Abstract
Mammals possess multiple insulin-like growth factor (IGF) binding proteins (IGFBPs), and related proteins, that modulate the activity of insulin/IGF signalling (IIS), a conserved neuroendocrine signalling pathway that affects animal lifespan. Here, we examine if increased levels of an IGFBP-like protein can extend lifespan, using Drosophila as the model organism. We demonstrate that Imaginal morphogenesis protein-Late 2 (IMP-L2), a secreted protein and the fly homologue of the human IGFBP7 tumour suppressor, is capable of binding at least two of the seven Drosophila insulin-like peptides (DILPs), namely native DILP2 and DILP5 as present in the adult fly. Increased expression of Imp-L2 results in phenotypic changes in the adult consistent with down-regulation of IIS, including accumulation of eIF-4E binding protein mRNA, increase in storage lipids, reduced fecundity and enhanced oxidative stress resistance. Increased Imp-L2 results in up-regulation of dilp2, dilp3 and dilp5 mRNA, revealing a feedback circuit that is mediated via the fly gut and/or fat body. Importantly, over-expression of Imp-L2, ubiquitous or restricted to DILP-producing cells or gut and fat body, extends lifespan. This enhanced longevity can also be observed upon adult-onset induction of Imp-L2, indicating it is not attributable to developmental changes. Our findings point to the possibility that an IGFBP or a related protein, such as IGFBP7, plays a role in mammalian aging.
Keywords: aging, Drosophila, IMP-L2, insulin/insulin-like growth factor signalling, insulin-like growth factor-binding protein
Introduction
The insulin/insulin-like growth factor (IGF) signalling (IIS) pathway is an evolutionarily conserved neuroendocrine signalling pathway that controls a variety of processes and traits in animals, including growth and development, energy metabolism, reproduction and stress resistance. Genetic manipulations of pathway components that result in dampened IIS extend lifespan in worms, flies and mice, and ameliorate age-dependent functional decline (Tatar et al., 2003; Piper et al., 2008). Genetic variation in several components of this pathway is strongly associated with human longevity (Kuningas et al., 2007; Willcox et al., 2008; Flachsbart et al., 2009; Pawlikowska et al., 2009), confirming the relevance of IIS to human aging.
Central to the pathway are insulin-like ligands, which include insulin, IGF-I and IGF-II in mammals (White, 2006); 38 insulin-like peptides in worms (Pierce et al., 2001) and the seven Drosophila insulin-like peptide (DILPs) in flies (Brogiolo et al., 2001). The ligands mediate cell-to-cell signalling by activating an insulin receptor-like receptor, leading to the activation of PI3-kinase – Akt, TOR and ERK intracellular signalling pathways (Tatar et al., 2003; White, 2006; Piper et al., 2008). Importantly, not all manipulations of the pathway result in lifespan extension (Clancy et al., 2001; Tatar et al., 2001; Hwangbo et al., 2004; Selman et al., 2008; Ikeya et al., 2009), suggesting that the pathway needs to be manipulated to a specific level of signal reduction and in specific tissues to achieve enhanced longevity.
In mammals, a layer of complexity is added to IIS by the presence of IGF binding proteins (IGFBPs). Six classic IGFBPs bind IGF-I and IGF-II with high affinity and act as modulators of IGF activity. They can both enhance and dampen IIS by prolonging the half-life of IGFs, altering their local and systemic availability and preventing them from binding to the receptor (Hwa et al., 1999). Furthermore, mammals possess IGFBP-related proteins, such as IGFBP7, that bind IGFs with lower affinity (Hwa et al., 1999). Notably, IGFBP7 has received attention as a potent secreted tumour suppressor acting in an autocrine/paracrine manner to block melanoma genesis (Wajapeyee et al., 2008).
Insects also possess IGFBP-like proteins, the first of which was discovered serendipitously, allowing the subsequent identification of the Drosophila Imaginal morphogenesis protein-Late 2 (Imp-L2) (Sloth Andersen et al., 2000; Alic & Partridge, 2008). IMP-L2 resembles IGFBP7 in sequence and it appears to be equivalent to an IGFBP in function, acting as a negative regulator of IIS during development, regulating growth cell-non-autonomously and antagonising dilp2 in genetic assays (Honegger et al., 2008). Interestingly, when the germline is ablated late in fly development, lifespan is increased with a concomitant increase in the levels of Imp-L2 mRNA (Flatt et al., 2008), indicating that one of the roles of Imp-L2 may be to mediate a lifespan-extending signal emanating from the gonad to IIS in the soma. However, the role of this gene in IIS in the adult fly, including its capacity to enhance longevity, has not been examined.
While the complex and important effects of IGFBPs on IIS in mammals and, in turn, the role of IIS in animal lifespan are both well established, no study has examined if IGFBPs or related proteins can alter animal physiology in such a way as to enhance longevity. To determine if increased function of an IGFBP can extend lifespan, we used Drosophila as a model; such an approach has been fruitful in the past, when the finding that a mutation in an insulin-receptor substrate extends lifespan in the fly (Clancy et al., 2001) was subsequently confirmed in mammals (Taguchi et al., 2007; Selman et al., 2008). Here, we show that the fly IGFBP homologue, IMP-L2, binds native DILP2 and DILP5, is involved in a feedback circuit with dilp2, dilp3 and dilp5, affects IIS-regulated traits in the adult and extends lifespan, pointing to the possibility that an IGFBP or a related protein could modulate mammalian aging.
Results
Eighty percent increase in Imp-L2 activates transcription of 4E-BP
We first determined if increasing the amount of Imp-L2 can modulate IIS in adult flies at a molecular level. Imp-L2 is expressed in multiple tissues during development and in the adult (Osterbur et al., 1988; Garbe et al., 1993; Honegger et al., 2008), and so we chose to initially over-express it ubiquitously. Since a strong ubiquitous over-expression of Imp-L2 is lethal (Honegger et al., 2008), we used the genetically weaker UAS-Imp-L2 transgene created by Honegger et al. (2008) and drove its expression with a heatshockGAL4 (hsGAL4) driver at 25°C. This manipulation resulted in viable flies and an 80% increase in the levels of Imp-L2 mRNA in the adult female (Fig. 1A), as detected by qPCR. To monitor protein levels, we raised an antibody against recombinant IMP-L2 protein and affinity purified the serum. On western blots, we confirmed the previously observed apparent molecular weight of IMP-L2 as ∼30 kDa (Garbe et al., 1993), and detected ∼80% increase in IMP-L2 protein in hsGAL4 > UAS-Imp-L2 flies compared to the pooled average of the two controls (Fig. 1B), an increase equivalent to the mRNA increase. The details of mRNA and protein quantification methods are given in Experimental procedures.
Fig. 1.
