Abstract
Background
Sulfatase 2 (SULF2), an extracellular heparan sulphate 6-O-endosulphatase, has an oncogenic effect in hepatocellular carcinoma (HCC) that is partially mediated through glypican 3, which promotes heparin-binding growth factor signalling and HCC cell growth. SULF2 also increases phosphorylation of the anti-apoptotic Akt kinase substrate GSK3β and SULF2 expression is associated with a decreased apoptotic index in human HCCs.
Methods
We investigated the functional and mechanistic effects of SULF2 on drug-induced apoptosis of HCC cells using immunohistochemistry, Western immunoblotting, gene transfection, real-time quantitative polymerase chain reaction, MTT and apoptosis assays and immunocytochemistry.
Results
The increased expression of SULF2 in human HCCs was confirmed by immunohistochemistry and immunoblotting. Treatment with inhibitors of MEK, JNK and PI3 kinases decreased the viability of SULF2-negative Hep3B HCC cells and induced apoptotic caspase 3 and 7 activity, which was most strongly induced by the PI3K inhibitor LY294002. Forced expression of SULF2 in Hep3B cells significantly decreased activity of the apoptotic caspases 3 and 7 and induced resistance to LY294002-induced apoptosis. As expected, LY294002 inhibited activation of Akt kinase by PI3K. Conversely, knockdown of SULF2 using an shRNA construct targeting the SULF2 mRNA induced profound cell growth arrest and sensitized the endogenously SULF2-expressing HCC cell lines Huh7 and SNU182 to drug-induced apoptosis. The effects of knockdown of SULF2 on HCC cells were mediated by decreased Akt phosphorylation, downregulation of cyclin D1 and the anti-apoptotic molecule Bcl-2, and upregulation of the pro-apoptotic molecule BAD.
Conclusion
The prosurvival, anti-apoptotic effect of SULF2 in HCC is mediated through activation of the PI3K/Akt pathway.
Keywords: Akt pathway, apoptosis, caspase, heparan sulphate glycosaminoglycan (HSGAG), heparan sulphate proteoglycan (HSPG), oncogene, SULF2
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide (1). Because of frequent de novo and acquired resistance of HCCs to chemotherapy, there are limited options for therapy of HCC (2, 3). There is therefore an urgent need for improved therapy of HCC. Consequently there is strong interest in identifying novel molecular targets for therapy of advanced HCC.
The role of the extracellular heparan sulphate 6-O-endosulphatases, sulfatase 1 (SULF1) and sulfatase 2 (SULF2) in human carcinogenesis has not been completely elucidated (4, 5). SULF1 has been shown to function as a tumour suppressor in HCC, head and neck cancer, ovarian cancer and pancreatic cancer (5–10). SULF1 and SULF2 have also been reported to inhibit tumour growth in multiple myeloma (11). In contrast, SULF2 is upregulated in breast cancer and functions as an oncogene in HCC, pancreas cancer, lung cancer and chronic lymphocytic leukemia (12–16).
Gene expression microarray analysis of 139 pairs of HCC tumour and adjacent benign tissue showed upregulation of SULF2 in 57% of HCCs (13). The 5-year survival rate for patients with HCCs with upregulated SULF2 was significantly worse than for those with down-regulated SULF2. Patients with upregulated SULF2 also had earlier recurrence of HCC after surgery. Immunohistochemical analysis of cell proliferation and apoptosis was performed in 30 of the HCCs (13). Tumours were classified into subclass A (poor prognosis) or subclass B (good prognosis) based on the prior gene expression profiling study by Lee et al. (17). Subclass A tumours were more frequent in the high SULF2 group (13 of 14 tumours) and less frequent in the low SULF2 group (two of 16 tumours; P=0.00001). HCCs with high SULF2 expression also had a significantly higher Ki-67 proliferation index (P= 0.007) and a significantly lower apoptosis index (P = 0.0001) than those with low SULF2 expression. SULF2 expression therefore correlated with increased proliferation and decreased apoptosis (13). In experiments to validate these results, we showed that SULF2 promoted proliferation and migration of HCC cells in vitro (13, 18). Mechanistically, SULF2 upregulated cell surface glypican 3 and promoted FGF signalling. Expression of SULF2 increased phosphorylation of Erk and Akt (13). SULF2 expression also increased phosphorylation of the anti-apoptotic Akt substrate GSK3β and stimulated Wnt/β-catenin signalling(19). Other investigators have also demonstrated that SULF2 promotes signalling by receptor tyrosine kinase ligands, Wnts and other growth factors (14, 20, 21).
