Abstract
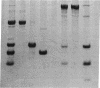
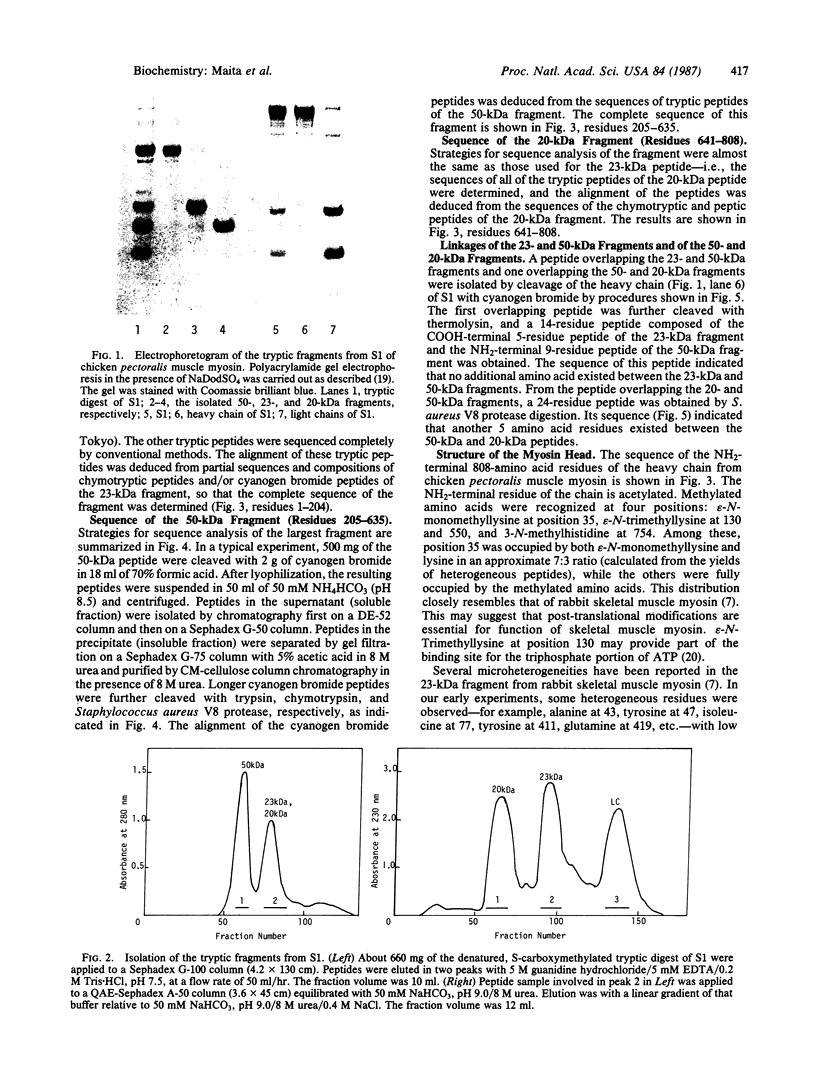
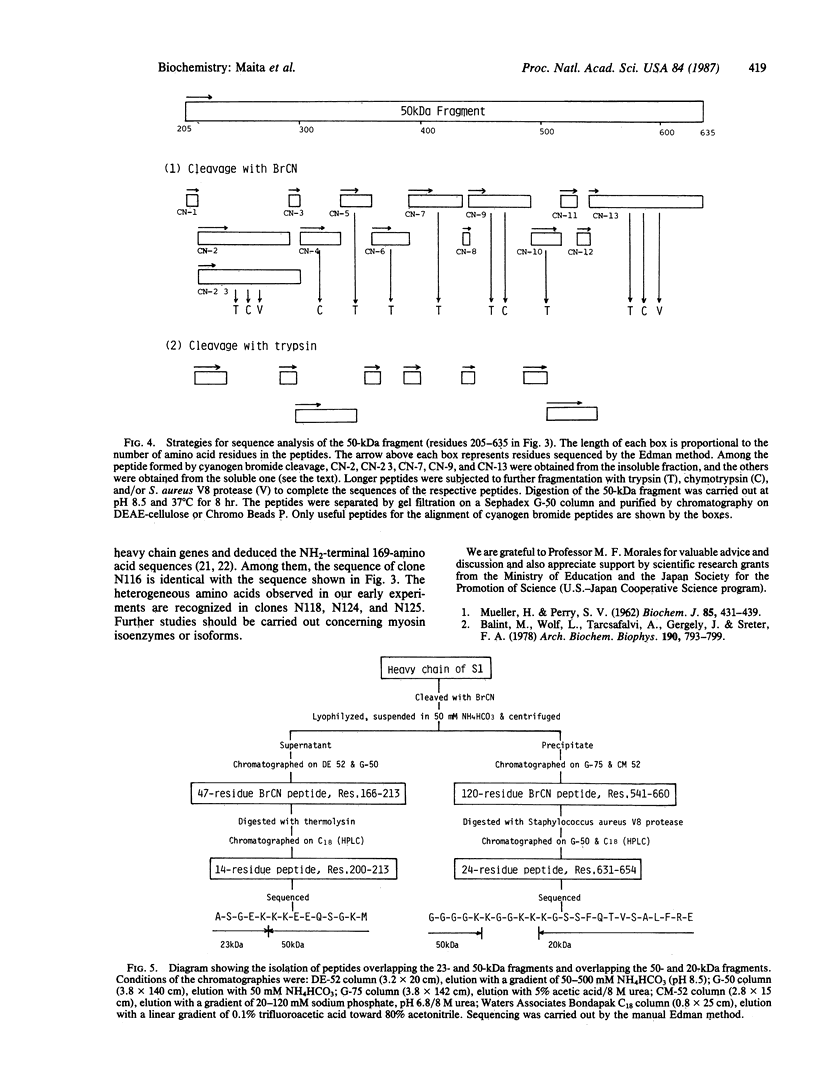
The sequence of the NH2-terminal 808 amino acid residues of chicken pectoralis muscle myosin head was determined. Three characteristic 20-, 23-, and 50-kDa fragments were isolated from a digest of myosin subfragment 1 (S1) by gel filtration on a Sephadex G-100 column in the presence of 5 M guanidine hydrochloride, followed by anion-exchange chromatography on a QAE-Sephadex A-50 column in the presence of 8 M urea. The fragments were sequenced completely by conventional methods. Peptides overlapping the 23- and 50-kDa fragments and also overlapping the 50- and 20-kDa fragments were obtained by cleaving S1 with cyanogen bromide. Comparison of the 23-kDa and 50-kDa sequences with that of the overlapping peptide indicated that no additional amino acid exists between the 23- and 50-kDa fragments and that 5 amino acids exist between the 50- and 20-kDa fragments of S1. Methylated amino acid residues were found at four positions: epsilon-N-monomethyllysine at position 35, epsilon-N-trimethyllysine residues at 130 and 550, and 3-N-methylhistidine at 754.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applegate D., Reisler E. Protease-sensitive regions in myosin subfragment 1. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7109–7112. doi: 10.1073/pnas.80.23.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bálint M., Wolf I., Tarcsafalvi A., Gergely J., Sréter F. A. Location of SH-1 and SH-2 in the heavy chain segment of heavy meromyosin. Arch Biochem Biophys. 1978 Oct;190(2):793–799. doi: 10.1016/0003-9861(78)90339-9. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Kropp K., Gulick J., Robbins J. A canonical sequence organization at the 5'-end of the myosin heavy chain genes. J Biol Chem. 1986 May 15;261(14):6613–6618. [PubMed] [Google Scholar]
- MUELLER H., PERRY S. V. The degradation of heavy meromyosin by trypsin. Biochem J. 1962 Dec;85:431–439. doi: 10.1042/bj0850431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maita T., Morokuma K., Matsuda G. Amino acid sequences of the tryptic peptides from carboxymethylated L-asparaginase from Escherichia coli. Hoppe Seylers Z Physiol Chem. 1979 Oct;360(10):1483–1495. doi: 10.1515/bchm2.1979.360.2.1483. [DOI] [PubMed] [Google Scholar]