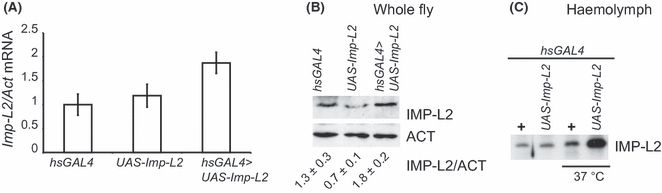
Over-expression of Imp-L2 with heatshockGAL4 driver. The flies were reared at 25°C and harvested on day 7. (A) The transcript levels of Imp-L2 were determined by qPCR, normalised to Act mRNA and the ratio in hsGAL4 set to one. Means and standard errors are shown with n = 6 for hsGAL4 > UAS-Imp-L2 and n = 7 for the two controls. The measurements for hsGAL4 > UAS-Imp-L2 were compared to those for hsGAL4 by t-test: P = 0.02, to UAS-Imp-L2: P = 0.05. (B) The levels of IMP-L2 protein were determined in whole flies by western blot, with actin as the loading control. The averages and standard errors of three independent measurements of IMP-L2 protein normalised to actin are given below the images, with the average of pooled controls set to one. The levels in hsGAL4 > UAS-Imp-L2 were significantly different from the pooled controls (P = 0.04) or UAS-Imp-L2 (P = 0.01) but not from hsGAL4, by t-test. (C) Levels of IMP-L2 protein were determined in haemolymph by western blot. All flies carried the hsGAL4 driver with or without UAS-Imp-L2 and were either kept at 25°C or placed at 37°C for 2 h prior to collection of haemolymph. Full genotypes of the flies tested were: w−/w−; hsGAL4/+; +/+, w−/w−; +/+; UAS-Imp-L2/+, w−/w−; hsGAL4/+; UAS-Imp-L2/+. Note that in A and B, the differences between the two controls were not statistically significant.
IMP-L2 is predicted to be a secreted protein (Honegger et al., 2008). Indeed, tagged IMP-L2 was efficiently secreted from S2 cells (data not shown). To confirm this in vivo, we looked for the native IMP-L2 in circulation. We could detect the protein in the adult haemolymph, both using antibodies previously described (data not shown) (Garbe et al., 1993) and the antibody we generated (Fig. 1C). hsGAL4 driven over-expression did not result in substantial increases in circulating IMP-L2 (Fig. 1C), probably because of low levels of induction at 25°C. To check that IMP-L2 protein could, in principle, be secreted from the transgene that we used, we increased the level of induction of hsGAL4 by incubating the flies at 37°C for 2 h. This process resulted in a ∼8-fold increase in the mRNA over the hsGAL4 control (data not shown) and a marked increase in the haemolymph IMP-L2 (Fig. 1C), indicating that IMP-L2 protein from the transgene could be correctly processed in our flies.
To determine if this weak, ubiquitous over-expression of Imp-L2 had an effect on molecular readouts of IIS status in the adult, we looked at the activation of the transcription factor thought to mediate the effects of IIS – dFOXO (Partridge & Bruning, 2008). The overall phosphorylation status of dFOXO, observed as slower migration on SDS-PAGE, reflects the activity of IIS in cell culture (Puig et al., 2003). In vivo in the adult female, strong down-regulation of IIS also reduces levels of phosphorylated dFOXO in whole-fly extracts (Ikeya et al., 2009). Over-expression of Imp-L2 did not result in an observable difference in dFOXO phosphorylation (Fig. 2A), indicating that a strong down-regulation of IIS did not occur in our flies. Indeed, ablation of the median neurosecretory cells (mNSC) that produce DILP2, DILP3 and DILP5, a model of IIS reduction that increases adult lifespan (Broughton et al., 2005), is also not enough to detectably alter whole-fly dFOXO phosphorylation status (data not shown). To detect more subtle changes in IIS, we examined the mRNA levels of 4E-BP, a target of dFOXO (Junger et al., 2003), which appears to mediate lifespan response to dietary restriction (Zid et al., 2009), since low but prolonged activation of the transcription factor could result in an observable accumulation of the target message. Over-expression of Imp-L2 led to a significant increase (∼80%) in 4E-BP mRNA (Fig. 2B), consistent with activation of dFOXO. The data indicated that a subtle down-regulation of IIS occurs upon weak over-expression of Imp-L2 in the adult.
Fig. 2.
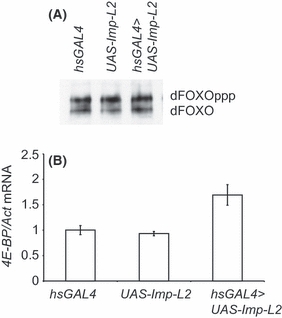
Increase in Imp-L2 induces 4E-BP transcript. (A) The phosphorylation of dFOXO in flies over-expressing Imp-L2. The phosphorylation was monitored as retardation on SDS-PAGE and dFOXO bands revealed by western blotting with an anti-dFOXO antibody. In separate wild-type extracts, the slower migrating band (dFOXOppp) was shown to disappear on treatment with calf intestinal phosphatase (data not shown). (B) The transcript levels of 4E-BP were determined by qPCR, normalised to Act mRNA and the ratio in hsGAL4 set to one. Means and standard errors are shown with n = 6 for hsGAL4 > UAS-Imp-L2 and n = 7 for the two controls, P < 10−3 to either control by t-test, while the differences between the two controls were not significant. Full genotypes of the flies tested were: w−/w−; hsGAL4/+; +/+, w−/w−; +/+; UAS-Imp-L2/+, w−/w−; hsGAL4/+; UAS-Imp-L2/+.
Secreted IMP-L2 binds native DILP2 and DILP5
IMP-L2 has been shown to interact with Flag-tagged DILP2 in insect cells (Arquier et al., 2008; Honegger et al., 2008). However, the increase in 4E-BP mRNA observed on over-expression of Imp-L2 is not observed upon deletion of only dilp2, but requires simultaneous deletion of dilp2, dilp3 and dilp5 (Gronke et al., 2010). Therefore, it is likely that IMP-L2 can also bind DILPs other than DILP2, prompting us to seek evidence for a physical interaction. It was also important to establish whether IMP-L2 interacts with the native version of DILP2, because modified (Flag-tagged) DILP2 was used previously (Arquier et al., 2008; Honegger et al., 2008).