In terms of associations with other known pro-apoptotic molecules, SULF2 has been shown to be a transcriptional target of p53 in colon cancer, lung cancer, ovarian cancer and HCC cells, but the direct or indirect effects of SULF2 on apoptosis and apoptosis-related pathways in HCC have not been reported (22, 23). ERK, PI3K/Akt and JNK pathway inhibitors and histone deacetylase (HDAC) inhibitors induce apoptosis and are currently in clinical trials for cancer therapy (24–26). We studied the expression of SULF2 in HCCs and determined the role of SULF2 in modulating apoptosis induced by these kinase and HDAC inhibitors in HCC cells. The questions addressed in this study were: Is SULF2 mRNA expression correlated to protein expression in HCCs? Do changes in SULF2 expression affect cell viability, caspase activation and induction of apoptosis of HCC cells by ERK, PI3K, JNK or HDAC inhibitors? Does knockdown of SULF2 inactivate the Akt pathway? Does knockdown of SULF2 inhibit cell cycle progression as measured by cyclin D1 expression? Does SULF2 mediate its effects by regulating apoptosis-related Bcl-2, Bcl-XL and BAD protein expression?
Materials and methods
Chemicals and antibodies
Complete Mini Protease Inhibitor Mixture, Protein G Sepharose, and 4′,6-diamidino-2-phenylindole (DAPI), antibody to β actin and horseradish peroxidase-conjugated mouse IgG were from Sigma Chemical Co. (St Louis, MO, USA); antiphospho-Akt ser 473 and total Akt antibodies from Cell Signaling (Beverly, MA, USA), and BAD (sc-7869 from Santa Cruz Biotechnology (Santa Cruz, CA, USA); rabbit IgG from Invitrogen Corp. (Carlsbad, CA, USA), and ECL reagents from Amersham/GE Healthcare (Piscataway, NJ, USA). The rabbit polyclonal antibody to SULF2 was reported previously (13). Plasmid vectors pSS-H1p and pG-SUPER were gifts from Dr Daniel D. Billadeau and Dr Shin-Ichiro Kojima respectively.
Tissue samples and immunohistochemistry for SULF2
Immunostaining was performed using antibody to SULF2 on sections of paraffin-embedded HCCs and adjacent benign liver tissues using the En Vision+system (Dako Corporation, Carpinteria, CA, USA) as described (8). A negative control was set up by replacement of the primary antibody with 1% BSA-TBS. Negative control specimens showed no non-specific staining.
HCC cell lines
The Hep3B and SNU182 HCC cell lines were from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured as recommended;. Huh-7 cells were from Dr Gregory J Gores (27).
RNA isolation and quantitative real-time PCR
Total RNA was extracted from HCC cell lines using the RNeasy kit (Qiagen, Valencia, CA, USA). cDNA synthesis was performed using Superscript II RNase H− reverse transcriptase (Life Technologies, Bethesda, MD, USA) to transcribe 2 μg of total RNA primed with 1 μl of 500 μg/ml random hexamers. For quantitative real-time PCR analysis, an ABI TaqMan assay (HS00378697) was used in an ABI 7300 system with the following profile: 95°C for 10min followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. SULF2 mRNA levels were normalized by comparison to 18S ribosomal RNA levels in the same samples. Each measurement was performed in quadruplicate; standard curves were prepared from synthesized SULF2 and 18S standards.
Establishment of SULF2 stable transfectant clones
Sulphatase 2-negative Hep3B cells were transfected with either SULF2-expressing plasmid DNA or pcDNA3.1 vector DNA (7, 13). SULF2-expressing Huh7 cells were stably transfected with plasmids expressing short hairpin RNA (shRNA) sequences targeting SULF2 (13).
Caspase 3/7 activity assay
Caspase 3/7 activity assays were performed as described (8).
Detection of apoptosis by fluorescence microscopy
Apoptosis was measured by using fluorescence microscopy to identify apoptotic nuclear changes (i.e. chromatin condensation and nuclear fragmentation) after staining with the DNA binding dye DAPI as described (8).
Immunocytochemistry and confocal microscopy
Huh7 cells were seeded on glass cover slips in six well plates and incubated for 24 h. Cells were transiently transfected with shRNA plasmid targeting SULF2 for 24 h, rinsed with Dulbecco’s PBS (D-PBS; 8.1 mM Na2HPO4, 1.2mM KH2PO4, pH 7.2, 138 mM NaCl, 2.7 mM KCl, 0.9 mM CaCl2, 0.5mM MgCl2) at room temperature and fixed for 20 min with 2.5% formaldehyde in PIPES buffer [0.1 M piperazine-N,N′-bis (2-ethanesulphonic acid), pH 6.95, 3 mM MgSO4, 1 mM EGTA]. After rinsing with D-PBS, cells were incubated in blocking buffer (5% normal goat serum and 5% glycerol in D-PBS) for 1 h at 37°C. After incubation with polyclonal antibody against phospho-Akt ser473 for 2 h at 37°C, the cells were rinsed 3 times, 10 min each time, with D-PBS and incubated with the appropriate TRITC-labelled anti-mouse or rabbit IgG for 1 h at 37°C. Cells were then washed three times for 5 min each time with D-PBS, rinsed briefly with distilled water, and mounted with DAPI on a glass slide. Confocal microscopy was performed as described (8).
