yang MJ, Dan Y. J Appl Polym Sci. 2005;96:2280. [Google Scholar]
- 14.Nieuwenhuis J, Tan YY, Vanekenstein G. Angew Makromol Chem. 1987;147:83. [Google Scholar]
- 15.Barbu E, Sarvaiya I, Green KL, Nevell TG, Tsibouklis J. J Biomed Mater Res, Part A. 2005;74A:598. doi: 10.1002/jbm.a.30329. [DOI] [PubMed] [Google Scholar]
- 16.Feldstein MM. Polymer Science Series A. 2004;46:1165. [Google Scholar]
- 17.Liu SX, Fang Y, Hu DD, Gao GL, Ma JB. J Appl Polym Sci. 2001;82:620. [Google Scholar]
- 18.Ponratnam S, Rao SP, Joshi SG, Kapur SL. J Macromol Sci-Chem. 1976;A10:1055. [Google Scholar]
- 19.Bekturov EA, Bimendina LA. Adv Polym Sci. 1981;43:100. [Google Scholar]
- 20.Polacco G, Cascone MG, Petarca L, Peretti A. Eur Polym J. 2000;36:2541. [Google Scholar]
- 21.Khare AR, Peppas NA. Biomaterials. 1995;16:559. doi: 10.1016/0142-9612(95)91130-q. [DOI] [PubMed] [Google Scholar]
- 22.Bianco G, Gehlen MH. J Photochem Photobiol, A. 2002;149:115. [Google Scholar]
- 23.Thomas JB, Creecy CM, McGinity JW, Peppas NA. Polym Bull. 2006;57:11. [Google Scholar]
- 24.Chen KS, Ku YA, Lin HR, Yan TR, Sheu DC, Chen TM, Lin FH. Mater Chem Phys. 2005;91:484. [Google Scholar]
- 25.Yaung JF, Kwei TK. J Appl Polym Sci. 1998;69:921. [Google Scholar]
- 26.Devine DM, Higginbotham CL. Polymer. 2003;44:7851. [Google Scholar]
- 27.Lazzari M, Kitayama T, Hatada K, Chiantore O. Macromolecules. 1998;31:8075. [Google Scholar]
- 28.Brandrup J, Immergut EH. Polymer Handbook. 3. John Wiley & Sons; New York: 1989. [Google Scholar]
- 29.Csaki KF, Nagy M, Csempesz F. Langmuir. 2005;21:761. doi: 10.1021/la047827h. [DOI] [PubMed] [Google Scholar]
- 30.Eichenbaum GM, Kiser PF, Dobrynin AV, Simon SA, Needham D. Macromolecules. 1999;32:4867. doi: 10.1021/ma970897t. [DOI] [PubMed] [Google Scholar]
- 31.Hasa J, Ilavsky M. J Polym Sci, Part B: Polym Phys. 1975;13:263. [Google Scholar]
- 32.Peppas NA. Drug Delivery Using Smart Polymers: Recent Advances. In: Galaev I, Mattiasson B, editors. Smart Polymers: Applications in Biotechnology and Biomedicine. CRC Press; Boca Raton: 2007. p. 331. [Google Scholar]
- 33.Lucht LM, Peppas NA. Chem Eng Commun. 1984;30:291. [Google Scholar]
- 34.Tarazona MP, Saiz E. J Biochem Biophys Methods. 2003;56:95. doi: 10.1016/s0165-022x(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 35.Blanchette J, Peppas NA. J Biomed Mater Res, Part A. 2005;72A:381. doi: 10.1002/jbm.a.30243. [DOI] [PubMed] [Google Scholar]
- 36.Peppas NA, Wood KM, Blanchette JO. Expert Opin Biolog Ther. 2004;4:881. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 37.Lowman AM, Peppas NA. Macromolecules. 1997;30:4959. [Google Scholar]
- 38.Hovgaard L, Jacobs H, Mazer NA, Kim SW. Int J Pharm. 1996;132:107. [Google Scholar]
- 39.Nakamura K, Murray RJ, Joseph JI, Peppas NA, Morishita M, Lowman AM. J Controlled Release. 2004;95:589. doi: 10.1016/j.jconrel.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Tuesca A, Nakamura K, Morishita M, Joseph J, Peppas N, Lowman A. J Pharm Sci. 2008;97:2607. doi: 10.1002/jps.21184. [DOI] [PubMed] [Google Scholar]