Abstract
Velocity vector imaging (VVI) is novel ultrasound image analysis software, enabling simultaneous evaluation of longitudinal and radial tissue motion. This study aimed to investigate the possible usefulness of VVI in evaluating the longitudinal vessel wall movement of the common carotid artery (CCA). Sixteen healthy volunteers and 16 patients with established coronary artery disease (CAD) were included in the study. CCA was scanned and standard B-mode ultrasound images were analysed off-line with VVI. In healthy volunteers, total longitudinal displacements (tLoD) of the right and left CCA were similar, as were the movements of the near- and far wall of the right CCA. The CAD group showed significantly lower tLoD compared to the healthy volunteers (0·543 ± 0·394 versus 0·112 ± 0·074, P<0·0001). VVI is a highly feasible technique in assessing longitudinal CCA wall motion, which may be of potential relevance as a novel vascular biomarker.
Keywords: arterial stiffness, cardiovascular disease, carotid artery ultrasound, longitudinal displacement, surrogate marker
Introduction
Arterial stiffness measured with different techniques is well known to be of importance for future cardiovascular morbidity and mortality (Franklin et al., 1999; Weber et al., 2004; Dolan et al., 2006; Willum-Hansen et al., 2006). The golden standard method to assess arterial stiffness is pulse wave velocity (PWV) (Laurent et al., 2006), which measures the time of a pulse wave along an arterial segment and relies on transfer functions for pressure estimation. PWV has been shown to be of prognostic relevance in different patient cohorts (Guerin et al., 2001; Boutouyrie et al., 2002; Cruickshank et al., 2002; Mattace-Raso et al., 2006). Local methods focusing on the diameter change during heart cycles, i.e. the radial movement has also been extensively studied in terms of local strain, distensibility and compliance. However, the longitudinal arterial movement has gained little research focus, and its clinical implications remains to be elucidated.
Increased intima-media thickness is a well-established marker of atherosclerotic diseases and provides a predictive value for cardiovascular events (O’Leary et al., 1999; Ali et al., 2006). In addition to the structural information, the carotid images are usually recorded in real time, which make it possible to study common carotid artery (CCA) wall motion, typically in the radial direction using e.g. M-mode. The concept to combine simultaneous morphological and functional measurement is highly attractive, especially when the imaging procedure is highly feasible, non-invasive and inexpensive.
Recently, (Persson et al., 2003) developed a new ultrasound-based technique with the ability to assess radial, as well as longitudinal CCA wall motion in man. With ultrasound technique, they demonstrated a distinct longitudinal vessel wall movement in healthy subjects (Cinthio et al., 2006). However, little is known about the longitudinal movements’ clinical correlates and possible usefulness. Velocity vector imaging (VVI) is new ultrasound software, based on multiple M-mode evaluations and speckle-tracking technique. The software has been used to study ventricle dyssynchronies of the heart (Cannesson et al., 2006). VVI allows simultaneous evaluation of longitudinal and radial velocity, strain, strain rate and displacement. In this study, we aimed to investigate the applicability and reproducibility of VVI in assessment of longitudinal CCA wall motion. Additionally, the potential difference in longitudinal displacement between healthy volunteers and a group of patients with established coronary artery disease (CAD) was investigated.
Materials and method
Study population
Sixteen young healthy volunteers free of symptomatic cardiovascular disease and any medications were recruited for study participation. As a control group, 16 patients with established atherosclerotic CAD were recruited with the inclusion criteria of prior CABG operation. All subjects gave their informed and written consent to study participation. The study was approved by the Local Ethics Committee in Gothenburg.