We wanted to examine the ability of IMP-L2 to bind native i.e. non-tagged and non-recombinant DILPs for two main reasons: the physical structure, including C-chain excision, of DILPs has not been characterised, precluding confirmation of the correct structure for a synthetic or recombinant DILP; and the presence of a tag may significantly alter the physical behaviour of DILPs because of their small size. To achieve this, we used a far-western blotting procedure, not requiring tagging or production of synthetic or recombinant proteins. To focus on DILP2, DILP3 and DILP5, we used proteins extracted from fly heads, where these DILPs are produced (Broughton et al., 2005). We expressed myc epitope-tagged IMP-L2 in S2 cells and, to insure correct folding of the protein, we only used the secreted IMP-L2-myc6, in the form of conditioned medium, to probe female head proteins separated by non-reducing Tris–Tricine PAGE and transferred to a nitrocellulose membrane. IMP-L2-myc6 binding was then localised with an anti-myc antibody.
The far-western blotting revealed IMP-L2 binding to two proteins of ∼8 and ∼12 kDa, and this specific binding was not observed in the mock control using conditioned medium from cells not expressing IMP-L2-myc6 (Fig. 3A,B). To identify the proteins bound by IMP-L2-myc6 we used head protein extracts from mutants deleted for each one of the three dilps produced in the mNSC (Fig. 3A,B), and also probed the blots for DILP2 (not shown), and for tubulin as a loading control (Fig. 3C). The 12 kDa band co-migrated with the band recognised by the anti-DILP2 antibody (data not shown) and was absent in dilp2Δ/dilp2Δ flies (Fig. 3A,B), confirming this is indeed DILP2. Hence, secreted IMP-L2 can bind native DILP2. Note that a faint non-specific band, migrating close to DILP2 and present in dilp2Δ/dilp2Δ extracts, appears on the mock far-western blot (Fig. 3A) and is probably attributable to non-specific binding of the anti-myc antibody. The other specific band was absent in dilp5Δ/dilp5Δ (Fig. 3A,B), indicating that IMP-L2 can also interact with DILP5. The binding to DILP5 was weaker, and could be more easily observed on a higher exposure of the far-western blot (Fig. 3B), possibly because of lower levels of expression of dilp5 compared to dilp2 (Broughton et al., 2005). Furthermore, there appeared to be more DILP5 in dilp2Δ/dilp2Δ fly heads (Fig. 3A,B), and this finding is consistent with the compensatory increase observed at the level of mRNA (Broughton et al., 2008; Gronke et al., 2010).
Fig. 3.
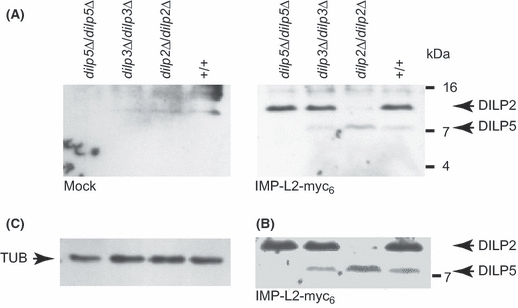
IMP-L2 binds DILP2 and DILP5. (A) Far-western blot was performed on head proteins from flies of the indicated genotypes using S2-cell conditioned media containing either IMP-L2-myc6 (right-hand panel) or not (mock; left-hand panel) and the binding of IMP-L2-myc6 visualised by an anti-myc antibody. Bands corresponding to DILP2 and DILP5 are indicated. (B) Higher exposure of the far-western shown above in A. (C) Tubulin used as loading control. Note that in all the panels the lanes shown align with the genotypes indicated in A. Full genotypes of the flies tested were: w−/w−; +/+; +/+, w−/w−; +/+; dilp2Δ/dilp2Δ, w−/w−; +/+; dilp3Δ/dilp3Δ, w−/w−; +/+; dilp5Δ/dilp5Δ.
Increased Imp-L2 feeds back onto dilp expression
As mentioned above, down-regulation or deletion of dilp2 results in up-regulation of dilp3 and dilp5 transcription (Broughton et al., 2008; Gronke et al., 2010). Such feedback regulates in part the transcription of dilp2, dilp3 and dilp5, and also encompasses Imp-L2 (Broughton et al., 2008; Gronke et al., 2010): simultaneous deletion of dilp2, dilp3 and dilp5 results in a drop in Imp-L2 transcript levels (Gronke et al., 2010), suggesting a compensatory down-regulation of Imp-L2 in response to IIS reduction. We wanted to examine if the inverse can also occur i.e. if increased levels of Imp-L2 led to an elevation of dilp expression levels. Indeed, driving Imp-L2 over-expression with the hsGAL4 resulted in significant increases (∼2-fold) in the mRNA for dilp2, dilp3 and dilp5 (Fig. 4). Hence, dilp2, dilp3 and dilp5, on one hand, and Imp-L2 on the other, reciprocally regulate each other’s expression, forming a circuit in which changes in one are compensated by changes in the other.
Fig. 4.
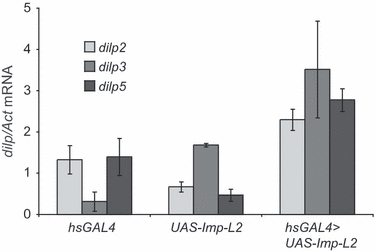
Increase in Imp-L2 results in increased dilp2, dilp3 and dilp5 transcription. The levels of dilp2, dilp3 and dilp5 transcripts were determined by qPCR in whole fly RNA, normalised to Act mRNA and the average ratio of the two control genotypes set to one for each transcript. Means and standard errors are shown with n = 4 for all measurements except for dilp5 in UAS-Imp-L2 where n = 3. Two-way anova showed that the effect of genotype was significant: P < 10−4, where hsGAL4 > UAS-Imp-L2 was different to both controls by t-test while the differences between the two controls were not significant, and there was no significant interaction between genotype and transcript. Full genotypes of the flies tested were: w−/w−; hsGAL4/+; +/+, w−/w−; +/+; UAS-Imp-L2/+, w−/w−; hsGAL4/+; UAS-Imp-L2/+.
Up-regulation of Imp-L2 increases lifespan
We next determined if weak, ubiquitous over-expression of Imp-L2 was sufficient to cause an observable phenotype. IIS phenotypes tend to be more pronounced in female flies (Clancy et al., 2001; Giannakou et al., 2004; Hwangbo et al., 2004; Broughton et al., 2005), so we used female flies in our tests. IIS regulates metabolism in the adult, and down-regulation of IIS increases levels of stored lipids, whole body trehalose content and circulating sugars (Broughton et al., 2005, 2008). Over-expression of Imp-L2 caused a significant increase (21%) in the levels of stored lipids, as measured by determining whole-fly triacylglycerol content (Fig. 5A). On the other hand, while there was a trend towards an increase in the whole-fly trehalose content and the levels of circulating trehalose, glucose or the combined sugars, these were not significantly altered (Fig. S1A,B). Furthermore, an increase in starvation resistance was observed in only one of two trials performed (Fig. S1C). Therefore, apart from an increase in stored lipids, the effect of increasing Imp-L2 on metabolic traits was not robust.
Fig. 5.
