Abstract
The adult human heart is an ideal target for regenerative intervention since it does not functionally restore itself after injury yet has a modest regenerative capacity that could be enhanced by innovative therapies. Adult cardiac cells with regenerative potential share gene expression signatures with early fetal progenitors that give rise to multiple cardiac cell types, suggesting that the evolutionarily conserved regulatory networks that drive embryonic heart development might also control aspects of regeneration. Here we discuss commonalities of development and regeneration, and the application of the rich developmental biology heritage to achieve therapeutic regeneration of the human heart.
Keywords: embryonic development, differentiation, myocyte regeneration, stem cells
I will remove from you your heart of stone and give you a heart of flesh.—Ezekiel 36:26.
The traditional view that the adult mammalian heart is incapable of myocyte renewal has given way in recent years to the notion that humans and other mammals exhibit a limited capacity for myocardial regeneration that is clearly measureable but insufficient to restore heart function after ischemic or other injury. Moreover, new insights into regenerative mechanisms, including the genes and cytokines that direct stem cell cardiogenesis, suggest strategies for stem cell therapies. Thus, like redemption for disciples of the prophet Ezekiel, efficient human cardiac regeneration remains elusive, but recent advances make it seem tantalizingly close at hand.
Numerous challenges must be resolved in order to achieve clinically meaningful regeneration, including the problems of producing enough cells and ensuring their functional integration. Insight into both issues comes from the study of embryonic cardiac development. The heart forms in response to stereotyped patterns of timing and concentrations and combinations of extracellular signaling proteins that guide pluripotent cells through successive steps of mesoderm induction, commitment to a cardiac fate, and the elaboration of specialized cardiac cells, including atrial and ventricular myocytes, conducting cells, fibroblasts, and vascular endothelial and smooth muscle cells (for reviews, see Olson and Schneider 2003; Olson 2006; Rosenthal and Harvey 2010). This review explores the idea that tapping systematically into the signaling and genetic networks that control embryonic cardiac differentiation might be an effective means to regulate proliferation and differentiation of adult cardiac stem or progenitor cells to achieve therapeutic regeneration.
Unassisted self-repair
The rationale for treating heart disease by regenerative intervention is the limited, but detectable, ability of mammalian cardiac muscle to reconstruct itself after cell death. The mismatch between cell death and restoration is clinically self-evident, standing in stark contrast with both the ability of a single hematopoietic stem cell to reconstitute the bone marrow (Matsuzaki et al. 2004) and the scarless healing of injured hearts in newts, axolotls, and other more regenerative species (Oberpriller and Oberpriller 1974). A robust capacity for cardiac self-repair has been explored extensively in zebrafish (Poss et al. 2002; Raya et al. 2003). Initially, regrowth of the injured zebrafish heart was envisioned to occur like that of an amputated limb, proceeding by epimorphic regeneration, with the creation of a primitive blastema at the margin of injury comprising undifferentiated progenitor cells (Lepilina et al. 2006). In more recent studies, however, lineage tracing by drug-induced Cre/lox fate-mapping indicates instead that cardiac regeneration in zebrafish occurs via pre-existing adult cardiomyocytes themselves, producing new cardiomyocytes through reactivation of the cell cycle (Jopling et al. 2010; Kikuchi et al. 2010). As one consequence of this paradigm shift, it has become less clear whether molecules and pathways implicated in fin regeneration—ranging from the requirement for a me(3)K27 H3 demethylase (Stewart et al. 2009) to antagonists of the imidazoline receptor (Oppedal and Goldsmith 2010)—are predictive of fruitful approaches for promoting heart repair, even in a robustly regenerative species such as zebrafish. Screens aimed at heart regeneration itself may be imperative, even though highly challenging technically.
In humans, despite the unquestionable lack of self-repair sufficient in magnitude to offset cell death in heart disease, conclusive proof of cardiomyocyte renewal has resulted from isotopic studies (Bergmann et al. 2009). The production of 14C in the atmosphere during the era of atomic weapons testing, followed by termination of aboveground testing in 1963, offers a geopolitical pulse-chase experiment from which the age of the body's various nuclei can be ascertained. The frequency of cardiomyocyte replacement ranged from 1% per year in young adults to 0.45% in the elderly (Bergmann et al. 2009). Providing the most objective evidence to date of human cardiac muscle cell renewal, this estimate is 20-fold to 40-fold less than claimed on the basis of other criteria (Kajstura et al. 2010).
While analogous 14C results have not yet been reported for human cardiac injury, a genetic pulse-chase study in mice provides a tantalizing indication of adult mammalian cardiac self-repair following infarction (Hsieh et al. 2007). A tamoxifen-dependent, cardiomyocyte-restricted Cre was activated transiently in healthy adult mice, causing a short-lived pulse of recombination that irreversibly activated the Cre-dependent reporter in >80% of cardiac muscle cells. In the absence of injury, no dilution of the reporter gene's expression occurred over time, indicating the lack of detectable cardiac muscle cell replacement during normal aging. However, dilution occurred 3 mo after ischemic damage, suggesting that as many as 18% of the cardiomyocytes bordering the injury arose de novo from formerly undifferentiated cells, either intrinsic or extrinsic to the heart (Hsieh et al. 2007). There was little evidence of replication of pre-existing myocytes, indicating that natural cardiac regeneration in the mouse occurs primarily through stem or progenitor cell contribution, rather than by cell cycle re-entry of differentiated cardiomyocytes, as predominates in zebrafish.
The cardiac cell cycle as a therapeutic target
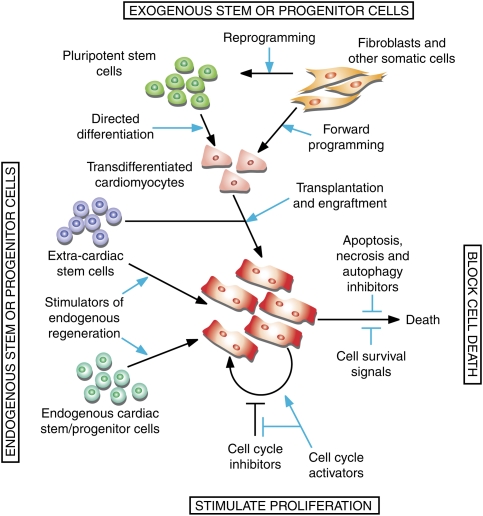
Conceptually, one might approach the task of augmenting cardiac muscle cell number by antagonizing cell death (Fig. 1; for review, see Whelan et al. 2010). Success in mice has been achieved by numerous genetic interventions that interfere with the machinery for apoptosis, necrosis, and autophagy (Pattingre et al. 2005; Nakayama et al. 2007; Diwan et al. 2008; Chen et al. 2010; Toko et al. 2010); with oxidative stress (Li et al. 2009; Kuroda et al. 2010); or with stress-activated cascades that culminate in myocyte death (Krishnan et al. 2009; Ling et al. 2009). Conversely, others have increased cardiac muscle cell number by enhancing cell survival pathways (Oh et al. 2001; Harada et al. 2005; Oshima et al. 2009; Takagi et al. 2010). However striking, though, as a means to prevent or lessen cell death, such interventions cannot reconstitute cardiac muscle cell number once cell death has transpired.