Carotid artery ultrasound examinations
Carotid artery B-mode ultrasound was conducted by an experienced sonographer according to a standardized protocol over the distal CCA. All examinations were performed with the study subject in the supine position after 10 min of rest. The Acuson Sequoia 512 ultrasound system (Siemens Medical Solutions Inc., Mountain View, USA), equipped with an 8- MHz transducer was used. CINE-looped images of consecutive cardiac cycles of the distal CCA were stored for later offline analysis. Intima-media thickness (IMT) was defined as the distance from the lumen-intimal interface to the medial-adventitial border and measured approximately one centimetre proximal to the bulb of the CCA far wall (Wendelhag et al., 1991). The presences of plaques were evaluated in long-axis view of the carotid bifurcation by manual delineation. As conventional local stiffness measurements, CCA strain, based on the radial diameter change during a heart cycle, was calculated using the formula:
and carotid stiffness index was calculated as:
 |
where ln is the natural logarithm and blood pressure was measured in the brachial artery by conventional oscillometric approach as an estimate (Kawasaki et al., 1987).
Velocity vector imaging of the common carotid artery
All VVI-measurements were made offline at a workstation by two other independent observers. VVI software (Research Arena 2; TomTec imaging systems GmbH, Unterschleissheim, Germany) was used to derive vessel wall velocity, strain, strain rate and displacement of both the near- and far wall of the right and left CCA. Further, the radial movement in terms of velocity and displacement was studied. Delineation of the lumen-vessel wall border was conducted using the leading-to-leading edge principle; edges were outlined approximately 1 cm distal to the carotid bulb in the right and left CCA. Five guiding points were equally distributed (0·25 cm apart) within a 1- cm segment both in the near wall and far walls, i.e. a total of ten measuring points were used. With available software, it is possible to evaluate the vessel wall movements of single wall segments; the outlined segments corresponding to the near- and far wall of the CCA were selected. The virtual transducer applied in the software was placed above the near wall. Figure 1 illustrates an outlined measurement. Software settings were positioned to cover peak systole and peak diastole values during one complete cardiac cycle. To be able to quantify the longitudinal movement, we calculated the total longitudinal displacement (tLoD) during a cardiac cycle as the sum of absolute values of maximal systolic plus the maximal diastolic displacements. Figure 2 displays an example of the processed measurement with the vectors indicating the movement of the CCA walls in the longitudinal direction.
Figure 1.
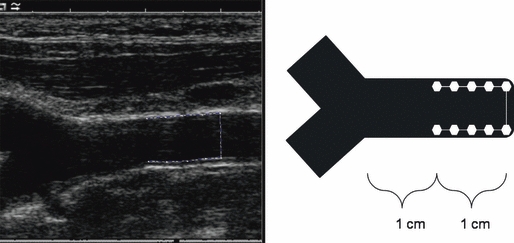
The left picture shows delineation of the lumen vascular borders using the VVI tool in a standard common carotid artery (CCA) image. The schematic picture to the right illustrates the guiding points in the near- and far wall of the CCA proximal to the carotid bulb.
Figure 2.
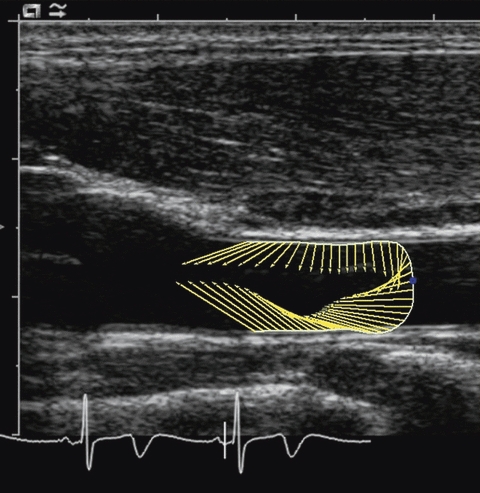
The image shows the common carotid artery (CCA) wall motion at the specific time point shown in the ECG recording. The vectors indicate the direction and magnitude of the CCA wall motion in the longitudinal and radial direction.