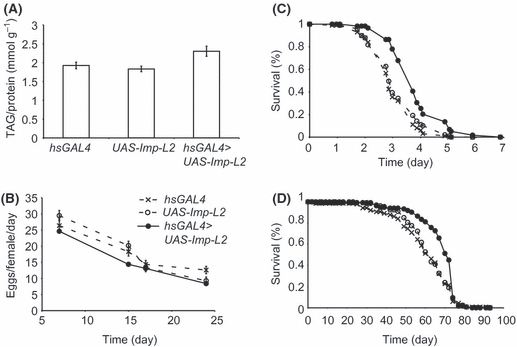
Over-expression of Imp-L2 increases lifespan, oxidative stress resistance and whole-fly lipid content, while reducing fecundity. (A) Means and standard errors of the measurements of whole-fly triacylglycerol (TAG) per protein are shown, with n = 8 for each genotype, where hsGAL4 > UAS-Imp-L2 is different to both controls by t-test (P = 0.01 to hsGAL4, P = 0.003 to UAS-Imp-L2). (B) The average number of eggs laid per female over 24 h was measured in ten separate vials per genotype at the times indicated. Means and standard errors are shown. Two Way anova showed that the effect of genotype was significant: P = 0.008, where hsGAL4 > UAS-Imp-L2 is different to both controls by t-test; effect of time: P < 10−4; no interaction of the two main effects. Estimate of the cumulative eggs laid per female: 72 for hsGAL4, 72 for UAS-Imp-L2 and 60 for hsGAL4 > UAS-Imp-L2. (C) Five-day old female flies (hsGAL4 > UAS-Imp-L2 n = 59, hsGAL4 n = 99, UAS-Imp-L2 n = 99) were placed on food containing 5% H2O2/suchrose and their survival determined over time. The survival of hsGAL4 > UAS-Imp-L2 was significantly different from either control by Log-rank test (P < 10−4). (D) Lifespans of hsGAL4 > UAS-Imp-L2 (n = 137, med = 71 days, max = 74 days) and the two genetic controls hsGAL4 and UAS-Imp-L2 (n = 142, med = 61 days, max = 74 days and n = 139, med = 61 days, max = 74 days). The survival of hsGAL4 > UAS-Imp-L2 was significantly different from either control by Log-rank test, P < 10−4. Note in B, C and D the same symbols are used for the genotypes and are indicated in B. Full genotypes of the flies tested were: w−/w−; hsGAL4/+; +/+, w−/w−; +/+; UAS-Imp-L2/+, w−/w−; hsGAL4/+; UAS-Imp-L2/+. Note that in all panels the differences between the two controls were not statistically significant.
On the other hand, increasing Imp-L2 did clearly affects other IIS-regulated traits. Over-expression of Imp-L2 resulted in slight, but significant, reduction (17%) in cumulative eggs laid by an average female fly per day over the first 25 days of adult life (Fig. 5B), showing that Imp-L2 could reduce fecundity. To examine their resistance to oxidative stress, we fed the flies 5% H2O2/sucrose food. The flies over-expressing Imp-L2 survived for significantly longer (Fig. 5C), with a 23% increase in median survival time, indicating that increasing Imp-L2 increases oxidative stress resistance. Most importantly, over-expression of Imp-L2 using the hsGAL4 driver significantly extended the lifespan of female flies at 25°C (Fig. 5D), with median lifespan extended by 15%, while the maximum lifespan remained unchanged with this driver. Note that, similar to over-expression of dfoxo (Giannakou et al., 2004), hsGAL4 > UAS-Imp-L2 had no effect on male lifespan (Fig. S2).
Adult-onset over-expression of Imp-L2 can extend lifespan
The hsGAL4 driver is expressed in both the pre-adult and adult periods, and the lifespan-extension upon over-expression of Imp-L2 achieved with this driver could thus be attributed to a developmental effect. To examine adult-onset over-expression, we used the inducible Actin GeneSwitch (ActGS) driver. ActGS drives ubiquitous transgene expression but only in the presence of the RU486 steroid drug (Ford et al., 2007). Addition of RU486 to food had no effect on lifespan of ActGS or UAS-Imp-L2 controls (Fig. S3), while in ActGS > UAS-Imp-L2 adult female flies it almost doubled the period where no deaths were observed and resulted in a 20% increase in median lifespan, as well as a smaller increase in maximal lifespan (Fig. 6A), demonstrating that effect of Imp-L2 on lifespan can be separated from its developmental effects.
Fig. 6.
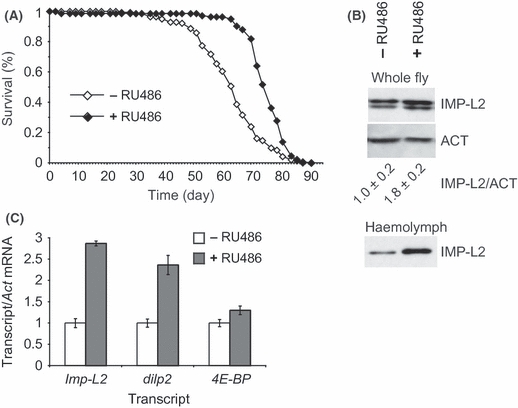
Adult-onset induction of Imp-L2 increases lifespan. (A) Lifespans of female ActGS > UAS-Imp-L2 flies induced to ubiquitously over-express Imp-L2 by feeding RU486-containing food from day 3 of adulthood (+RU486, n = 143, med = 75 days, max = 84) or the uninduced controls (−RU486, n = 143, med = 63 days, max = 79 days). The survival of ActGS > UAS-Imp-L2+ RU486 was significantly different from the −RU486 control by Log-rank test (P < 10−4). Maximal lifespan was also extended upon Imp-L2 over-expression (P < 0.05 by Log-rank test on the final 10% survivors). (B) Levels of Imp-L2, 4E-BP and dilp2 mRNA relative to Act mRNA were determined by qPCR in ActGS > UAS-Imp-L2 female flies after 4 days of induction or in the uninduced control. Means and standard errors are shown, with values for the uninduced control set to 1 and with n = 7 for all measurements except for Imp-L2 mRNA in +RU486 where n = 6. In each case −RU486 was significantly different to +RU486 by t-test (Imp-L2: P < 10−4, dilp2: P = 5 × 10−4, 4E-BP: P = 0.04). (C) The levels of IMP-L2 protein were determined in whole flies (top) or in haemolymph (bottom) by western blot. For whole-fly extracts, actin was used as the loading control, and the averages and standard errors of three independent measurements of IMP-L2 protein normalised to actin are given below the images, with the levels in the uninduced control set to one; these were different by t-test (P = 0.04). Full genotype of ActGS > UAS-Imp-L2 flies: w−/w−; ActGS/+; UAS-Imp-L2/+.