- Maita T., Umegane T., Kato Y., Matsuda G. Amino-acid sequence of the L-1 light chain of chicken cardiac-muscle myosin. Eur J Biochem. 1980 Jun;107(2):565–575. doi: 10.1111/j.1432-1033.1980.tb06064.x. [DOI] [PubMed] [Google Scholar]
- Maita T., Umegane T., Matsuda G. Amino-acid sequence of the L-4 light chain of chicken skeletal-muscle myosin. Eur J Biochem. 1981;114(1):45–49. doi: 10.1111/j.1432-1033.1981.tb06169.x. [DOI] [PubMed] [Google Scholar]
- Matsuda G., Suzuyama Y., Maita T., Umegane T. The L-2 light chain of chicken skeletal muscle myosin. FEBS Lett. 1977 Dec 1;84(1):53–56. doi: 10.1016/0014-5793(77)81055-7. [DOI] [PubMed] [Google Scholar]
- Mornet D., Ue K., Morales M. F. Proteolysis and the domain organization of myosin subfragment 1. Proc Natl Acad Sci U S A. 1984 Feb;81(3):736–739. doi: 10.1073/pnas.81.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y., Yount R. G. Identification of an active site peptide of skeletal myosin after photoaffinity labeling with N-(4-azido-2-nitrophenyl)-2-aminoethyl diphosphate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1575–1579. doi: 10.1073/pnas.82.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Winkelmann D. A. Crystallization of myosin subfragment 1. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4378–4380. doi: 10.1073/pnas.81.14.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J., Horan T., Gulick J., Kropp K. The chicken myosin heavy chain family. J Biol Chem. 1986 May 15;261(14):6606–6612. [PubMed] [Google Scholar]
- Tong S. W., Elzinga M. The sequence of the NH2-terminal 204-residue fragment of the heavy chain of rabbit skeletal muscle myosin. J Biol Chem. 1983 Nov 10;258(21):13100–13110. [PubMed] [Google Scholar]
- Tsunasawa S., Narita K. Micro-identification of amino-terminal acetylamino acids in proteins. J Biochem. 1982 Sep;92(3):607–613. doi: 10.1093/oxfordjournals.jbchem.a133971. [DOI] [PubMed] [Google Scholar]
- Umegane T., Maita T., Matsuda G. Amino-acid sequence of the L-1 light chain of chicken fast skeletal-muscle myosin. Hoppe Seylers Z Physiol Chem. 1982 Nov;363(11):1321–1330. doi: 10.1515/bchm2.1982.363.2.1321. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Winkelmann D. A., Mekeel H., Rayment I. Packing analysis of crystalline myosin subfragment-1. Implications for the size and shape of the myosin head. J Mol Biol. 1985 Feb 20;181(4):487–501. doi: 10.1016/0022-2836(85)90422-x. [DOI] [PubMed] [Google Scholar]