Western immunoblotting
Western immunoblotting using whole cell lysates was performed as described (8).
Blots were probed with polyclonal or monoclonal antibodies against SULF2, p-Aktser473, p-ERK44/42, cyclin D1, Bcl-2, Bcl-XL and BAD. The levels of total ERK, total Akt and β-actin were also measured to control for equal loading.
Statistical analysis
All data represent at least three independent experiments using cells from separate cultures and are expressed as the mean ± SEM. Differences between groups were compared using an unpaired two-tailed t-test.
Results
SULF2 protein is upregulated in HCC tumour tissue when compared with surrounding benign liver tissue
We have previously shown that SULF2 is frequently upregulated in HCCs and predicts a more aggressive tumour phenotype by measuring mRNA expression levels (13). To confirm high SULF2 protein expression in tumour tissue as compared with surrounding benign tissue, we performed immunohistochemistry in 45 resected HCCs and adjacent benign liver tissues (Fig. 1A and B). We also performed Western blotting in three pairs of benign and HCC tissues (Fig. 1C), which further confirmed the high SULF2 protein expression level in tumour tissue when compared with surrounding benign liver tissue.
Fig. 1.
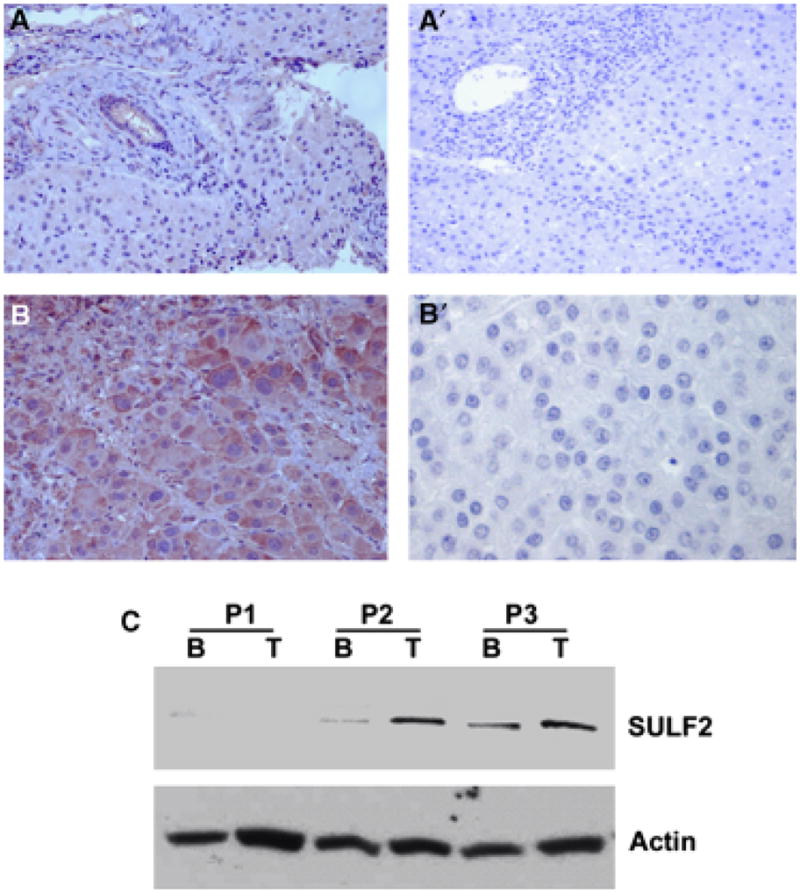
Sulfatase 2 (SULF2) expression in hepatocellular carcinomas (HCCs) and their adjacent benign liver. (A, B) Immunohistochemistry of SULF2 and negative control in benign (A) and HCC (B) samples: left panels A and B are stained with antibody to SULF2, right panels A′ and B′ are negative controls in which the antibody to SULF2 was replaced with 1% BSA-TBS. (C) Confirmation of SULF2 levels in pairs of benign (B) and tumour (T) tissue from three HCC patients by Western immunoblotting. Panel 1 (P1) in (C) is from a tumour with low SULF2 mRNA expression; panels P2 and P3 are from tumours with higher SULF2 mRNA in the tumour than in adjacent benign tissue.