Interestingly, while ActGS > UAS-Imp-L2 female flies showed an almost 3-fold increase in Imp-L2 mRNA upon RU486 feeding (Fig. 6B), the levels of IMP-L2 protein were only 80% increased (Fig. 6C). This apparent block to translation is indicative of translational control that appears exerted on both the native Imp-L2 and the transgene we used (Honegger et al., 2008). The reason why the ActGS driver had a more substantial effect on lifespan than hsGAL4 may be because, in the case of the latter, the levels of IMP-L2 were increased in circulation (Fig. 6C). Adult-specific ubiquitous induction of Imp-L2 also resulted in significantly increased levels of dilp2 and 4E-BP mRNA (Fig. 6B), as well as a decrease in fecundity (Fig. S4A) and an increase in H2O2 resistance (Fig. S4B). However, the increase in 4E-BP observed here (∼30%, Fig. 6B) was lower than with hsGAL4 (∼80%, Fig. 2B), indicating that prolonged IIS down-regulation or down-regulation during development may be required for a pronounced effect on 4E-BP expression, and that the magnitude of lifespan extension is not proportional to the levels of 4E-BP mRNA.
Tissue-specific over-expression of Imp-L2 can extend lifespan
Since IMP-L2 is a secreted protein, we were interested in determining if tissue-restricted over-expression can extend lifespan. In both the larvae and the adult, IMP-L2 is produced in distinct cells of both brain hemispheres (Honegger et al., 2008). However, driving UAS-Imp-L2 expression with the pan-neuronal elavGAL4 driver (Luo et al., 1994) did not extend lifespan (Fig. S5). IMP-L2 is also produced in the corpora cardiaca, part of the ring gland (Honegger et al., 2008), but driving UAS-Imp-L2 with the corpora cardiaca-specific akhGAL4 driver (Kim & Rulifson, 2004) also failed to extend lifespan (Fig. S5). IMP-L2 is also expressed in the mNSCs, together with DILP2, DILP3 and DILP5 (Honegger et al., 2008). Driving UAS-Imp-L2 expression with the dilp2GAL4 driver, expressed only in the mNSCs starting from the third-instar larval stage (Broughton et al., 2005), significantly extended lifespan of female flies (Fig. 7A), prolonging the median survival time by ∼10%. The magnitude of the extension was similar to the one observed with hsGAL4, suggesting that the majority of the effect on lifespan of ubiquitous over-expression can be recapitulated by increased expression at the site of DILP2, DILP3 and DILP5 production.
Fig. 7.
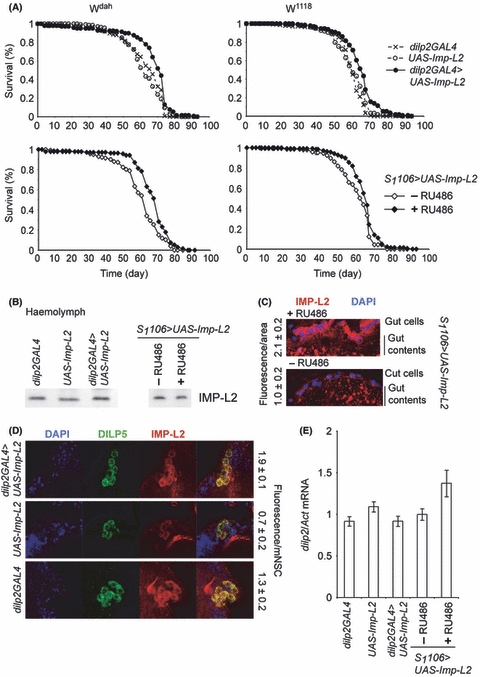
Tissue-restricted over-expression of Imp-L2 increases lifespan. (A) Lifespans of female flies over-expressing Imp-L2 in the mNSC (dilp2GAL4 > UAS-Imp-L2, Wdahn = 142, med = 71 days, max = 77 days; w1118n = 141, med = 65 days, max = 72 days) and the two genetic controls (dilp2GAL4, Wdahn = 149, med = 66 days, max = 74 days; w1118n = 140, med = 59 days, max = 67 days; UAS-Imp-L2, Wdahn = 142, med = 66 days, max = 74 days; w1118n = 142, med = 59 days, max = 67 days; top two panels) or female flies in which over-expression of Imp-L2 in the gut and fat body by the S1106 driver was induced at day 3 of adulthood by RU486 (S1106 > UAS-Imp-L2+ RU486, Wdahn = 143. med = 69 days, max = 77 days; w1118n = 144, med = 65 days, max = 74 days) or the uninduced controls (S1106 > UAS-Imp-L2−RU486, Wdahn = 143, med = 62, max = 74; w1118n = 150, med = 63 days, max = 70 days; lower two panels), in outbred Wdah (two panels on the left) and the inbred w1118 (two panels on the right). The survival of dilp2GAL4 > UAS-Imp-L2 was significantly different from the two controls in both backgrounds by Log-rank test (P < 10−4). Log-rank test also showed the survival of S1106 > UAS-Imp-L2 significantly altered by addition of RU486, with P < 10−6 for Wdah, P = 0.003 for w1118. In all cases, the maximal lifespan was extended upon Imp-L2 over-expression (P < 0.05 by Log-rank test on the final 10% survivors). (B) IMP-L2 was measured in the haemolymph of dilp2GAL4 > UAS-Imp-L2 female flies and the two genetic controls, or in S1106 > UAS-Imp-L2 female flies fed or not with RU486, by western blotting. (C) IMP-L2 was visualised with immunofluorescence in the guts of S1106 > UAS-Imp-L2 female flies fed or not with RU486. IMP-L2 is indicated in red, DAPI-stained nuclei in blue. Note the red fluorescence of the gut contents. The numbers next to the images give the mean and standard error of relative fluorescence intensity per unit area of gut as quantified, after background subtraction, from at least three animals (P = 0.01 by t-test). (D) IMP-L2 was visualised with immunofluorescence in the mNSC of wandering third-instar dilp2GAL4 > UAS-Imp-L2 larvae or the two genetic controls. mNSC were identified with an anti-DILP5 antibody (green), IMP-L2 is indicated in red, DAPI in blue. The numbers next to the images give the mean and standard error of relative fluorescence intensity per mNSC quantified, after background subtraction, and averaged over at least three cells from four animals (n = 4, t-test dilp2GAL4 > UAS-Imp-L2 to dilp2GAL4 P = 0.04, to UAS-Imp-L2 P = 0.001). (E) Levels of dilp2 mRNA relative to Act mRNA were determined in the flies of the indicated genotypes/treatments, with n = 6 and the levels set to 1 in relevant controls. The levels in S1106 > UAS-Imp-L2 were significantly altered by addition of RU486 (t-test: P = 0.05). Full genotypes of the flies used: w−/w−; +/+; dilp2GAL4/+, w−/w−; +/+; UAS-Imp-L2/+, w−/w−; +/+; dilp2GAL4/UAS-Imp-L2, w−/w−; S1106/+; UAS-Imp-L2/k. Note that in all cases the differences between dilp2GAL4 and UAS-Imp-L2 controls were not statistically significant.