Figure 1.
Strategies to increase cardiac muscle cell number as a therapeutic target. In principle, the limited ability of the heart to replace cardiomyocytes can be improved by reactivating cell division of pre-existing cardiomyoctes and/or inhibiting cell death or augmenting survival. Alternatively, new myocytes can be produced from multipotent stem or progenitors that reside within niches in the myocardium, circulating stem cells with cardiac potency, or ex vivo cells transplanted into the injured heart. Challenges to regeneration include an endogenous restorative capacity that appears limited by an insufficient number of available stem or progenitor cells, and the need to develop efficient means to produce or deliver exogenous cells. Developmental signals are being investigated for use in enhancing therapeutic regeneration from endogenous and exogenous sources.
For this reason, investigators also have sought to override the “post-mitotic” phenotype of mammalian ventricular muscle, by forced expression of cell cycle activators or inactivation of cell cycle inhibitors (MacLellan and Schneider 2000; Rubart and Field 2006). In many instances, persistent cycling of cardiomyocytes has been associated with concurrent apoptosis, defective differentiation, and lethal heart failure; e.g., after combined deletion of Rb plus p130 (MacLellan et al. 2005), forced expression of Myc (Lee et al. 2009), or combined deletion of miR-133a-1 and miR-133a-2 (Liu et al. 2008). This might not be the obligatory consequence of cycling itself, but rather a reflection of specific genes' dual roles in growth and differentiation. Other manipulations successfully conferred proliferative growth in adult myocytes without overt dysregulation of cardiac genes; e.g., by forced expression of cyclin D2 (Pasumarthi et al. 2005; Rubart and Field 2006) or deletion of Dusp6 (Maillet et al. 2008). Hence, enhancing cardiomyocyte proliferation seems to protect the organ-level function of the heart as a biomechanical pump against the decrements of muscle cell demise.
A limitation is that most of the genetic manipulations reported to augment cardiomyocyte cycling are active in the cardiomyocyte lineage prior to terminal differentiation and normal cell cycle exit. Few studies have attempted cell cycle activation in normal adult myocytes within the intact adult heart, and even fewer have done so reversibly. Moreover, it is important to distinguish the activation of DNA synthesis from karyokinesis and cytokinesis and evaluate the adverse effects on apoptosis. In one report, conditional activation of Myc evoked DNA synthesis with endoreduplication, not proliferation (Xiao et al. 2001). In a second, viral delivery of E2F-1 to myocardium caused extensive apoptosis (Agah et al. 1997), which might be surmountable by the use of E2F-2 instead (Ebelt et al. 2008). Overall, the evidence of translational promise is still scant, notwithstanding the inherent value of experiments to unmask the genetic mechanisms underlying cardiac growth arrest.
Alternative approaches to inducing cycling in adult cardiomyocytes make use of defined mitogens, pharmacological manipulations, or their combination, despite the expected refractory state of the cells. As an example, the Notch pathway can drive cell cycle re-entry by neonatal ventricular myocytes and prolong the proliferation of cardiomyocytes derived from mouse embryonic stem cells (ESCs) (Campa et al. 2008; Collesi et al. 2008). In contrast, in older, quiescent ventricular myocytes even 5 d after birth, Notch signaling activates DNA damage checkpoint kinases and causes G2/M arrest, attributed to abnormal DNA synthesis in S phase (Campa et al. 2008). Signals that provoke cycling of adult cardiomyocytes, even in vivo, include FGF1 plus a p38 MAP kinase inhibitor (Engel et al. 2006), periostin (Kuhn et al. 2007), and the epidermal growth factor relative neuregulin-1 (NRG1) (Bersell et al. 2009). The incidence of cycling cells in these studies is lower (just a few percent or less) than for the genetic methods mentioned, and the incidence of dividing cells is even lower. Nonetheless, the number of new muscle cells accruing over time might account for the beneficial effects of periostin and NRG1 seen in rodent models of myocardial infarction (Liu et al. 2006; Kuhn et al. 2007). In another study (Bersell et al. 2009), only mononuclear myocytes were found replicating, suggesting that replicative competence is lost as myocytes become multinuclear. Unfortunately, the aging human heart has few remaining mononuclear cardiomyocytes, and infarction exacerbates this decline (Olivetti et al. 1995; Herget et al. 1997); thus, the desirable pool of cycling-competent cells might be diminished in the neediest individuals.
Heart induction
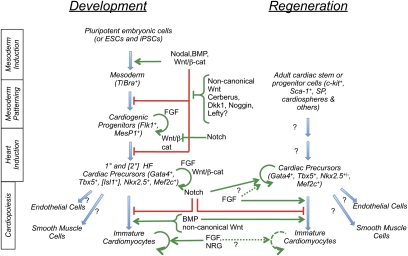
The alternative to stimulating regeneration from pre-existing differentiated cells is to stimulate the production of new ones from stem or progenitor cells of either endogenous or exogenous sources. Strategies to mobilize endogenous stem or progenitor cells include increasing the size of the stem/progenitor pool and enhancing the efficiency of differentiation. Exogenous sources include human ESCs (hESCs) and induced pluripotent stem cells (hiPSCs), the latter offering immunocompatible replacement, but both raising issues of delivery modality, persistence after transplantation, integration into the patient's heart, and tumorigenicity of pluripotent cells. Regardless of the starting cell type, directing efficient myocardial differentiation has been a major research goal and is broadly based on activating developmental programs (Fig. 2; Noseda et al. 2011).
Figure 2.
Development and regeneration. Extracellular signaling molecules positively (green) and negatively (red) control mesoderm induction to cardiopoietic differentiation in the embryo. Adult cardiac precursors share genetic markers with their developmental counterparts, and emerging data indicate that developmental signals also control regeneration, suggesting parallels between development and the control of adult cardiomyocyte renewal.
Within 5–7 d after fertilization, depending on the species, mammalian embryos undergo gastrulation, during which future mesoderm and definitive endoderm cells delaminate from the ectoderm along a furrow—the primitive streak—to form distinct germ layers. Not only are the germ layers (ectoderm, mesoderm, and endoderm) established, but cell fates are also laid down in a pattern that presages organotypic differentiation. Wnts and the TGFβ family member Nodal play evolutionarily conserved roles in inducing mesoderm and endoderm; thus, they are important to promote cardiogenic differentiation and, for this reason, are widely used to initiate cardiogenesis in ESC cultures (for example, see Xu et al. 2006; Yang et al. 2008). Interestingly, these factors are inhibitory as gastrulation ensues. Nodal has a graded influence at this time, such that high levels induce definitive endoderm and anterior mesoderm, including cardiac mesoderm, while lower levels favor more lateral and posterior derivatives; thus, specification of cardiogenic cells requires a particular dose and temporal window (for example, see Brennan et al. 2001). Similarly biphasic and concentration effects exist for BMPs and Wnts. Wnts promote cardiogenesis during mesoderm induction, but inhibit it subsequently as progenitors become committed to the cardiac lineage (Naito et al. 2006; Kwon et al. 2007). Although inductive earlier (Behfar et al. 2002), BMP2 and BMP4 inhibit cardiogenesis during mesodermal patterning, whereas reduced BMP enhances neural ectoderm and dorsal (including cardiogenic) mesoderm (Yuasa et al. 2005; Hao et al. 2008). Importantly, BMPs, Wnts, and Nodal inhibit when uncommitted Flk1+/MesP1+ progenitors adopt a cardiac fate, but at least BMPs are needed subsequently for differentiation of committed precursors (Schultheiss et al. 1997). In short, exposure to these factors must be tightly regulated.