Reproducibility measurements
We evaluated intra-observer and inter-observer variability of the measurements at separate occasions at least 4 weeks apart. For intra- and inter-observer variability, coefficients of variance were calculated using the formula:
where x and y represent values of the first and the second measurement, respectively.
Statistics
A P-value of <0·05 was considered significant. To determine differences in vessel wall motion between different wall segments and the left and right side, a Student’s paired T-test was used. To study differences between genders and healthy and diseased subjects, Student’s t-test was performed.
Results
Healthy volunteers
Table 1 shows clinical data of the study groups with comparison by gender. The 16 healthy subjects were aged 25·4 ± 5·1 years (range 20–38 years) and were normotensive. They all displayed a distinct measureable longitudinal movement during cardiac cycles. There was no significant difference between far wall mean tLoD of the right CCA (mean 0·543 ± 0·394 mm, range 0·090–1·516 mm) and the left CCA (mean 0·401 ± 0·274 mm, range 0·036 ± 0·824 mm). There was no difference in tLoD between men and women (0·532 ± 0·460 mm versus 0·557 ± 0·325 mm, P = NS). Table 2 displays the VVI-generated variables of the CCA with comparisons of both the different walls in the right CCA and comparisons of the right and left CCA. The radial movement amplitude was similar to that of the longitudinal.
Table 1.
Clinical characteristics of the study groups with gender comparisons
| Healthy volunteers (n = 16) | CAD-group (n = 16) | |||||
|---|---|---|---|---|---|---|
| Males | Females | P | Males | Females | P | |
| Gender (%) | 56·3 | 43·7 | NS | 60·0 | 40·0 | NS |
| Age (years) | 26·8 ± 6·5 | 23·6 ± 6·9 | NS | 66·8 ± 9·6 | 70·2 ± 3·1 | NS |
| HR (bpm) | 54·4 ± 6·1 | 62·3 ± 8·9 | NS | 65·1 ± 8·4 | 57·3 ± 9·9 | NS |
| BMI (kg m−2) | 24·2 ± 3·2 | 21·5 ± 1·3 | 0·05 | 26·7 ± 1·6 | 21·6 ± 10·9 | NS |
| SBP (mmHg) | 112·2 ± 8·3 | 101·4 ± 14·4 | NS | 150·9 ± 10·3 | 155·8 ± 37·5 | NS |
| DBP (mmHg) | 66·9 ± 7·5 | 63·0 ± 8·4 | NS | 86·6 ± 11·1 | 76·6 ± 6·1 | NS |
| PP (mmHg) | 46·3 ± 11·6 | 46·0 ± 9·6 | NS | 64·4 ± 17·7 | 79·2 ± 31·7 | NS |
| CCA dS (cm) | 0·64 ± 0·06 | 0·61 ± 0·04 | NS | 0·65 ± 0·07 | 0·67 ± 0·11 | NS |
| CCA dD (cm) | 0·57 ± 0·06 | 0·54 ± 0·02 | NS | 0·61 ± 0·07 | 0·61 ± 0·11 | NS |
| CCA IMT (cm) | 0·029 ± 0·008 | 0·027 ± 0·004 | NS | 0·057 ± 0·010 | 0·060 ± 0·011 | NS |
| CCA strain | 0·122 ± 0·052 | 0·142 ± 0·055 | NS | 0·067 ± 0·028 | 0·111 ± 0·068 | NS |
| CCA stiffness index | 5·8 ± 3·9 | 3·7 ± 0·8 | NS | 9·34 ± 4·39 | 7·53 ± 2·56 | NS |
Values are displayed as means ± SD. HR, heart rate; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; CCA dS, common carotid artery diameter in systole; CCA dD, common carotid artery diameter in diastole; IMT, intima-media thickness.
Table 2.