Forced expression of SULF2 attenuates the effects of ERK, PI3K, JNK inhibitors on cell viability and caspase 3 and 7 activation and attenuates LY294002-induced apoptosis
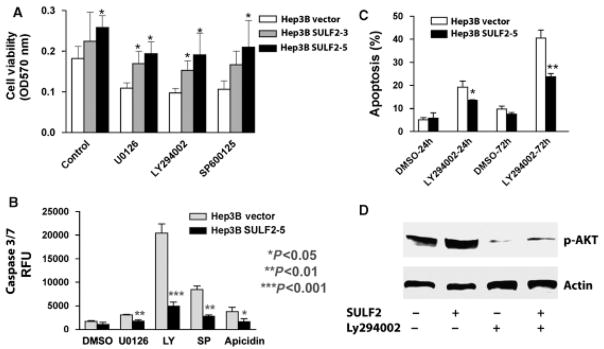
The MAPK pathway plays an important role in regulation of cell proliferation and migration (7, 8, 27). The PI3 and JNK kinase pathways are also important in regulation of apoptosis (28). We have shown that forced expression of SULF2 increased both mRNA and protein levels of SULF2 and resulted in increased phosphorylation of both ERK and Akt in Hep3B cells (13). Further, we have shown that phosphorylation of GSK3β increased following forced expression of SULF2 in Hep3B cells (19). We have also shown that the HDAC inhibitor apicidin induced apoptosis with activation of caspase 3 and 7 in HCC cells (27). In the present study we compared cell viability in Hep3B Vector or Hep3B SULF2-5 cells after treatment with the ERK inhibitor U0126, the PI3 kinase inhibitor LY294002, and the JNK inhibitor SP600125 and caspase 3/7 activity after treatment with the three kinase inhibitors and the HDAC inhibitor apicidin. We found that all three kinase inhibitors decreased Hep3B cell viability and the three kinase inhibitors and the HDAC inhibitor apicidin all increased activated caspase 3/7 as compared with control cells treated with DMSO; these effects were significantly abrogated by SULF2 expression (Fig. 2A & B). Of the four inhibitors, the PI3 kinase inhibitor LY294002 had the most profound effect on cell viability and caspase 3/7 activation. LY294002 induced apoptosis in Hep3B Vector cells at 24 and 72 h after treatment was significantly but not completely attenuated by SULF2 expression in Hep3B SULF2-5 cells (Fig. 2C). To evaluate the effects of SULF2 on Akt phosphorylation, Hep3B cells were transfected with or without SULF2 and treated with or without LY294002. Forced expression of SULF2 increased Akt phosphorylation and, as expected, this effect was inhibited by LY294002 (Fig. 2D). Thus, forced expression of SULF2 partially protected Hep3B cells from drug-induced apoptosis, and SULF2 most likely functions upstream of multiple pro-survival kinase pathways.
Fig. 2.
Expression of sulfatase 2 (SULF2) protects hepatocellular carcinoma (HCC) cells against drug-induced effects on cell viability and apoptosis and increases AKT phosphorylation. (A) Hep3B vector, Hep3B SULF2-3 and Hep3B SULF2-5 cells were plated into 96 well plates at 5000 cells per well using six replicates for each condition. Cells were treated with control DMSO, 25 μM U0126, 20 μM Ly294002 and 25 μM SP600125 for 24 h. Cell viability was evaluated using the MTT assay. (B) In a parallel experiment to A, the activity of caspases 3 and 7 was measured in relative fluorescence units (RFU). Apicidin induced activation of caspase 3/7 was used as a control (8). (C) Hep3B vector or Hep3B SULF2-5 cells were plated into six well plates at 2 × 105 cells per well and treated with 20 μM Ly294002. Apoptosis was evaluated after DAPI staining. (D) Whole cell lysates were collected at 72 h after treatment of Hep3B vector or Hep3B SULF2-5 cells with Ly294002. Akt phosphorylation was then evaluated by Western immunoblotting using antibodies against p-Akt and actin.
LY294002-induced HCC cell apoptosis is enhanced by knockdown of SULF2
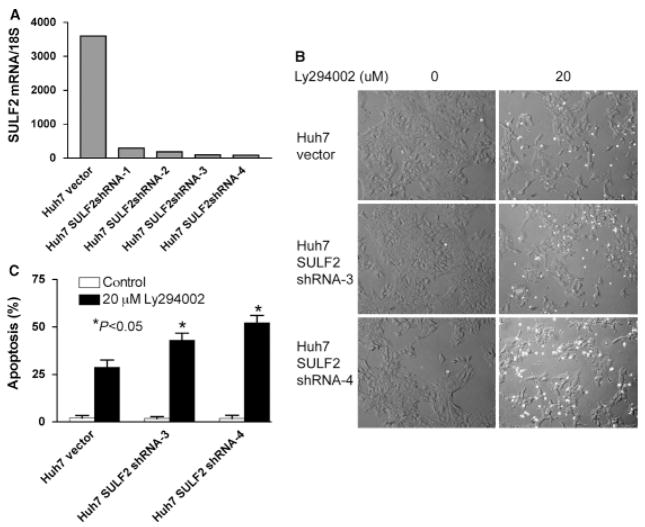
Huh7 and SNU182 HCC cells endogenously overexpress SULF2 (13). To explore the effects of knockdown of SULF2 on apoptosis of HCC cells, we generated stable clones transfected with shRNA expressing constructs targeting SULF2. SULF2 expression was significantly decreased by shRNA targeting SULF2 in four separate clones as compared with an empty vector transfected clone (Fig. 3A). Because the PI3 kinase inhibitor LY294002 produced the most profound apoptotic effect on HCC cells, we evaluated the effects of knockdown of SULF2 on apoptosis induced by LY294002. Apoptosis was evaluated by DAPI staining and fluorescence microscopy. At 48 h after treatment with 20 μM of LY294002, we found that Huh7 clones with knockdown of SULF2 showed increased sensitivity to LY294002-induced apoptosis as compared with the empty vector transfected clone of Huh7 cells (P < 0.05) (Fig. 3B & C). Similar effects of knockdown of SULF2 on the sensitivity of SNU182 cells to Ly294002-induced apoptosis and of both SNU182 and Huh7 cells to apicidin-induced apoptosis were also observed (data not shown). We have previously shown that knockdown of SULF2 in Huh7 cells decreased phosphorylated GSK3β (19). The enhancement of Ly294002-induced apoptosis of Huh7 cells by SULF2 knockdown may be mediated at least partly by the decrease in phosphorylated GSK3β.