In this context, extracellular inhibitors of BMPs, Wnts, and Nodal are the principal inducers that commit multipotent progenitors (Flk1+/MesP1+) to a cardiac fate, as marked by heart-forming transcription factors Nkx2.5, Isl1, Tbx1, Tbx5, and Mef2c. Natural inhibitors Dickkopf-1 (Dkk1), which blocks canonical Wnt signaling; Noggin, which blocks BMP; and Cerberus-1 (Cer1), which blocks both BMP and Nodal, are evolutionarily conserved inducers of cardiac mesoderm (for example, see Foley and Mercola 2005; Yuasa et al. 2005). Dkk1 and other cytokines have been incorporated into an efficient protocol for directing hESCs to recapitulate embryonic mesoderm induction and cardiogenesis (Yang et al. 2008). Based on data in ESCs, Cer1 permits cardiomyocyte differentiation of Flk1+/MesP1+ progenitors by overcoming the inhibitory effect of Nodal and BMP (M Mercola and MD Schneider, unpubl.). Nodal, BMP, and Wnt are broadly expressed in the embryo and do not block differentiation of vascular endothelial and smooth muscle cells, which arise from the same progenitor pool; thus, Cer1 and Dkk1 (and possibly other antagonists) effectively distinguish areas of potency for heart versus systemic vasculature.
Once the heart field is established, proliferation and fate of competent cardiac progenitors are controlled by signals such as FGFs (see below) and Notch. Notch is particularly fascinating because it acts at different times in nearly all tissues within the developing cardiovascular system (High and Epstein 2008). During specification of cardiogenic cells, Notch (through RBPJ) suppresses cardiogenesis, as seen in ESC cultures and Xenopus embryos (Rones et al. 2000; Schroeder et al. 2003, 2006). Conversely, deleting Notch1 using Cre driven by Isl-1 led to an expanded number of committed Nkx2.5+ cardiac precursors (Kwon et al. 2009). In this case, the knockout increased the level of transcriptionally active β-catenin in the cells, suggesting that Notch might normally attenuate the proliferative effect of canonical Wnt signaling. Later-acting knockouts and targeted misexpression reveal an additional role for Notch in supporting proliferation and trabeculation in the ventricular wall, in part by controlling production of Neuregulin, EphrinB2, and Bmp10 in the endocardium (Watanabe et al. 2006; Grego-Bessa et al. 2007). Since Notch can sustain proliferation of progenitor cells while blocking their differentiation, it is not surprising that the absence of Notch has been linked to precocious differentiation and exhaustion of resident progenitors or precursors, such as skeletal muscle satellite cells (Conboy and Rando 2002) and epithelial precursors of the developing pancreas (Jensen et al. 2000). In summary, Notch plays an important gatekeeper role that is the conceptual basis for efforts to manipulate Notch signaling to expand the cardiopoietic progenitor pool in the adult (see below).
Not all cardiac progenitors develop identically. Seminal cell lineage-tracing studies (de la Cruz et al. 1977) in the developing chick showed that the linear heart tube gives rise to the atria and left ventricle, while the outflow tract and right ventricle are added from a different source as the heart tube loops (for review, see Dyer and Kirby 2009). It is now appreciated that these cardiac structures in avians and mammals arise from two distinct fields in the lateral mesoderm. The first heart field gives rise to the linear heart tube, whereas the second heart field lies just caudal and medial and forms the outflow tract and right ventricle (Kelly et al. 2001; Mjaatvedt et al. 2001; Waldo et al. 2001). Since the first and second heart fields lie contiguously along their entire length (Abu-Issa and Kirby 2008), it is likely that the molecules discussed above induce both fields at the same time. Even if specified simultaneously, differentiation of the second field is delayed relative to the first, so that its cells are added later, when the heart has begun to loop (Meilhac et al. 2003, 2004; Prall et al. 2007). What is responsible for this delay? At least Wnt/β-catenin signaling maintains second heart field cells in a proliferative state until they are needed to build the right ventricle and outflow tract (Ai et al. 2007; Cohen et al. 2007; Klaus et al. 2007; Lin et al. 2007). Given that a source of Wnts at this time is the neural plate (e.g., Tzahor and Lassar 2001), the medially located second heart field cells would be expected to be exposed to higher and sustained Wnt levels relative to their more lateral first heart field counterparts. The spatial organization also suggests that a shift in the balance of Wnts, BMPs, and Nodals to their inhibitors during evolution of lower vertebrates might have titrated signaling sufficiently to expand the domain of cardiac potency to create the second heart field, and that this enabled the development of paired atria and ventricles needed for anatomically separated pulmonary and systemic blood flow.
Directed differentiation of myocardial precursors
Once progenitors become cardiac-committed, additional signals support their proliferation and terminal differentiation into the cardiac lineages. Although inhibitory during gastrula-stage patterning, BMPs potently promote differentiation of committed precursors in first and second heart field explants (Schultheiss et al. 1997; Monzen et al. 1999; Schlange et al. 2000; Shi et al. 2000; Waldo et al. 2001). The prodifferentiation effect of BMPs contrasts with the effects of FGFs, which have long been implicated in the survival and proliferation of cardiac precursors, although proliferation and differentiation are not necessarily mutually exclusive and can be hard to distinguish experimentally (Sugi et al. 1993; Sugi and Lough 1995; Alsan and Schultheiss 2002). For example, FGFs in the epicardium control myocardial proliferation (Lavine et al. 2005; Merki et al. 2005), while FGF8 appears to be a critical pharyngeal endodermal factor needed for the proliferation of second heart field progenitors and, consequently, alignment of the outflow tract with the ventricles (Ilagan et al. 2006; Park et al. 2006). Moreover, reduced FGF8 and FGF10 expression in mice lacking Tbx-1 suggests that attenuated proliferation is the disease mechanism for diGeorge syndrome in humans (Vitelli et al. 2002). A recent informatics analysis proposes a model in which the homeodomain transcription factor Msx1 operates at the fulcrum between differentiation and proliferation, since the data show that BMPs induce Msx1 to suppress FGF expression, thus attenuating proliferation and promoting differentiation (Tirosh-Finkel et al. 2010).