Comparisons between various VVI-derived CCA wall motion measurements in healthy volunteers
| RCCA | LCCA | ||||
|---|---|---|---|---|---|
| NW | FW | P-valuea | FW | P-valueb | |
| Longitudinal variables | |||||
| Velocity S (cm s−1) | 0·278 ± 0·230 | 0·290 ± 0·230 | NS | 0·166 ± 0·116 | NS |
| Velocity D (cm s−1) | 0·168 ± 0·097 | 0·173 ± 0·093 | NS | 0·134 ± 0·093 | NS |
| Strain S (%) | 7·519 ± 5·412 | 6·991 ± 6·274 | NS | 4·732 ± 3·920 | NS |
| Strain D (%) | 1·443 ± 1·722 | 1·696 ± 1·632 | NS | 1·159 ± 1·708 | NS |
| Strain rate S (l s−1) | 0·422 ± 0·348 | 0·419 ± 0·376 | NS | 0·234 ± 0·162 | NS |
| Strain rate D (1 s−1) | 0·283 ± 0·192 | 0·266 ± 0·202 | NS | 0·205 ± 0·129 | NS |
| Displacement S (mm) | 0·457 ± 0·347 | 0·474 ± 0·356 | NS | 0·332 ± 0·262 | NS |
| Displacement D (mm) | 0·075 ± 0·086 | 0·069 ± 0·088 | NS | 0·069 ± 0·180 | NS |
| Total displacement (mm) | 0·531 ± 0·372 | 0·543 ± 0·394 | NS | 0·401 ± 0·274 | NS |
| Radial variables | |||||
| Velocity S (cm s−1) | 0·100 ± 0·071 | 0·100 ± 0·071 | NS | 0·070 ± 0·061 | NS |
| Velocity D (cm s−1) | 0·133 ± 0·093 | 0·080 ± 0·064 | 0·005 | 0·053 ± 0·050 | 0·04 |
| Displacement S (mm) | 0·429 ± 0·314 | 0·162 ± 0·160 | 0·001 | 0·109 ± 0·120 | NS |
| Displacement D (mm) | 0·027 ± 0·041 | 0·030 ± 0·031 | NS | 0·037 ± 0·062 | NS |
P-value indicates significance between near wall and far wall of the RCCA.
P-value shows significance between RCCA and LCCA far walls.
Patients with established coronary artery disease
Patients who previously underwent a CABG and hence are considered to exhibit established CAD showed significantly lower tLoD of both the near- and far wall of the CCA as shown in Table 3. No significant difference between men and women in tLoD could be found (0·093 ± 0·078 versus 0·126 ± 0·073, P = NS). Of the patients who underwent CABG, 50% (8/16) were on ongoing treatment with ACE inhibitors, 81·3% (13/16) on beta blockers, 68·8% (11/16) on statins, 75% (12/16) on aspirin and 37·5% (6/16) were treated with clopidogrel. Further, 12 of the 16 patients exhibited plaques in the CCA bifurcation; the average plaque area in this study group was 0·151 ± 0·209 cm. Figure 3 illustrates detailed examples from the software of tLoD in healthy volunteers and patients with CAD.
Table 3.
Longitudinal displacements in healthy volunteers and patients with CAD
| Healthy subjects (n = 16) | CAD-patients (n = 16) | P-values | |
|---|---|---|---|
| Longitudinal variables | |||
| Displacement S NW (mm) | 0·457 ± 0·347 | 0·078 ± 0·065 | <0·0001 |
| Displacement D NW (mm) | 0·075 ± 0·086 | 0·032 ± 0·062 | NS |
| tLoD NW (mm) | 0·531 ± 0·372 | 0·101 ± 0·079 | <0·0001 |
| Displacement S FW (mm) | 0·474 ± 0·354 | 0·093 ± 0·075 | <0·0001 |
| Displacement D FW (mm) | 0·069 ± 0·088 | 0·019 ± 0·024 | 0·04 |
| tLoD FW (mm) | 0·543 ± 0·394 | 0·112 ± 0·074 | <0·0001 |
S, systole; D, diastole; NW, near wall; FW, far wall; tLoD, total longitudinal displacement.