Fig. 3.
Knockdown of sulfatase 2 (SULF2) enhances Ly294002-induced apoptosis. (A) Plasmid constructs expressing an shRNA targeting the SULF2 mRNA or the empty vector plasmid pSS-H1pwere stably transfected into Huh7 cells, which express high levels of SULF2 mRNA. SULF2 levels were quantitated by real-time RT-PCR in Huh7 vector and SULF2shRNA clones, designated Huh7 SULF2 shRNA– 1 to –4. SULF2 mRNA levels were profoundly suppressed by the shRNA plasmid. (B) Cells of Huh7 vector, Huh7 SULF2 shRNA-3 and Huh7 SULF2 shRNA-4were plated into six well plates at 2 × 105 cells per well and treated with 0 (DMSO control) or 20 μM Ly294002 for 48 h. Cell morphology was randomly imaged after DAPI staining. (C) Ly 294002-induced apoptosis was quantitated and showed a significantly increased rate of apoptosis in Huh7 SULF2 shRNA-3 and Huh7 SULF2 shRNA-4 cells when compared with Huh7 vector cells (P < 0.05 for both clones).
Knockdown of SULF2 decreases p-Akt, cyclin D1 and anti-apoptotic Bcl-2 and increases pro-apoptotic BAD protein expression
To investigate the effects of knockdown of SULF2 on activation of the Akt survival pathway and expression of the anti-apoptotic Bcl-2 family members Bcl-2 and Bcl-XL and the pro-apoptotic family member BAD, we evaluated Akt phosphorylation by immunocytochemistry and the levels of p-Akt, total Akt, cyclin D1, Bcl-2, Bcl-XL and BAD by Western immunoblotting. We found strong nuclear and cytoplasmic expression of phospho-Akt in Huh7 cells that endogenously express high levels of SULF2. In contrast, knockdown of SULF2 with SULF2 targeting shRNA decreased both nuclear and cytoplasmic phospho-Akt (Fig. 4A). Western immunoblot analysis confirmed the decrease in phospho-Akt after knockdown of SULF2 and also showed decreased expression of cyclin D1 and bcl-2 but not of Bcl-XL (Fig. 4B). However, knockdown of SULF2 in Huh7 cells increased the level of expression of the pro-apoptotic bcl-2 family member BAD (Fig. 4C).
Fig. 4.
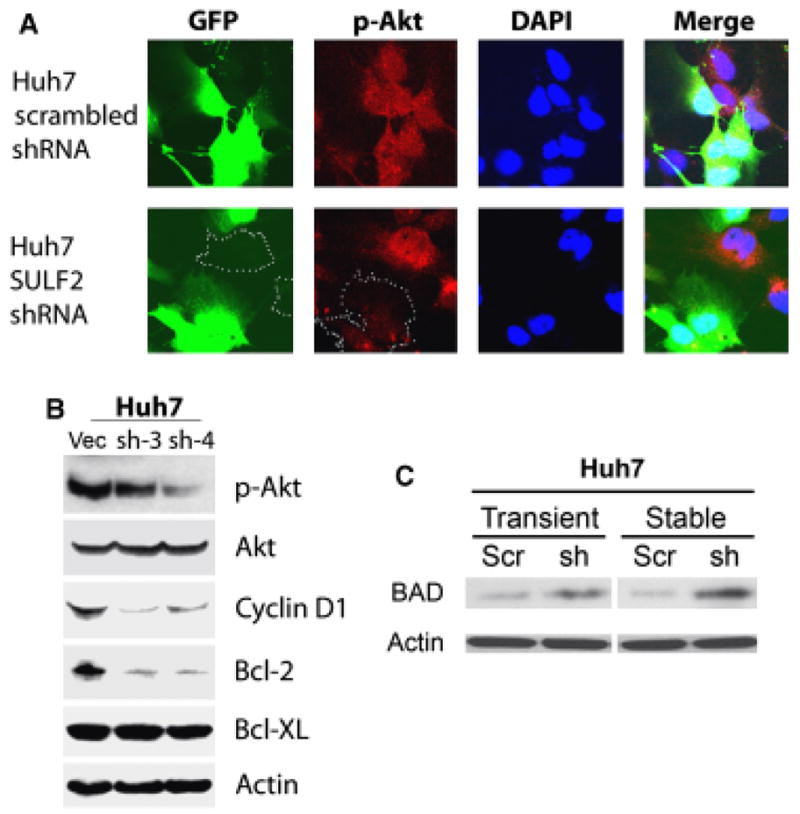
Knockdown of SULF2 downregulates p-Akt, cyclin D1 and Bcl-2 and upregulates BAD in Huh7 cells. (A) Huh7 cells were plated onto cover slips in six-well plates and transiently transfected with either GFP-expressing plasmids expressing a scrambled shRNA sequence or plasmids expressing an shRNA targeting SULF2 mRNA. After 24 h, cells were immunostained for phospho-Akt ser473 and examined by confocal microscopy. Nuclei were counterstained with DAPI. (B) Whole cell lysates of Huh7 vector, Huh7 SULF2 shRNA-3 and Huh7 SULF2 shRNA-4 cells were used for Western immunoblotting using antibody against phospho-Akt, cyclin D1, Bcl-2 and Bcl-XL. Total Akt and actin were used as loading controls. (C) Huh7 cells were either transiently or stably transfected with plasmids expressing a scrambled shRNA sequence or a sequence targeting SULF2. For the transient transfection, the transfection efficiency was monitored by transfection of a GFP plasmid and was 70–80%. Whole cell lysates were prepared and Western immunoblotting performed using antibody to BAD at 1:250 dilution; actin was used as a loading control. The BAD level was increased in Huh7 cells with both transient and stable knockdown of SULF2.
Discussion