Emerging data have elucidated FGFs, Sonic Hedgehog (Shh), NRG1, and other factors as mediators of the effects of epicardium and endocardium that support proliferation and differentiation of cardiomyocytes, as well as enhance the acquisition of mature physiological properties (Manner et al. 2001; Limana et al. 2010). The epicardium has received particular attention in the past few years, since emerging data suggest that it is not only a rich source of diffusible factors during development, but it might retain beneficial paracrine functions in the adult that are enhanced after injury, or even be a source of cardiopoietic cells capable of differentiation into vascular cells or cardiomyocytes (Limana et al. 2010).
Cardiopoietic signals for postnatal heart-forming cells
Numerous types of adult progenitor and stem cells have been deployed for cardiac repair, and the scope of clinical trials in humans now entails >30 published studies and 1000 enrolled patients (Wollert and Drexler 2010). The very first cells tried were skeletal myoblasts, which proved ineffective, and subsequent trials focused on bone marrow cells and their circulating derivatives (Dimmeler et al. 2005). For these, the results have been highly reproducible despite differing methods of cell purification, preparation, and delivery, with two or more years of clinical follow-up now available in some cases (Assmus et al. 2010). However the quantitative benefit to the heart's function as a biomechanical pump is small, at the margin of clinicians' ability to measure with accuracy. Why so meager? One explanation is that early exuberant claims of bone marrow's trans-differentiation into cardiac muscle have been subject to intense dispute, regardless of whether the injected population is c-kit+ cells or mesenchymal stem cells (MSCs) (Reinecke et al. 2008). An equally damning challenge to the trans-differentiation hypothesis is the lack of persistence of injected cells within recipient hearts, even though functional improvements can be seen weeks or months after injection (Wollert and Drexler 2010). These counterarguments undercut the hypothetical need for trans-differentiation and have refocused attention on other mechanisms, principally cell-nonautonomous effects mediated by even a short-lived presence in the treated hearts, including benefits to host cell survival, wound healing, angiogenesis, and activation of newly discovered endogenous cardiac stem or progenitor cells.
The revelation that cardiac progenitor or stem cells exist in the postnatal mammalian myocardium was unanticipated, given the very small extent of verifiable regeneration. However, the finding of such cells, now reproduced widely, constitutes one of the major breakthroughs of the past decade, and suggests that mobilizing these cells could be a novel avenue to augmenting heart repair. No consensus view has emerged on markers that precisely define the cells, nor on their capacity for self-renewal and potential for forming cardiomyocytes versus other lineages (e.g., endothelial or smooth muscle cells). Hence, here we refer to them as stem or progenitor cells. Part of the challenge has been that purity and potency are hard to gauge, since differentiation is inefficient in vitro and is difficult to quantify in vivo. Nonetheless, the populations express an overlapping set of cardiogenic markers—including GATA4, NKX2.5, TBX5, and MEF2c—regardless of the markers used for their enrichment: c-kit (Beltrami et al. 2003; Bearzi et al. 2007), stem cell antigen-1 (Sca-1) (Oh et al. 2003; Matsuura et al. 2009), an unknown human epitope that cross reacts with anti-Sca1 (Goumans et al. 2007), the side population (SP) dye efflux phenotype (Martin et al. 2004; Pfister et al. 2008), residual expression of the transcription factor gene Isl1 (Laugwitz et al. 2005; Domian et al. 2009), epicardial-derived progenitor cells (Limana et al. 2007), or growth in tissue culture as “cardiospheres” analogous to the neurospheres produced by neural stem cells (Messina et al. 2004; Smith et al. 2007).
Like Janus, the two-faced Roman god, adult cardiac progenitor cells possess two aspects, having a variety of stem cell markers (e.g., MSC markers and telomerase, in addition to features already mentioned) but being unambiguously distinguished from bone marrow cells by expressing many heart-forming transcription factors (including NKX2.5, MEF2C, and GATA4) even in their basal undifferentiated state. Arguably, therefore, cardiac progenitor/stem cells resemble a forme fruste of cardiac mesoderm, an intermediate that exists transiently in embryos and ESC-derived embryoid bodies.
From a developmental perspective, the existence of such cells poses several paradoxes. If cardiac transcription factors of known importance are already present, why aren't their target genes turned on? And, given that little is known of the normal developmental circuits that control this final transition into the cardiac muscle fate, what molecules or genes might be tested most fruitfully to trigger this transition for therapeutic purposes, by cell activation ex vivo or within the diseased heart muscle itself?
To date, in comparison with the wealth of information concerning directed differentiation of mouse ESCs and hESCs, there has been a paucity of mechanistic studies on cardiac lineage decisions by cells from the adult bone marrow, circulation, or heart (Fig. 2). As one early sign that developmental signals of cardiogenesis might be reused in an adult context, an essential role was shown for the BMP receptor Alk3 in mouse cardiac Sca-1+ cells, as differentiation in culture is abolished by Cre-mediated deletion of the floxed receptor gene (Oh et al. 2003). Sca-1+ cells also require FGF2, but, in contrast to predictions from developmental and ESC biology, FGF2 in adult progenitor cells is predominantly in differentiation, not in pool expansion (Rosenblatt-Velin et al. 2005), suggesting that similar factors may play differential roles in different contexts.
Potential involvement of a third pathway, Notch, in adult cardiac progenitor cells has also been suggested. The receptor Notch1; its ligand, Jagged1; its mediator, RBPJ; and its target, Hes1 were all up-regulated during the phases of pathological heart growth and, later, heart failure in both cardiomyocytes and nonmyocytes. An in vivo function of Notch1 might be to dampen pathological remodeling after hypertensive stress, as revealed by an exacerbated response in cardiomyocyte-specific Notch1 deletion by Mlc2v-Cre (Croquelois et al. 2008). In addition, a regenerative response was suggested by the presence of activated Notch1 on cycling nonmyocytes, some of which expressed Nkx2.5. Treatment with a γ-secretase inhibitor, which suppresses the cleavage of full-length transmembrane Notch to the active intracellular form, enhanced differentiation of these noncardiomyocytes in culture. Thus, two functions are proposed for Notch: evoking a cardiomyocyte-autonomous beneficial effect on pathological remodeling, and promoting proliferation of putative progenitors with regenerative potential.
Presently, nothing has been reported of the potential role for other known positive and negative regulators of cardiac myogenesis on postnatal heart-derived cells, including Activin, Nodal, Wnts, Hedgehogs, Dkk1, Cer1, and retinoic acid. Reports have shown differentiation in response to less-defined culture conditions; for instance, 5-aza-cytidine plus TGFβ (Smits et al. 2009). 5-Aza-cytidine nonselectively activates genes due to DNA promoter hypomethylation; thus, the specific mediators are unknown, but the system is amenable to exploration and may involve autocrine BMPs (Oh et al. 2003). A number of developmentally familiar pathways have been scrutinized in adult bone marrow cells or their circulating derivatives in pursuit of means to promote cardiac muscle creation more efficiently than by just grafting noncardiac cells in their native state. Cardiac differentiation was triggered in human circulating progenitor cells by coculture with rat cardiomyocytes, was associated with Notch activation, and was blocked by interfering with Notch signals (Koyanagi et al. 2007). In endothelial progenitor cells (EPCs), the β-catenin-independent Wnt5a and Wnt11 induce cardiac differentiation (Koyanagi et al. 2005), whereas human circulating mesoangioblasts already express cardiogenic transcription factors and progress to cardiac differentiation if treated instead with a β-catenin-dependent Wnt, Wnt3a (Koyanagi et al. 2009).