IMP-L2 is also expressed in the fly gut and fat body (Honegger et al., 2008). The S1106 driver activates expression in these tissues upon addition of RU486 steroid drug to food (Poirier et al., 2008). Adult-onset induction of S1106 > UAS-Imp-L2 also significantly extended female fly lifespan (Fig. 7A), while addition of RU486 to food of S1106 or UAS-Imp-L2 controls had no effect (Fig. S3). Hence, IMP-L2 production in the gut/fat body can also contribute to longevity. With both the dilp2GAL4 and S1106 drivers, the maximum lifespan was also extended (Fig. 7A), confirming that Imp-L2 over-expression can extend maximum lifespan. In both cases, the enhanced longevity was observed in two different fly strains, the outbred Wdah and the inbred w1118 (Fig. 7A), indicating that it is robust to genetic background.
Similar to the situation in hsGAL4 > UAS-Imp-L2, the level of circulating IMP-L2 was not substantially increased in S1106 > UAS-Imp-L2 flies fed RU486-containing food, or in dilp2GAL4 > UAS-ImpL2 compared to controls (Fig. 7B). On the other hand, there were detectable increases in IMP-L2 protein in the gut of S1106 > UAS-Imp-L2 flies in presence of RU486 (Fig. 7C; no significant increase was detectable in the fat body, Fig. S6), or in the mNSC of the third-instar dilp2GAL4 > UAS-Imp-L2 larvae compared to controls (Fig. 7D), confirming the induction of the transgene. Note that certain other cells in the brain normally express much higher levels of IMP-L2 than those attained in the mNSC with dilp2GAL4 (Fig. S7), so that the contribution from mNSC to the total pool of IMP-L2, even in the dilp2GAL4 > UAS-Imp-L2 flies, is likely to be negligible, and not proportionate to its effect on lifespan. Hence, the site of expression, rather than the levels of IMP-L2, is relevant.
Tissue-specific induction of Imp-L2 appeared to selectively target lifespan and not other IIS regulated traits since, with both dilp2GAL4 and S1106 drivers, there was no effect on fecundity, stress resistance or 4E-BP expression (Fig. S8). Intriguingly, the levels of dilp2 mRNA were only increased when Imp-L2 was induced in the gut/fat body, and not when it was induced in the mNSC (Fig. 7E), indicating that the feedback to dilp expression may occur via the former tissue(s).
Discussion
In this study we determined the function of Imp-L2 in adulthood, and found that its over-expression caused phenotypic changes consistent with negative regulation of IIS. Importantly, we found that this genetic manipulation of IIS could extend lifespan, which is not the case for all IIS-targeted interventions (Clancy et al., 2001; Tatar et al., 2001; Hwangbo et al., 2004; Selman et al., 2008; Ikeya et al., 2009). It will be interesting to determine if this longevity-enhancing role of Imp-L2 can be performed by an IGFBP or an IGFBP-rP, such as IGFBP7, in mammals. IGFBP7 has been characterised as a tumour suppressor, acting as a secreted senescence/apoptosis factor (Wajapeyee et al., 2008). Establishing and examining the role of IGFBP7 in lifespan may shed light on the relationship between cellular senescence, cancer and whole organism aging in mammals, an important emerging field of study (Campisi & Yaswen, 2009).
Our data are consistent with IMP-L2 regulating IIS by sequestering the DILP ligands. In this respect, it is important that our binding assay was performed with native DILP proteins, revealing for the first time that IMP-L2 can bind native DILP5 as well as native DILP2. Interestingly, we did not observe binding to any other fly head proteins, despite the expression of DILP3 and DILP4 in the head (Ikeya et al., 2002; Broughton et al., 2005; Gronke et al., 2010), implying that IMP-L2 can discriminate amongst different DILPs. However, this lack of observable binding may have also resulted from differential levels of expression of native DILPs e.g. from the very low levels of expression of dilp3 in the mNSC (Broughton et al., 2005). IMP-L2 can probably also bind DILPs other than DILP2 and DILP5, because strong over-expression of the protein is lethal (Honegger et al., 2008), but simultaneous deletion of dilp2, dilp3 and dilp5 is not (Gronke et al., 2010). The dilp2Δ/dilp2Δ dilp3Δ/dilp3Δ dilp5Δ/dilp5Δ dilp6Δ/dilp6Δ quadruple mutant is lethal (Gronke et al., 2010), indicating that IMP-L2 may bind DILP6. Alternatively, IMP-L2 may also have DILP-independent effects, in a similar way that some IGFBPs appear to have IGF-independent functions (Mohan & Baylink, 2002).
Interestingly, sequence analysis indicates that DILPs are cleaved and processed like insulin (Gronke et al., 2010), however, no study to date has physically observed these processed forms of DILPs. While we have previously observed DILP2 on a western blot (Broughton et al., 2008), this is the first time that the native DILP5 has been observed on an SDS-PAGE. For both of these DILPs, the apparent molecular weight is too large for the processed DILPs (predicted molecular weight of DILP2 and DILP5 processed like insulin is ∼6 kDa) and is closer to the predicted molecular weight of the pro-peptide or an uncleaved IGF-like peptide (∼13 and ∼10 kDa for DILP2 and DILP5 respectively). IMP-L2′s ability to bind these uncleaved forms is consistent with its binding to human pro-insulin, IGF-I and IGF-II, as well as insulin (Sloth Andersen et al., 2000), also indicating that these uncleaved forms of DILPs may be functional in the fly.
Removal of dilp2, dilp3 and dilp5 results in down-regulation of Imp-L2 transcription (Gronke et al., 2010), and we show here that, reciprocally, up-regulation of Imp-L2 results in up-regulation of the mRNAs for all three dilps. This compensatory feedback loop is, in both cases, not sufficient to completely correct the disturbance of the fly IIS status since both genetic manipulations result in phenotypes consistent with down-regulation of IIS including lifespan extension. Interestingly we find that over-expression of Imp-L2 in the mNSC does not result in increased dilp2 mRNA, indicating that this feedback does not occur via an/a autocrine/paracrine mechanism. Rather, the feedback occurs via effector(s) produced in the gut/fat body, since up-regulation of Imp-L2 in these tissues resulted in increased dilp2 transcript levels. Hence, peripheral tissues, such as the fat body, are not only in charge of regulating DILP release from the mNSC in response to nutritional changes, as has been observed in larvae (Geminard et al., 2009), but are also main regulators of dilp synthesis in response to changes to adult IIS status.