Figure 3.
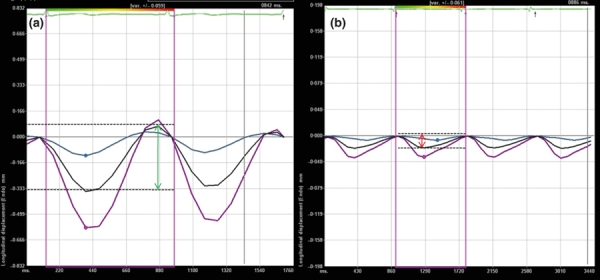
Shows detailed examples from the output of the VVI-software. Displayed are the longitudinal displacement curves during heart cycles. The blue and purple curves correspond to the common carotid artery far wall; the black curve is the average of the blue and purple curves. The purple vertical lines denote the measured cardiac cycle. The ECG recording can be seen above the displacement curves. (a) Displays a normal longitudinal wall motion of the CCA far wall in a healthy volunteer. The green arrow indicates tLoD, which in this patient was found to be 0·438 mm. (b) Shows a patient with low tLoD. The red arrow indicates the tLoD to be 0·019 mm.
Reproducibility measurements
Intra-observer reproducibility of the total longitudinal displacement was found to be 10·5% for the CCA far wall and 12·2% for the CCA near wall. Table 4 shows the intra-observer and inter-observer variability at repeated measurements.
Table 4.
Intra- and inter-observer coefficients of variance
| Near wall | Far wall | |
|---|---|---|
| Intra-observer (%) | 12·2 | 10·5 |
| Inter-observer (%) | 18·1 | 9·1 |
Discussion
This study shows tLoD to be measurable with VVI-technique. Further, our data shows the longitudinal movement of the CCA near- and far wall to be of similar magnitude in healthy volunteers. Additionally, no differences in movements could be seen between the right and left CCA. Patients with CAD displayed significantly lower total longitudinal displacement compared to healthy volunteers. The intra-observer and inter-observer reproducibility of the right CCA far wall tLoD was found to be 10·5% and 9·1%, respectively.
Measuring longitudinal displacement with velocity vector imaging technique
As the arterial system is complex and susceptible to a variety of different physiological conditions, characterization of arterial stiffness has faced practical problems (O’Rourke & Mancia, 1999). The longitudinal wall motion is known to be very small studied in experimental models (Patel et al., 1961; Patel & Fry, 1966) and has not been under any specific research focus. This is probably due to lack of an adequate tool to assess this small movement. Now, VVI-technique provides a potential method, which is readily available. We found a visually good tracking ability of wall movements, which is confirmed by previous experimental validation of the VVI-technique (Pirat et al., 2008). The technique reveals the longitudinal CCA movement of the near- and far wall to be of about the same magnitude in healthy volunteers. Further, also the movements of the right and left CCA are of about the same size. CCA movements in healthy volunteers reveal somewhat large individual variations, and this could be because of both physiological and methodological reasons. Nevertheless, we suggest that the measurements of the small movement must be very carefully conducted in a standardized manner. With the present available VVI-software version and the reproducibility data provided in this study; we calculated the tLoD of the CCA during a heart cycle, which seems to be a robust read-out variable of CCA wall motion. Further, we conducted the measurements on the far wall of the right CCA, usually with best 2D image quality, to provide a standardized measuring concept.
Concerning the VVI-technique, it is based on speckle-tracking technique and multiple M-mode evaluations. In the current VVI setting, the longitudinal wall motion was averaged from a 1-cm segment at the lumen-vessel boundary of the CCA far wall. There is thus no particular region of interest (ROI) that is evaluated, but several speckles that are integrated and then averaged. This averaging approach using VVI is probably not sensitive enough to detect multiphasic motions at a particular ROI in the vessel wall. However, this probably makes the VVI-technique less susceptible for local variations throughout the CCA. This fact is probably the main reason that the technique launched by Cinthio et al. is better suited to study the multiphasic longitudinal wall motion.