A complex cocktail comprising Activin A, BMP4, FGF2, insulin-like growth factor-1 (IFG-1), interleukin-6 (IL-6), retinoic acid, α-thrombin, and TGFβ1, guided by prior work in ESCs, was found to direct human MSCs to cardiopoiesis (Behfar et al. 2007, 2010). Interestingly, the combination of the three TGFβ family members plus retinoic acid could induce NKX2.5 and MEF2C as stable proteins, but the transcription factors remained cytosolic unless IGF-1 and FGF2 were added. This combination, in turn, was growth-arrested, necessitating the addition of α-thrombin and IL-6 to promote proliferation. The ultimate combination induced sarcomeric proteins (α-actinin and cardiac troponin I), mitochondrial maturation, and rhythmic calcium transients in response to electrical pacing.
Forward reprogramming
The discovery that a minimal number of transcription factors can reprogram somatic cells to iPSCs, which resemble ESCs in terms of totipotency, toppled the view that development can only proceed unidirectionally, and suggest that it might be possible to convert noncardiomyocytes directly into cardiomyocytes. Takeuchi and Bruneau (2009) found that GATA4 and a BAF (BRG/brm-associated factor) chromatin remodeling protein, BAF60c, can initiate ectopic cardiogenic differentiation in mouse embryos. BAF60c functioned better than did other BAF complex proteins in permitting GATA4 to bind cardiac genes, suggesting that the cardiac-selective BAF60c might recognize chromatin of cardiac genes to enhance target selectivity, resembling the proposed mechanism of BAF complex subunits in directing lineage-specific transcription (Wu et al. 2009). Inclusion of Tbx5 in the transfection cocktail caused further differentiation and formation of ectopic-beating foci, suggesting that GATA4 and TBX5 plus BAF60c can bootstrap cardiogenic differentiation, at least in competent progenitors. Accordingly, GATA4, TBX5, and MEF2c were found to induce cardiomyocyte-like cells from postnatal cardiac and dermal fibroblasts (Ieda et al. 2010), resembling successes in reprogramming of pancreatic exocrine into β cells and fibroblasts into hematopoietic progenitors (Zhou et al. 2008; Szabo et al. 2010). Since induced cardiomyocytes directly reprogrammed in vitro or in vivo might be applicable for regenerative purposes (Fig. 1), it will be of great interest to learn whether key physiological properties are faithfully reproduced after reprogramming by these or other factors, especially since Ieda et al. (2010) found substantial differences between the gene profiles of induced and native cardiomyocytes, and since vestiges of the starting cell type and/or persistence of reprogramming factor expression might compromise differentiated cell function. Nonetheless, these pioneering studies provide the basis for exploring the therapeutic potential of reprogramming for heart disease.
Challenges and strategies for implementing a stem cell therapy
A key concern in implementing a stem cell transplantation therapy for heart disease is the selection of the cell, in particular its developmental stage, since this will determine safety and efficacy after engraftment. Segers and Lee (2008) argued that additional preclinical research is needed to understand whether and/or how each cell type affects cardiac performance as a function of cardiac pathologies. In addition, new methods for delivery and enhancing functional engraftment are needed, since most studies to date cite poor persistence of cells after engraftment, with the physical movement of the ventricular wall and the inflammatory environment of infarcted myocardium as potential factors in low retention rates (Laflamme and Murry 2005; Segers and Lee 2008). Tissue engineering of grafts to support appropriate structural and electromechanical integration of myocytes with host tissue and development of vasculature seems essential to achieve long-term benefits from a transplantation approach. Although beyond the scope of this review, notable advances in engineering of extracellular matrices, including some that can be injected via catheter, suggest that development of materials should improve the survival and integration of transplanted cells so that they can contribute to functional heart muscle (Davis et al. 2005; Chen et al. 2008).
A pharmacological approach shares many of the same questions, such as what cell type to target and whether stimulating proliferation or differentiation of progenitors and immature myocytes will be best to regenerate functional myocardium. Ultimately, a combination of pharmacological approaches, tissue engineering, and cell transplantation might yield the most promising therapies.
Conclusion and prospects
If adult cardiac stem cells reside in mammalian hearts, including human ones, with a convincingly proven ability to adopt the cardiac muscle fate, the question arises: Why is cardiac injury so devastating? Why don't cardiac stem cells work better than they do? The answers are speculative but informative in the context of this review. An evolutionary perspective might emphasize that no selection pressure occurred for most of human existence to rebuild the myocardium after myocardial infarction, largely a recent disorder of affluent societies in which the causes of earlier demise have been obviated. A numerical perspective might emphasize the rarity, for instance, of SP cells, which comprise just 300 cells per million in adult mouse hearts (Oh et al. 2003), and the progenitor cells' greater abundance in the atria than the left ventricle (Itzhaki-Alfia et al. 2009), which is by far the most relevant chamber for ischemic injury. A topographic perspective might question the distance over which the relevant recruitment signals can operate within the heart, or seek to define inhibitory signals in the cardiac stem cells' microenvironment (cf. Lymperi et al. 2010; Voog and Jones 2010).
The expedient question is how to achieve therapeutic regeneration. Given the utility of applying developmental insights, additional research is needed to fill the gaps in myocardial development that are most pertinent to stimulating proliferation and enhancing differentiation of adult cardiac stem and progenitor cells. Much is known about creating cardiogenic progenitors during development, but less is known about the signals that subsequently convert committed cells into cardiomyocytes themselves, and nearly nothing is known about mechanisms that drive myocyte maturation, including acquisition of the adult electrical and physical properties characteristic of the heart as a biomechanical pump, although interactions with nonmyocytes are important clues to the latter (Narmoneva et al. 2004; Kim et al. 2010). Accelerated progress will undoubtedly come from applying high-throughput technologies to the comprehensive screening of signaling molecules and pathways for cardiogenic activity using hESC- and hiPSC-derived progenitors, and even using isolated progenitors from adult heart, followed by application of these novel signals and pathways to restoring heart function. Unbiased genetic and chemical biology approaches might expose restorative mechanisms for creating cardiomyocytes from adult progenitor cells or even reverting adult cardiomyocytes to a primitive phenotype that is competent for cycling, suggested by lineage analysis of cardiosphere outgrowths (Zhang et al. 2010). Hence, an auspicious approach to this problem incorporates adult progenitors, and even terminally differentiated cells, in large-scale screens of chemical, siRNA, and microRNA libraries to identify critical signaling pathways and druggable targets that control regenerative processes. Beyond merely improving cardiomyocyte production ex vivo as research reagents and potential products for therapeutic grafting, the ultimate goal is the development of pharmacological interventions that enhance the heart's own regenerative response.