Because we had previously found that upregulation of the SULF2 correlates with a decreased apoptosis index in human HCCs, we investigated the role of SULF2 in modulating sensitivity of HCC cells to apoptosis and the potential mechanisms underlying this regulation. The principal findings of this study are: (i) SULF2 protects HCC cells against drug-induced apoptosis through activation of multiple pro-survival pathways, including the PI3K-Akt pathway; (ii) knockdown of SULF2 using shRNA targeting SULF2 decreases phosphorylation of Akt and sensitizes HCC cells to apoptosis induced by the PI3 kinase inhibitor LY294002 and (iii) the effects of modulation of SULF2 on HCC cell apoptosis are associated with changes in the levels of the Bcl-2 family proteins Bcl-2 and BAD, while there is no significant change in Bcl-XL levels.
SULF1 downregulates FGF signalling through desulphation of HSGAGs and subsequent inactivation of the PI3K and MAPK growth signalling pathways. In contrast to the tumour suppressor function of SULF1, we have shown that SULF2 activates the PI3K and MAPK pathways and promotes cell proliferation in vitro and tumour growth in vivo. SULF1 and SULF2 therefore appear to have opposing actions in HCC cells, however, the role of SULF2 in breast cancer remains controversial (12, 29). It was recently suggested that SULF2 may be a transcriptional target of the tumour suppressor p53, but the mechanistic role of SULF2 in modulation of apoptosis has not been reported (22). Inhibitors of prosurvival pathways including the ERK, JNK and Akt pathways and HDAC inhibitors induce tumour cell apoptosis and are currently in clinical trials for cancer treatment (24–26).
In the present study, we first confirmed the expression of SULF2 in HCC tumour samples by immunohistochemistry and Western immunoblotting. HCCs are highly heterogeneous tumours and the HCCs we have tested have shown both low and high levels of SULF2 expression, with approximately 60% of HCCs showing upregulation of SULF2 compared to adjacent benign liver tissue. Consistent with our previous real-time PCR results, SULF2 protein was upregulated in a proportion of HCC tumour tissues when compared to surrounding benign liver tissue (13).
To further characterize the role of SULF2 in HCC tumourigenesis, we now show here for the first time that SULF2 increases the resistance of HCC cells to apoptosis induced by inhibition of multiple cell growth and survival pathways. We found that inhibitors of ERK kinase, PI3 kinase, JNK and HDAC all decreased HCC cell viability and induced HCC cell apoptosis, as measured by caspase 3/7 activation. The effects of the inhibitors were significantly abrogated by SULF2 expression. The PI3 kinase inhibitor LY294002 had the greatest effect on caspase 3/7 activation. We therefore evaluated the effects of SULF2 on Akt phosphorylation and found that SULF2 increased Akt phosphorylation and this effect was inhibited by LY294002. We have previously shown that forced expression of SULF2 in SULF2-negative Hep3B cells increased phosphorylation of the Akt substrate GSK3β (19). Thus, it is possible that forced expression of SULF2 activated ERK and Akt, increased phosphorylated GSK3β and protected Hep3B cells from drug-induced apoptosis.