Radial wall motion
Using VVI-technique to evaluate the radial wall movements, it provides the radial velocity and displacement. Radial wall movements in terms of diameter changes are more easily performed by established methods. Our data indicate the radial CCA displacement to be of about equal size compared to the longitudinal movement in the CCA near wall and of about twice the size in the far wall. This finding confirms the data from previous observations (Golemati et al., 2003; Cinthio et al., 2006). Further, in consistence with the findings by (Persson et al., 2003), we found that the CCA near wall had greater radial wall movement than the far wall. However, the nature of this phenomenon still remains to be determined. Several local arterial stiffness methods, most based on ultrasound investigations, relay on the radial diameter changes and often take blood pressure into account. Calculation of the carotid stiffness index is a well-known local measure of arterial stiffness (O’Rourke et al., 2002). As can be confirmed in our study; patients with CAD show increased stiffness index (Hirai et al., 1989).
Aspects on vessel wall mechanical properties
Considering arterial biomechanics, wall shear stress and the circumferential stress acting on the arterial wall traditionally account for vessel wall remodelling during pathological circumstances. Altered blood flow is known to impact on vessel wall radius (Langille et al., 1989; Sho et al., 2004), whereas blood pressure acts on vessel wall thickness (Courtman et al., 1998; Hu et al., 2007) to adapt with the goal of re-achieving arterial vessel wall homeostasis (Davies, 1995). The fundamental axial stress acting on the vessel in the longitudinal direction is less well studied in comparison, and increasing interest is aimed at evaluation of the biaxial forces in experimental settings. However, axial stretch in vivo has been shown to be reduced with increasing wall thickness or hypertension (Wolinsky, 1970; Eberth et al., 2009). Elastin has been proposed to play a crucial role in providing an axial prestretch in vivo owing to the fact that elastin is stretched during development (Dobrin et al., 1990). Increased collagen deposition that follows hypertension will consequently result in increased wall thickness and a reduced axial retraction. This becomes evident when calculating the C:E ratio (collagen/elastin) where a larger ratio is clearly associated with lower in vivo axial stretch (Wolinsky, 1970; Berry & Greenwald, 1976). It has been shown both theoretically and experimentally that following vessel wall thickening, the axial stress is decreased to larger extent than the circumferential stress. This may suggest that, compared to the traditional radial wall strain, the longitudinal wall motion might be an earlier and more sensitive measure of vascular wall remodelling (Humphrey et al., 2009). Not surprisingly, patients who underwent CABG in the present study display significantly greater IMT than the healthy subjects because of their advanced systemic atherosclerotic disease. This could be one of the important mechanism underlying the lower tLoD in patients who underwent CABG compared to the healthy volunteers.
Potential clinical values of the CCA longitudinal wall motion
At present, great research efforts are made to define risk markers of early atherosclerotic disease with the ambition to predict future cardiovascular (CV) events. The CCA longitudinal wall motion could be a new potential risk marker with additional values on top of the CCA morphological information. Also, physiological and molecular determinants of tLoD need to be investigated further.
This study showed the longitudinal wall movement in patients with CAD to be significantly decreased in all measured segments compared to healthy volunteers. The comparison was performed to demonstrate potential biological significance of this measure in this very initial study. Obviously, no adjustments for age and traditional CV risk factors were made at this point. Nevertheless, tLoD is highly distinct between the two groups. If tLoD could be used as an independent factor for CV risk, stratification still remains to be addressed in future prospective studies in a relevant CV patient cohort. At this point, the found difference in tLoD could be caused by various factors such as age, blood pressure, CV disease burden and different medications.