Acknowledgments
M.M. acknowledges support from NIH (R37HL059502, R33HL088266, R01HL083463, and P01HL098053), the California Institute for Regenerative Medicine (RC1-000132), Mathers Charitable Foundation, and the Sanford Children's Health Center, Sanford-Burnham Medical Research Institute. P.R.-L. acknowledges NIH R01HL065484, R01HL086879, and P01HL098053. M.D.S. is supported by the British Heart Foundation Simon Marks Chair in Regenerative Cardiology, the British Heart Foundation Centre of Research Excellence, the EU FP7 CardioCell Integrated Project, a European Research Council Advanced Investigator Grant, the Fondation Leducq Transatlantic Network of Excellence for Cardiac Regeneration, and a Medical Research Council-British Heart Foundation Cardiovascular Stem Cell Research Strategic Development Grant.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.2018411.
References
- Abu-Issa R, Kirby ML 2008. Patterning of the heart field in the chick. Dev Biol 319: 223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agah R, Kirshenbaum LA, Abdellatif M, Truong LD, Chakraborty S, Michael LH, Schneider MD 1997. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest 100: 2722–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF 2007. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci 104: 9319–9324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsan BH, Schultheiss TM 2002. Regulation of avian cardiogenesis by Fgf8 signaling. Development 129: 1935–1943 [DOI] [PubMed] [Google Scholar]
- Assmus B, Rolf A, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Tillmanns H, Yu J, Corti R, Mathey DG, et al. 2010. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail 3: 89–96 [DOI] [PubMed] [Google Scholar]
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, et al. 2007. Human cardiac stem cells. Proc Natl Acad Sci 104: 14068–14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M 2002. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J 16: 1558–1566 [DOI] [PubMed] [Google Scholar]
- Behfar A, Perez-Terzic C, Faustino RS, Arrell DK, Hodgson DM, Yamada S, Puceat M, Niederlander N, Alekseev AE, Zingman LV, et al. 2007. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med 204: 405–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A 2010. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol 56: 721–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. 2003. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 763–776 [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, et al. 2009. Evidence for cardiomyocyte renewal in humans. Science 324: 98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersell K, Arab S, Haring B, Kuhn B 2009. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138: 257–270 [DOI] [PubMed] [Google Scholar]
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ 2001. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411: 965–969 [DOI] [PubMed] [Google Scholar]
- Campa VM, Gutierrez-Lanza R, Cerignoli F, Diaz-Trelles R, Nelson B, Tsuji T, Barcova M, Jiang W, Mercola M 2008. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J Cell Biol 183: 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QZ, Harding SE, Ali NN, Lyon AR, Boccaccini AR 2008. Biomaterials in cardiac tissue engineering: Ten years of research survey. Mat Sci Eng R 59: 1–37 doi: 10.1016/j.mser.2007.08.001 [Google Scholar]
- Chen Y, Lewis W, Diwan A, Cheng EH, Matkovich SJ, Dorn GW II 2010. Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc Natl Acad Sci 107: 9035–9042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE 2007. Wnt/β-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest 117: 1794–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collesi C, Zentilin L, Sinagra G, Giacca M 2008. Notch1 signaling stimulates proliferation of immature cardiomyocytes. J Cell Biol 183: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Rando TA 2002. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell 3: 397–409 [DOI] [PubMed] [Google Scholar]
- Croquelois A, Domenighetti AA, Nemir M, Lepore M, Rosenblatt-Velin N, Radtke F, Pedrazzini T 2008. Control of the adaptive response of the heart to stress via the Notch1 receptor pathway. J Exp Med 205: 3173–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT 2005. Custom design of the cardiac microenvironment with biomaterials. Circ Res 97: 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz MV, Sanchez Gomez C, Arteaga MM, Arguello C 1977. Experimental study of the development of the truncus and the conus in the chick embryo. J Anat 123: 661–686 [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM, Schneider MD 2005. Unchain my heart: The scientific foundations of cardiac repair. J Clin Invest 115: 572–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan A, Wansapura J, Syed FM, Matkovich SJ, Lorenz JN, Dorn GW 2nd 2008. Nix-mediated apoptosis links myocardial fibrosis, cardiac remodeling, and hypertrophy decompensation. Circulation 117: 396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, Shao Y, Wu SM, Parker KK, Chien KR 2009. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science 326: 426–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer LA, Kirby ML 2009. The role of secondary heart field in cardiac development. Dev Biol 336: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebelt H, Zhang Y, Kampke A, Xu J, Schlitt A, Buerke M, Muller-Werdan U, Werdan K, Braun T 2008. E2F2 expression induces proliferation of terminally differentiated cardiomyocytes in vivo. Cardiovasc Res 80: 219–226 [DOI] [PubMed] [Google Scholar]
- Engel FB, Hsieh PC, Lee RT, Keating MT 2006. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci 103: 15546–15551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley AC, Mercola M 2005. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev 19: 387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans MJ, de Boer TP, Smits AM, van Laake LW, van Vliet P, Metz CH, Korfage TH, Kats KP, Hochstenbach R, Pasterkamp G, et al. 2007. TGF-β1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res 1: 138–149 [DOI] [PubMed] [Google Scholar]
- Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, et al. 2007. Notch signaling is essential for ventricular chamber development. Dev Cell 12: 415–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC 2008. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS ONE 3: e2904 doi: 10.1371/journal.pone.0002904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, et al. 2005. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med 11: 305–311 [DOI] [PubMed] [Google Scholar]
- Herget GW, Neuburger M, Plagwitz R, Adler CP 1997. DNA content, ploidy level and number of nuclei in the human heart after myocardial infarction. Cardiovasc Res 36: 45–51 [DOI] [PubMed] [Google Scholar]
- High FA, Epstein JA 2008. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet 9: 49–61 [DOI] [PubMed] [Google Scholar]
- Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT 2007. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med 13: 970–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D 2010. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142: 375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN 2006. Fgf8 is required for anterior heart field development. Development 133: 2435–2445 [DOI] [PubMed] [Google Scholar]
- Itzhaki-Alfia A, Leor J, Raanani E, Sternik L, Spiegelstein D, Netser S, Holbova R, Pevsner-Fischer M, Lavee J, Barbash IM 2009. Patient characteristics and cell source determine the number of isolated human cardiac progenitor cells. Circulation 120: 2559–2566 [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD 2000. Control of endodermal endocrine development by Hes-1. Nat Genet 24: 36–44 [DOI] [PubMed] [Google Scholar]