Our study indicates that an agent present in circulation has an effect on lifespan; a finding that may have important therapeutic applications. Indeed, the genetic manipulation that led to increased IMP-L2 levels in the haemolymph (ActGS > UAS-Imp-L2+ RU486) was the one that resulted in the most substantial increase to longevity. Interestingly, in other cases where we could observe an extension of lifespan, we did not observe a substantial increase in the levels of circulating IMP-L2. It is possible that IMP-L2 was secreted but then retained in a target tissue, so that no net increase was observed in circulation. Alternatively, IMP-L2 may have predominantly acted in an/a autocrine/paracrine manner. In either case, IMP-L2 could have been retained in specific tissue(s) based on its interactions with components of extracellular matrix or cell surface proteins, as is known for IGFPBs (Hwa et al., 1999; Mohan & Baylink, 2002). In the case of dilp2GAL4 > UAS-Imp-L2, the over-expression of IMP-L2 might have been very efficient in sequestering DILPs at the site of their production. In the case of S1106 driven over-expression, the gut may be the most relevant tissue since it is here that we could observe a substantial increase in IMP-L2. Increased activity of dFOXO using the same S1106 driver is sufficient to extend lifespan (Giannakou et al., 2004), and the gut may be the relevant tissue in this case as well. Interestingly, out of all the IIS-regulated adult traits, lifespan was most sensitive to increased Imp-L2, since no other phenotypes examined were responsive to tissue-specific Imp-L2 induction.
Experimental procedures
Fly stocks and husbandry, phenotypic tests
UAS-Imp-L2 this is the genetically weaker transgene generated by Honegger et al. (2008), heatshockGAL4 (Bloomington Stock Center), dilp2GAL4 (Broughton et al., 2005), S1106 (Giannakou et al., 2004), ActGS (Ford et al., 2007), dilp2Δ/dilp2Δ, dilp3Δ/dilp3Δ and dilp5Δ/dilp5Δ (Gronke et al., 2010) were backcrossed at least six times into the outbred Dahomey background carrying the w1118 mutation (Wdah) (Giannakou et al., 2004), which had been cured of the Wolbachia infection; or the inbred w1118 background, which is Wolbachia free. Note that the ActGS line used (255B) is thought to have multiple insertions of the driver (Ford et al., 2007), however, after more than six backcrosses we did not observe any segregation of different dye colours. All experiments were performed at 25°C, 12-hour light/dark cycle, and controlled humidity, on female flies in Wdah background, unless otherwise noted. Flies were reared at standard density on SYA food (5% sucrose, 10% yeast, 1.5% agar), female flies sorted on day 3 and kept ten per vial. Where required, induction by RU486 was performed as described previously (Giannakou et al., 2004). For starvation, flies were kept on 1% agar, and for H2O2 treatment on food containing 1% agar, 5% sucrose, 5% H2O2, starting on day 5. For RNA or protein extraction the flies were frozen in liquid nitrogen on day 7. Lifespan experiments, whole-fly trehalose, circulating sugar (Parrou & Francois, 1997; Broughton et al., 2008) or whole-fly lipid (Gronke et al., 2005) quantifications were performed as described.
Antibody production, western blots and immunofluorescence
cDNA coding for IMP-L2 without the signal peptide was amplified from Wdah cDNA using the primers: CACCAGAGCCGTGGACCTGGTAGACG and TTAGTCTTCCTCATTAAGTACGGGA-TAC, cloned into pENTR/D-TOPO vector (Invitrogen, Paisley, UK), sequenced and transferred into pDEST17 vector (Invitrogen) adding a His6 tag. The protein was expressed in BL21(DES3) E. coli, recovered in the insoluble fraction and purified under denaturing conditions on Ni-NTA agarose (Quagen, Crawley, UK), followed by preparative SDS-PAGE. Anti-IMP-L2 antibody was raised in rabbits by Eurogentec (Eurogentec, Fawley, UK), and affinity purified against rIMP-L2. Fly protein extractions and western blots were performed as described (Giannakou et al., 2007; Broughton et al., 2008). Affinity purified anti-Imp-L2 was used at 1:2000 dilution. For quantification, the blots were developed with ECL, images captured with LAS-1000 cooled CCD (Fujifilm; Fujifilm UK Ltd, Bedfordshire, UK) and band intensities determined with ImageJ (freeware from Research Services Branch, National Institute of Mental Health, Bethesda, Maryland, USA). The quantity of IMP-L2 was expressed relative to ACT, with this ratio set to one in the control genotypes/untreated flies. Haemolymph was extracted from 7-day old female flies as described (Broughton et al., 2008) and 1 μL used for western blots. Images presented were taken on film. For immunofluorescence, flies were dissected in ice-cold PBS and stained as described (Broughton et al., 2010) with the affinity purified anti-IMP-L2 antibody at 1:1000 dillution and TexasRed conjugated secondary antibody, and co-stained where required with rat anti-DILP5 and AlexaFluor488 conjugated secondary antibody as described (Broughton et al., 2010). Images were captured on Zeiss LSM 700 (Carl Zeiss Ltd, Hertfordshire, UK), and quantified using ImageJ.
Far-western blotting
The last two exons of Imp-L2, which include the signal peptide, were amplified from Wdah genomic DNA with the primers: CACCATGAATTTACATGTGTGCGCCTTAG and GTCTTCCTCATT-AAGTACGGGATAC, cloned in to pENTR/D-TOPO vector, sequenced and transferred into pTWM (giving UASt-Imp-L2-myc6). S2 cells were cultured in serum-free medium (Invitrogen) and co-transfected using Cellfectin (Invitrogen) with plasmids encoding either Act5C-GAL4, UASp-GFP and UASt-Imp-L2-myc6 (producing IMP-L2-myc6) or Act5C-GAL4 and UASp-GFP (mock control), and the conditioned media harvested after 3 days. Hundred micrograms of fly-head proteins were separated on non-reducing Tris–Tricine PAGE and transferred to nitrocellulose membranes as described (Broughton et al., 2008). The membranes were blocked in PBST (PBS + 0.2% Tween-20) with 5% BSA, probed over-night with a 1 in 10 dilution of the conditioned media in the same buffer at 4°C and IMP-L2-myc6 binding visualised with an anti-myc (Sigma, Dorset, UK) western blot.
qPCR
RNA extraction, cDNA synthesis and qPCR were performed as described (Broughton et al., 2008), except for dilp qPCR which was performed on 7900HT Fast Real-Time PCR System using Fast Syber Green Master Mix (Applied Biosystems). The Act, dilp2, dilp3 and dilp5 primers have been described (Broughton et al., 2008). The following primers were used for Imp-L2: CCTCATTAAGTACGGGATAC and CTTCTGATCTCCGAGATCAAG; for 4E-BP: CACTCCTGGAGGCACCA and GAGTTCCCCTCAGCAAGCAA. The amount of the relevant mRNA was expressed relative to Act mRNA and this ratio set to one in the control genotypes/untreated flies.