In advanced atherosclerotic vascular disease, it is conceivable that the radial as well as the longitudinal wall motion is impaired. However, it is likely that the longitudinal wall motion indices are more sensitive to detect early vessel wall pathology as well as to study adaptive vascular change and possible treatment effects, based on the aforementioned theoretical analysis. The more prominent differences in the longitudinal versus the radial wall motion between CAD and healthy subjects in this initial study may support this hypothesis.
Study limitations
The VVI software used in this study has the ability to generate peak systolic and diastolic values during a cardiac cycle. As a measuring concept, the software currently serves for cardiac wall motion evaluation, but can most likely be even further developed and specifically adapted for vessel wall function evaluation. The longitudinal CCA movement has previously been shown to be multiphasic (Cinthio et al., 2006). For the time being with available VVI-software, the multiphasic nature of the longitudinal wall motion does not seem to be evaluable. Instead of the approach by Cinthio et al. to assess detailed wall motion pattern in a localized region of interest, we observed rather reproducible measurement by averaging wall motion values from a 1-cm vessel segment and thus suggest measuring of peak systolic and diastolic values to provide the most stable vessel wall motions values using current the VVI software.
As a result of the very limited study population and a number of confounders, the difference between the healthy controls and patients with CAD should be interpreted with great caution. Potential clinical relevance of this technique remains to be addressed in a much larger and more homogenous clinically relevant population in the future.
Conclusions
This study demonstrates CCA longitudinal wall motion to be measurable with VVI-technique with acceptable accuracy. Patients with established CAD appear to exhibit impaired longitudinal wall motion compared to healthy volunteers. Future studies should be directed to further address the longitudinal wall motion, regarding its clinical correlates and potential role as a novel vascular biomarker for cardiovascular diseases.
References
- Ali YS, Rembold KE, Weaver B, Wills MB, Tatar S, Ayers CR, Rembold CM. Prediction of major adverse cardiovascular events by age-normalized carotid intimal medial thickness. Atherosclerosis. 2006;187:186–190. doi: 10.1016/j.atherosclerosis.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Berry CL, Greenwald SE. Effects of hypertension on the static mechanical properties and chemical composition of the rat aorta. Cardiovasc Res. 1976;10:437–451. doi: 10.1093/cvr/10.4.437. [DOI] [PubMed] [Google Scholar]
- Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- Cannesson M, Tanabe M, Suffoletto MS, Schwartzman D, Gorcsan J. Velocity vector imaging to quantify ventricular dyssynchrony and predict response to cardiac resynchronization therapy. Am J Cardiol. 2006;98:949–953. doi: 10.1016/j.amjcard.2006.04.045. [DOI] [PubMed] [Google Scholar]
- Cinthio M, Ahlgren AR, Bergkvist J, Jansson T, Persson HW, Lindström K. Longitudinal movements and resulting shear strain of the arterial wall. Am J Physiol Heart Circ Physiol. 2006;291:H394–H402. doi: 10.1152/ajpheart.00988.2005. [DOI] [PubMed] [Google Scholar]
- Courtman DW, Cho A, Langille L, Wilson GJ. Eliminating arterial pulsatile strain by external banding induces medial but not neointimal atrophy and apoptosis in the rabbit. Am J Pathol. 1998;153:1723–1729. doi: 10.1016/S0002-9440(10)65687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrin PB, Schwarcz TH, Mrkvicka R. Longitudinal retractive force in pressurized dog and human arteries. J Surg Res. 1990;48:116–120. doi: 10.1016/0022-4804(90)90202-d. [DOI] [PubMed] [Google Scholar]