Consistent with the anti-apoptotic effect of SULF2, knockdown of SULF2 in Huh7 cells reversed the SULF2-induced Akt pathway activation and decreased both nuclear and cytoplasmic phospho-Akt, as assessed by immunocytochemistry. Using Western immunoblot analysis, we showed that downregulation of SULF2 decreased the levels of phospho-Akt, the Akt downstream molecule and cell cycle regulator cyclin D1, and the anti-apoptotic molecule Bcl-2. There was no change in Bcl-XL levels. Next, we measured the expression level of the pro-apoptotic Bcl-2 family member BAD and showed that knockdown SULF2 in Huh7 cells increased the expression of BAD.
Thus, knockdown of SULF2 in Huh7 cells leads to a decrease in p-Akt and Bcl-2 and an increase in total BAD. The expression level of Bcl-xL does not change. BAD forms heterodimers with the survival proteins Bcl-2 and Bcl-xL and induces apoptosis. Thus knockout of SULF2 may increase total BAD, which binds to and sequesters the anti-apoptotic proteins Bcl-xL or Bcl-2, leading to increased apoptosis. In addition, knockdown of SULF2 decreases the level of the anti-apoptotic protein Bcl-2, which presumably enhances the sensitivity of the cells to apoptotic stimuli. The mechanism for this decrease in Bcl-2 is not clear; there may be alternate mechanisms mediated by SULF2 that regulate Bcl-2 levels, which deserve further investigation.
In summary, SULF2 decreases caspase 3/7 activation and increases HCC cell resistance to apoptosis. Conversely, knockdown of SULF2 decreases phosphorylation of the survival molecule Akt, downregulates expression of both cyclin D1 and the anti-apoptotic molecule Bcl-2, increases expression of the pro-apoptotic molecule BAD, and sensitizes HCC cells to drug induced apoptosis. Our data also demonstrated that the impact of SULF2 on HCC cells apoptosis is mediated by multiple pathways including AKT, ERK, JNK and HDAC, consequently, targeting SULF2 may be a rational therapeutic strategy for human HCCs.
Acknowledgments
Supported by NIH grants CA100882 and CA128633 (to L. R. R.), and P30DK084567 (Mayo Clinic Center for Cell Signaling in Gastroenterology). The authors thank Dr Shin-Ichiro Kojima for provision of the pG-SUPER vector, Dr Daniel D. Billadeau for provision of the pSSH1p plasmid vector, Patrick L. Splinter for technical assistance with shRNA design and Vicki Campion for secretarial assistance.
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole dihydrochloride
- ERK
extracellular signal-regulated kinases
- GFP
green fluorescent protein
- GPC 3
glypican 3
- HCC
hepatocellular carcinoma
- HRP
horseradish peroxidase
- HSGAG
heparan sulfate glycosaminoglycan
- HSPG
heparan sulphate proteoglycan
- MAPK/MAP kinase
mitogen activated protein kinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NP-40
nonidet P-40
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Sandhu DS, Tharayil VS, Lai JP, Roberts LR. Treatment options for hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2008;2:81–92. doi: 10.1586/17474124.2.1.81. [DOI] [PubMed] [Google Scholar]
- 3.Roberts LR, Gores GJ. Emerging drugs for hepatocellular carcinoma. Expert Opin Emerg Drugs. 2006;11:469–87. doi: 10.1517/14728214.11.3.469. [DOI] [PubMed] [Google Scholar]
- 4.Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002;277:49175–85. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai J, Chien J, Staub J, et al. Loss of HSulf-1 up-regulates heparin-binding growth factor signaling in cancer. J Biol Chem. 2003;278:23107–17. doi: 10.1074/jbc.M302203200. [DOI] [PubMed] [Google Scholar]
- 6.Lai JP, Chien J, Strome SE, et al. HSulf-1 modulates HGF-mediated tumor cell invasion and signaling in head and neck squamous carcinoma. Oncogene. 2004;23:1439–47. doi: 10.1038/sj.onc.1207258. [DOI] [PubMed] [Google Scholar]
- 7.Lai JP, Chien JR, Moser DR, et al. hSulf1 Sulfatase promotes apoptosis of hepatocellular cancer cells by decreasing heparin-binding growth factor signaling. Gastroenterology. 2004;126:231–48. doi: 10.1053/j.gastro.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Lai JP, Yu C, Moser CD, et al. SULF1 inhibits tumor growth and potentiates the effects of histone deacetylase inhibitors in hepatocellular carcinoma. Gastroenterology. 2006;130:2130–44. doi: 10.1053/j.gastro.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Kleeff J, Abiatari I, et al. Enhanced levels of Hsulf-1 interfere with heparin-binding growth factor signaling in pancreatic cancer. Mol Cancer. 2005;4:14. doi: 10.1186/1476-4598-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abiatari I, Kleeff J, Li J, et al. Hsulf-1 regulates growth and invasion of pancreatic cancer cells. J Clin Pathol. 2006;59:1052–8. doi: 10.1136/jcp.2005.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai Y, Yang Y, Macleod V, et al. HSulf-1 and HSulf-2 are potent inhibitors of myeloma tumor growth in vivo. J Biol Chem. 2005;280:40066–73. doi: 10.1074/jbc.M508136200. [DOI] [PubMed] [Google Scholar]