- Jopling C, Sleep E, Raya M, Marti M, Raya A, Belmonte JC 2010. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464: 606–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogorek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, et al. 2010. Cardiomyogenesis in the adult human heart. Circ Res 107: 305–315 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kelly RG, Brown NA, Buckingham ME 2001. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell 1: 435–440 [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD 2010. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature 464: 601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Majdi M, Xia P, Wei KA, Talantova M, Spiering S, Nelson B, Mercola M, Chen HS 2010. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev 19: 783–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W 2007. Distinct roles of Wnt/β-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci 104: 18531–18536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M., Haendeler J., Badorff C., Brandes RP, Hoffmann J., Pandur P, Zeiher AM., Kuhl M, and Dimmeler S 2005. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J Biol Chem 280: 16838–16842 [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Bushoven P, Iwasaki M, Urbich C, Zeiher AM, Dimmeler S 2007. Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ Res 101: 1139–1145 [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Iwasaki M, Haendeler J, Leitges M, Zeiher AM, Dimmeler S 2009. Wnt5a increases cardiac gene expressions of cultured human circulating progenitor cells via a PKCδ activation. PLoS ONE 4: e5765 doi: 10.1371/journal.pone.0005765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, et al. 2009. Activation of a HIF1α-PPARγ axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab 9: 512–524 [DOI] [PubMed] [Google Scholar]
- Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT 2007. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 13: 962–969 [DOI] [PubMed] [Google Scholar]
- Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J 2010. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci 107: 15565–15570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D 2007. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci 104: 10894–10899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D 2009. A regulatory pathway involving Notch1/β-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol 11: 951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE 2005. Regenerating the heart. Nat Biotechnol 23: 845–856 [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, et al. 2005. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433: 647–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM 2005. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell 8: 85–95 [DOI] [PubMed] [Google Scholar]
- Lee HG, Chen Q, Wolfram JA, Richardson SL, Liner A, Siedlak SL, Zhu X, Ziats NP, Fujioka H, Felsher DW, et al. 2009. Cell cycle re-entry and mitochondrial defects in myc-mediated hypertrophic cardiomyopathy and heart failure. PLoS ONE 4: e7172 doi: 10.1371/journal.pone.0007172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD 2006. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127: 607–619 [DOI] [PubMed] [Google Scholar]
- Li Q, Guo Y, Ou Q, Cui C, Wu WJ, Tan W, Zhu X, Lanceta LB, Sanganalmath SK, Dawn B, et al. 2009. Gene transfer of inducible nitric oxide synthase affords cardioprotection by upregulating heme oxygenase-1 via a nuclear factor-κB-dependent pathway. Circulation 120: 1222–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limana F, Zacheo A, Mocini D, Mangoni A, Borsellino G, Diamantini A, De Mori R, Battistini L, Vigna E, Santini M, et al. 2007. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res 101: 1255–1265 [DOI] [PubMed] [Google Scholar]
- Limana F, Capogrossi MC, Germani A 2010. The epicardium in cardiac repair: From the stem cell view. Pharmacol Ther 129: 82–96 [DOI] [PubMed] [Google Scholar]
- Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, et al. 2007. β-Catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci 104: 9313–9318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, et al. 2009. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest 119: 1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gu X, Li Z, Li X, Li H, Chang J, Chen P, Jin J, Xi B, Chen D, et al. 2006. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol 48: 1438–1447 [DOI] [PubMed] [Google Scholar]
- Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN 2008. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev 22: 3242–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperi S, Ferraro F, Scadden DT 2010. The HSC niche concept has turned 31. Has our knowledge matured? Ann N Y Acad Sci 1192: 12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan WR, Schneider MD 2000. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol 62: 289–320 [DOI] [PubMed] [Google Scholar]
- MacLellan WR, Garcia A, Oh H, Frenkel P, Jordan MC, Roos KP, Schneider MD 2005. Overlapping roles of pocket proteins in the myocardium are unmasked by germ line deletion of p130 plus heart-specific deletion of Rb. Mol Cell Biol 25: 2486–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet M, Purcell NH, Sargent MA, York A, Bueno OF, and Molkentin J.D 2008. Dusp6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem 283: 31246–31255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manner J, Perez-Pomares JM, Macias D, Munoz-Chapuli R 2001. The origin, formation and developmental significance of the epicardium: A review. Cells Tissues Organs 169: 89–103 [DOI] [PubMed] [Google Scholar]
- Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ 2004. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol 265: 262–275 [DOI] [PubMed] [Google Scholar]
- Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N, et al. 2009. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest 119: 2204–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y, Kinjo K, Mulligan RC, Okano H 2004. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity 20: 87–93 [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Kelly RG, Rocancourt D, Eloy-Trinquet S, Nicolas JF, Buckingham ME 2003. A retrospective clonal analysis of the myocardium reveals two phases of clonal growth in the developing mouse heart. Development 130: 3877–3889 [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME 2004. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell 6: 685–698 [DOI] [PubMed] [Google Scholar]
- Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Belmonte JC, et al. 2005. Epicardial retinoid X receptor α is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci 102: 18455–18460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, et al. 2004. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95: 911–921 [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR 2001. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol 238: 97–109 [DOI] [PubMed] [Google Scholar]
- Monzen K, Shiojima I, Hiroi Y, Kudoh S, Oka T, Takimoto E, Hayashi D, Hosoda T, Habara-Ohkubo A, Nakaoka T, et al. 1999. Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Mol Cell Biol 19: 7096–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I 2006. Developmental stage-specific biphasic roles of Wnt/β-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci 103: 19812–19817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, et al. 2007. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest 117: 2431–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT 2004. Endothelial cells promote cardiac myocyte survival and spatial reorganization: Implications for cardiac regeneration. Circulation 110: 962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda M, Peterkin T, Simões FC, Patient R, Schneider MD 2011. Cardiopoietic factors: Extracellular signals for cardiac lineage commitment. Circ Res 108: 129–152 [DOI] [PubMed] [Google Scholar]
- Oberpriller JO, Oberpriller JC 1974. Response of the adult newt ventricle to injury. J Exp Zool 187: 249–253 [DOI] [PubMed] [Google Scholar]
- Oh H, Taffet GE, Youker KA, Entman ML, Overbeek PA, Michael LH, Schneider MD 2001. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc Natl Acad Sci 98: 10308–10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, et al. 2003. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci 100: 12313–12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P 1995. Gender differences and aging: Effects on the human heart. J Am Coll Cardiol 26: 1068–1079 [DOI] [PubMed] [Google Scholar]
- Olson EN 2006. Gene regulatory networks in the evolution and development of the heart. Science 313: 1922–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Schneider MD 2003. Sizing up the heart: Development redux in disease. Genes Dev 17: 1937–1956 [DOI] [PubMed] [Google Scholar]