- Dolan E, Thijs L, Li Y, Atkins N, McCormack P, McClory S, O’Brien E, Staessen JA, Stanton AV. Ambulatory arterial stiffness index as a predictor of cardiovascular mortality in the Dublin Outcome Study. Hypertension. 2006;47:365–370. doi: 10.1161/01.HYP.0000200699.74641.c5. [DOI] [PubMed] [Google Scholar]
- Eberth JF, Gresham VC, Reddy AK, Popovic N, Wilson E, Humphrey JD. Importance of pulsatility in hypertensive carotid artery growth and remodeling. J Hypertens. 2009;27:2010–2021. doi: 10.1097/HJH.0b013e32832e8dc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- Golemati S, Sassano A, Lever MJ, Bharath AA, Dhanjil S, Nicolaides AN. Carotid artery wall motion estimated from B-mode ultrasound using region tracking and block matching. Ultrasound Med Biol. 2003;29:387–399. doi: 10.1016/s0301-5629(02)00760-3. [DOI] [PubMed] [Google Scholar]
- Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation. 1989;80:78–86. doi: 10.1161/01.cir.80.1.78. [DOI] [PubMed] [Google Scholar]
- Hu JJ, Fossum TW, Miller MW, Xu H, Liu JC, Humphrey JD. Biomechanics of the porcine basilar artery in hypertension. Ann Biomed Eng. 2007;35:19–29. doi: 10.1007/s10439-006-9186-5. [DOI] [PubMed] [Google Scholar]
- Humphrey JD, Eberth JF, Dye WW, Gleason RL. Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech. 2009;42:1–8. doi: 10.1016/j.jbiomech.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res. 1987;21:678–687. doi: 10.1093/cvr/21.9.678. [DOI] [PubMed] [Google Scholar]
- Langille BL, Bendeck MP, Keeley FW. Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol. 1989;256:H931–H939. doi: 10.1152/ajpheart.1989.256.4.H931. [DOI] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Leone N, Ducimetière P, Gariépy J, Courbon D, Tzourio C, Dartigues J-F, Ritchie K, Alpérovitch A, Amouyel P, Safar ME, Zureik M. Distension of the carotid artery and risk of coronary events: the three-city study. Arterioscler Thromb Vasc Biol. 2008;28:1392–1397. doi: 10.1161/ATVBAHA.108.164582. [DOI] [PubMed] [Google Scholar]
- Mattace-Raso FU, van derCammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Mancia G. Arterial stiffness. J Hypertens. 1999;17:1. doi: 10.1097/00004872-199917010-00001. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426–444. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- Patel DJ, Fry DL. Longitudinal tethering of arteries in dogs. Circ Res. 1966;19:1011–1021. doi: 10.1161/01.res.19.6.1011. [DOI] [PubMed] [Google Scholar]
- Patel DJ, Mallos AJ, Fry DL. Aortic mechanics in the living dog. J Appl Physiol. 1961;16:293–299. doi: 10.1152/jappl.1961.16.2.293. [DOI] [PubMed] [Google Scholar]
- Persson M, Ahlgren AR, Jansson T, Eriksson A, Persson HW, Lindström K. A new non-invasive ultrasonic method for simultaneous measurements of longitudinal and radial arterial wall movements: first in vivo trial. Clin Physiol Funct Imaging. 2003;23:247–251. doi: 10.1046/j.1475-097x.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- Pirat B, Khoury DS, Hartley CJ, Tiller L, Rao L, Schulz DG, Nagueh SF, Zoghbi WA. A novel feature-tracking echocardiographic method for the quantitation of regional myocardial function: validation in an animal model of ischemia-reperfusion. J Am Coll Cardiol. 2008;51:651–659. doi: 10.1016/j.jacc.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sho E, Nanjo H, Sho M, Kobayashi M, Komatsu M, Kawamura K, Xu C, Zarins CK, Masuda H. Arterial enlargement, tortuosity, and intimal thickening in response to sequential exposure to high and low wall shear stress. J Vasc Surg. 2004;39:601–612. doi: 10.1016/j.jvs.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- Wendelhag I, Gustavsson T, Suurküla M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol. 1991;11:565–577. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- Wolinsky H. Response of the rat aortic media to hypertension. Morphological and chemical studies. Circ Res. 1970;26:507–522. doi: 10.1161/01.res.26.4.507. [DOI] [PubMed] [Google Scholar]