- 12.Morimoto-Tomita M, Uchimura K, Bistrup A, et al. Sulf-2, a proangiogenic heparan sulfate endosulfatase, is upregulated in breast cancer. Neoplasia. 2005;7:1001–10. doi: 10.1593/neo.05496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai JP, Sandhu DS, Yu C, et al. Sulfatase 2 up-regulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma. Hepatology. 2008;47:1211–22. doi: 10.1002/hep.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nawroth R, Van Zante A, Cervantes S, et al. Extracellular sulfatases, elements of the Wnt signaling pathway, positively regulate growth and tumorigenicity of human pancreatic cancer cells. PLoS One. 2007;2:e392. doi: 10.1371/journal.pone.0000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemjabbar-Alaoui H, Van Zante A, Singer MS, et al. Sulf-2, a heparan sulfate endosulfatase, promotes human lung carcinogenesis. Oncogene. 2010;29:635–46. doi: 10.1038/onc.2009.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moussay E, Palissot V, Vallar L, et al. Determination of genes and microRNAs involved in the resistance to fludarabine in vivo in chronic lymphocytic leukemia. Mol Cancer. 9:115. doi: 10.1186/1476-4598-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JS, Chu IS, Heo J, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–76. doi: 10.1002/hep.20375. [DOI] [PubMed] [Google Scholar]
- 18.Lai JP, Thompson JR, Sandhu DS, Roberts LR. Heparin-degrading sulfatases in hepatocellular carcinoma: roles in pathogenesis and therapy targets. Future Oncol. 2008;4:803–14. doi: 10.2217/14796694.4.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai J, Oseini A, Moser C, et al. The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent activation of the Wnt/β-catenin pathway. Hepatology. 2010 doi: 10.1002/hep.23848. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchimura K, Morimoto-Tomita M, Bistrup A, et al. HSulf-2, an extracellular endoglucosamine-6-sulfatase, selectively mobilizes heparin-bound growth factors and chemokines: effects on VEGF, FGF-1, and SDF-1. BMC Biochem. 2006;7:2. doi: 10.1186/1471-2091-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ai X, Kitazawa T, Do AT, et al. SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation. Development. 2007;134:3327–38. doi: 10.1242/dev.007674. [DOI] [PubMed] [Google Scholar]
- 22.Adamsen BL, Kravik KL, Clausen OP, De Angelis PM. Apoptosis, cell cycle progression and gene expression in TP53-depleted HCT116 colon cancer cells in response to short-term 5-fluorouracil treatment. Int J Oncol. 2007;31:1491–500. [PubMed] [Google Scholar]
- 23.Chau BN, Diaz RL, Saunders MA, et al. Identification of SULF2 as a novel transcriptional target of p53 by use of integrated genomic analyses. Cancer Res. 2009;69:1368–74. doi: 10.1158/0008-5472.CAN-08-2742. [DOI] [PubMed] [Google Scholar]
- 24.Granville CA, Memmott RM, Gills JJ, Dennis PA. Handicapping the race to develop inhibitors of the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2006;12(Part 1):679–89. doi: 10.1158/1078-0432.CCR-05-1654. [DOI] [PubMed] [Google Scholar]
- 25.Siu LL, Pili R, Duran I, et al. Phase I study of MGCD0103 given as a three-times-per-week oral dose in patients with advanced solid tumors. J Clin Oncol. 2008;26:1940–7. doi: 10.1200/JCO.2007.14.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly WK, Marks PA. Drug insight: histone deacetylase inhibitors–development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol. 2005;2:150–7. doi: 10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]
- 27.Lai J-P, Sandhu DS, Moser C, et al. Additive effect of apicidin and doxorubicin in sulfatase 1 (SULF1)-expressing hepatocellular carcinoma in vitro and in vivo. J Hepatol. 2009;50:1112–21. doi: 10.1016/j.jhep.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen KF, Yeh PY, Yeh KH, et al. Down-regulation of phospho-Akt is a major molecular determinant of bortezomib-induced apoptosis in hepatocellular carcinoma cells. Cancer Res. 2008;68:6698–707. doi: 10.1158/0008-5472.CAN-08-0257. [DOI] [PubMed] [Google Scholar]
- 29.Hampton OA, Den Hollander P, Miller CA, et al. A sequence-level map of chromosomal breakpoints in the MCF-7 breast cancer cell line yields insights into the evolution of a cancer genome. Genome Res. 2009;19:167–77. doi: 10.1101/gr.080259.108. [DOI] [PMC free article] [PubMed] [Google Scholar]