Statistical analysis
Statistical analysis was performed either in jmp (SAS, Cary, NC, USA) or Excel. Details are given in figure legends.
Acknowledgments
We would like to thank H. Stocker and E. Hafen for reagents and helpful discussions, S. Grönke for supplying dilp deletion flies, I. Bjedov for primer design, J.R.W. Davis for technical assistance and J. Tower for ActGS flies. We acknowledge funding by the Wellcome Trust (L.P.) and EMBO and Marie Curie post-doctoral fellowships (N.A.).
Author contributions
N.A. and L.P. designed the experiments and wrote the manuscript; N.A., M.P.H and G.V. performed the experiments.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1 Whole body trehalose content, circulating sugars and starvation resistance in hsGAL4 > UAS-Imp-L2 flies.
Fig. S2 Life spans of hsGAL4 > UAS-Imp-L2 male flies.
Fig. S3 Lack of effect of RU486 addition to ActGS, UAS-Imp-L2 or S1106 controls.
Fig. S4 Reduced fecundity and increased H2O2 resistance upon induction of Imp-L2 with the ActGS driver.
Fig. S5 Lifespans of elavGAL4 > UAS-Imp-L2 and akhGAL4 > UAS-Imp-L2 flies.
Fig. S6 IMP-L2 produced in the fat body of induced S1106 > UAS-Imp-L2 adult female flies.
Fig. S7 IMP-L2 distribution in larval brains.
Fig. S8 Lack of effect on fecundity, H2O2 resistance and 4E-BP expression upon induction of Imp-L2 with the dilp2GAL4 or the S1106 driver.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Alic N, Partridge L. Stage debut for the elusive Drosophila insulin-like growth factor binding protein. J. Biol. 2008;7:18. doi: 10.1186/jbiol79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arquier N, Geminard C, Bourouis M, Jarretou G, Honegger B, Paix A, Leopold P. Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell Metab. 2008;7:333–338. doi: 10.1016/j.cmet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl Acad. Sci. USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE. 2008;3:e3721. doi: 10.1371/journal.pone.0003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton SJ, Slack C, Alic N, Metaxakis A, Bass TM, Driege Y, Partridge L. DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell. 2010;9:336–346. doi: 10.1111/j.1474-9726.2010.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Yaswen P. Aging and cancer cell biology, 2009. Aging Cell. 2009;8:221–225. doi: 10.1111/j.1474-9726.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc. Natl Acad. Sci. USA. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T, Min KJ, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl Acad. Sci. USA. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D, Hoe N, Landis GN, Tozer K, Luu A, Bhole D, Badrinath A, Tower J. Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp. Gerontol. 2007;42:483–497. doi: 10.1016/j.exger.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe JC, Yang E, Fristrom JW. IMP-L2: an essential secreted immunoglobulin family member implicated in neural and ectodermal development in Drosophila. Development. 1993;119:1237–1250. doi: 10.1242/dev.119.4.1237. [DOI] [PubMed] [Google Scholar]
- Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Jacobson J, Vinti G, Leevers SJ, Partridge L. Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell. 2007;6:429–438. doi: 10.1111/j.1474-9726.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- Gronke S, Mildner A, Fellert S, Tennagels N, Petry S, Muller G, Jackle H, Kuhnlein RP. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005;1:323–330. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Gronke S, Clarke D-F, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterisation of Drosophila insulin-like peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, Stocker H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J. Biol. 2008;7:10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr. Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Ikeya T, Broughton S, Alic N, Grandison R, Partridge L. The endosymbiont Wolbachia increases insulin/IGF-like signalling in Drosophila. Proc. Biol. Sci. 2009;276:3799–3807. doi: 10.1098/rspb.2009.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger MA, Rintelen F, Stocker H, Wasserman JD, Vegh M, Radimerski T, Greenberg ME, Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Kuningas M, Magi R, Westendorp RG, Slagboom PE, Remm M, van Heemst D. Haplotypes in the human Foxo1a and Foxo3a genes; impact on disease and mortality at old age. Eur. J. Hum. Genet. 2007;15:294–301. doi: 10.1038/sj.ejhg.5201766. [DOI] [PubMed] [Google Scholar]
- Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J. Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- Osterbur DL, Fristrom DK, Natzle JE, Tojo SJ, Fristrom JW. Genes expressed during imaginal discs morphogenesis: IMP-L2, a gene expressed during imaginal disc and imaginal histoblast morphogenesis. Dev. Biol. 1988;129:439–448. doi: 10.1016/0012-1606(88)90391-0. [DOI] [PubMed] [Google Scholar]
- Parrou JL, Francois J. A simplified procedure for a rapid and reliable assay of both glycogen and trehalose in whole yeast cells. Anal. Biochem. 1997;248:186–188. doi: 10.1006/abio.1997.2138. [DOI] [PubMed] [Google Scholar]
- Partridge L, Bruning JC. Forkhead transcription factors and ageing. Oncogene. 2008;27:2351–2363. doi: 10.1038/onc.2008.28. [DOI] [PubMed] [Google Scholar]
- Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, Psaty BM, Atzmon G, Barzilai N, Cummings SR, Browner WS, Kwok PY, Ziv E. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, Heller J, Platt DM, Pasquinelli AA, Liu LX, Doberstein SK, Ruvkun G. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001;15:672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Selman C, McElwee JJ, Partridge L. Separating cause from effect: how does insulin/IGF signalling control lifespan in worms, flies and mice? J. Intern. Med. 2008;263:179–191. doi: 10.1111/j.1365-2796.2007.01906.x. [DOI] [PubMed] [Google Scholar]
- Poirier L, Shane A, Zheng J, Seroude L. Characterization of the Drosophila gene-switch system in aging studies: a cautionary tale. Aging Cell. 2008;7:758–770. doi: 10.1111/j.1474-9726.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- Puig O, Marr MT, Ruhf ML, Tjian R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Sloth Andersen A, Hertz Hansen P, Schaffer L, Kristensen C. A new secreted insect protein belonging to the immunoglobulin superfamily binds insulin and related peptides and inhibits their activities. J. Biol. Chem. 2000;275:16948–16953. doi: 10.1074/jbc.M001578200. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MF. Regulating insulin signaling and beta-cell function through IRS proteins. Can. J. Physiol. Pharmacol. 2006;84:725–737. doi: 10.1139/y06-008. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc. Natl Acad. Sci. USA. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