- Oppedal D, Goldsmith MI 2010. A chemical screen to identify novel inhibitors of fin regeneration in zebrafish. Zebrafish 7: 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y, Ouchi N, Shimano M, Pimentel DR, Papanicolaou KN, Panse KD, Tsuchida K, Lara-Pezzi E, Lee SJ, Walsh K 2009. Activin A and follistatin-like 3 determine the susceptibility of heart to ischemic injury. Circulation 120: 1606–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM 2006. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development 133: 2419–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ 2005. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res 96: 110–118 [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B 2005. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122: 927–939 [DOI] [PubMed] [Google Scholar]
- Pfister O, Oikonomopoulos A, Sereti KI, Sohn RL, Cullen D, Fine GC, Mouquet F, Westerman K, Liao R 2008. Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circ Res 103: 825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT 2002. Heart regeneration in zebrafish. Science 298: 2188–2190 [DOI] [PubMed] [Google Scholar]
- Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H, et al. 2007. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell 128: 947–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya A, Koth CM, Buscher D, Kawakami Y, Itoh T, Raya RM, Sternik G, Tsai HJ, Rodriguez-Esteban C, Izpisua-Belmonte JC 2003. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc Natl Acad Sci 100: 11889–11895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke H, Minami E, Zhu WZ, Laflamme MA 2008. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res 103: 1058–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rones MS, McLaughlin KA, Raffin M, Mercola M 2000. Serrate and Notch specify cell fates in the heart field by suppressing cardiomyogenesis. Development 127: 3865–3876 [DOI] [PubMed] [Google Scholar]
- Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T 2005. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest 115: 1724–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N, Harvey RP, ed. 2010. Heart development and regeneration. Academic Press, New York [Google Scholar]
- Rubart M, Field LJ 2006. Cardiac regeneration: Repopulating the heart. Annu Rev Physiol 68: 29–49 [DOI] [PubMed] [Google Scholar]
- Schlange T, Andree B, Arnold HH, Brand T 2000. BMP2 is required for early heart development during a distinct time period. Mech Dev 91: 259–270 [DOI] [PubMed] [Google Scholar]
- Schroeder T, Fraser ST, Ogawa M, Nishikawa S, Oka C, Bornkamm GW, Honjo T, Just U 2003. Recombination signal sequence-binding protein Jκ alters mesodermal cell fate decisions by suppressing cardiomyogenesis. Proc Natl Acad Sci 100: 4018–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T, Meier-Stiegen F, Schwanbeck R, Eilken H, Nishikawa S, Hasler R, Schreiber S, Bornkamm GW, Just U 2006. Activated Notch1 alters differentiation of embryonic stem cells into mesodermal cell lineages at multiple stages of development. Mech Dev 123: 570–579 [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Burch JB, Lassar AB 1997. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev 11: 451–462 [DOI] [PubMed] [Google Scholar]
- Segers VF, Lee RT 2008. Stem-cell therapy for cardiac disease. Nature 451: 937–942 [DOI] [PubMed] [Google Scholar]
- Shi Y, Katsev S, Cai C, Evans S 2000. BMP signaling is required for heart formation in vertebrates. Dev Biol 224: 226–237 [DOI] [PubMed] [Google Scholar]
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E 2007. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115: 896–908 [DOI] [PubMed] [Google Scholar]
- Smits AM, van Vliet P, Metz CH, Korfage T, Sluijter JP, Doevendans PA, Goumans MJ 2009. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: An in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc 4: 232–243 [DOI] [PubMed] [Google Scholar]
- Stewart S, Tsun ZY, Izpisua Belmonte JC 2009. A histone demethylase is necessary for regeneration in zebrafish. Proc Natl Acad Sci 106: 19889–19894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi Y, Lough J 1995. ActivinA and FGF-2 mimic the inductive effects of anterior endoderm on terminal cardiac myogenesis in vitro. Dev Biol 168: 567–574 [DOI] [PubMed] [Google Scholar]
- Sugi Y, Sasse J, Lough J 1993. Inhibition of precardiac mesoderm cell proliferation by antisense oligodeoxynucleotide complementary to fibroblast growth factor-2 (FGF-2). Dev Biol 157: 28–37 [DOI] [PubMed] [Google Scholar]
- Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M 2010. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 468: 521–526 [DOI] [PubMed] [Google Scholar]
- Takagi H, Hsu CP, Kajimoto K, Shao D, Yang Y, Maejima Y, Zhai P, Yehia G, Yamada C, Zablocki D, et al. 2010. Activation of PKN mediates survival of cardiac myocytes in the heart during ischemia/reperfusion. Circ Res 107: 642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi JK, Bruneau BG 2009. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature 459: 708–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh-Finkel L, Zeisel A, Brodt-Ivenshitz M, Shamai A, Yao Z, Seger R, Domany E, Tzahor E 2010. BMP-mediated inhibition of FGF signaling promotes cardiomyocyte differentiation of anterior heart field progenitors. Development 137: 2989–3000 [DOI] [PubMed] [Google Scholar]
- Toko H, Takahashi H, Kayama Y, Oka T, Minamino T, Okada S, Morimoto S, Zhan DY, Terasaki F, Anderson ME, et al. 2010. Ca2+/calmodulin-dependent kinase IIδ causes heart failure by accumulation of p53 in dilated cardiomyopathy. Circulation 122: 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, Lassar AB 2001. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev 15: 255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitelli F, Taddei I, Morishima M, Meyers EN, Lindsay EA, Baldini A 2002. A genetic link between Tbx1 and fibroblast growth factor signaling. Development 129: 4605–4611 [DOI] [PubMed] [Google Scholar]
- Voog J, Jones DL 2010. Stem cells and the niche: A dynamic duo. Cell Stem Cell 6: 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML 2001. Conotruncal myocardium arises from a secondary heart field. Development 128: 3179–3188 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kokubo H, Miyagawa-Tomita S, Endo M, Igarashi K, Aisaki K, Kanno J, Saga Y 2006. Activation of Notch1 signaling in cardiogenic mesoderm induces abnormal heart morphogenesis in mouse. Development 133: 1625–1634 [DOI] [PubMed] [Google Scholar]
- Whelan RS, Kaplinskiy V, Kitsis RN 2010. Cell death in the pathogenesis of heart disease: Mechanisms and significance. Annu Rev Physiol 72: 19–44 [DOI] [PubMed] [Google Scholar]
- Wollert KC, Drexler H 2010. Cell therapy for the treatment of coronary heart disease: A critical appraisal. Nat Rev Cardiol 7: 204–215 [DOI] [PubMed] [Google Scholar]
- Wu JI, Lessard J, Crabtree GR 2009. Understanding the words of chromatin regulation. Cell 136: 200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Mao S, Baumgarten G, Serrano J, Jordan MC, Roos KP, Fishbein MC, MacLellan WR 2001. Inducible activation of c-Myc in adult myocardium in vivo provokes cardiac myocyte hypertrophy and reactivation of DNA synthesis. Circ Res 89: 1122–1129 [DOI] [PubMed] [Google Scholar]
- Xu C, Police S, Hassanipour M, Gold JD 2006. Cardiac bodies: A novel culture method for enrichment of cardiomyocytes derived from human embryonic stem cells. Stem Cells Dev 15: 631–639 [DOI] [PubMed] [Google Scholar]
- Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, et al. 2008. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453: 524–528 [DOI] [PubMed] [Google Scholar]
- Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S-i, Shimazaki T, Okano H, et al. 2005. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol 23: 607–611 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, Malliaras K, Abraham MR, Wang C, Marban E 2010. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS ONE 5: e12559 doi: 10.1371/journal.pone.0012559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA 2008. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455: 627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]