Abstract
Endothelin, the most potent vasoactive peptide known to date, has been suggested to play a potential role in the pathogenesis of open-angle glaucoma. Open-angle glaucoma is the most common optic nerve head neuropathy and is associated with a loss of retinal ganglion cells and visual field damage. Although an increased intraocular pressure is a major risk factor for glaucomatous optic neuropathy, other factors such as a reduced ocular blood flow play an important role for appearance of the disease. Thus, treatment of glaucoma is focused on lowering of intraocular pressure and preventing the occurrence or progression of glaucomatous optic neuropathy. Endothelin participates in the regulation of intraocular pressure by an effect on trabecular outflow, the main route for aqueous humour outflow from the eye. Trabecular outflow is modulated by trabecular meshwork contractility which is affected by endothelin. In addition to the effects of endothelin in the anterior part of the eye, the vasoconstrictor causes a decrease in ocular blood flow followed by pathological changes in the retina and the optic nerve head which is assumed to contribute to the degeneration of retinal ganglion cells. In sum, inhibition of endothelin signalling leads to lowering of intraocular pressure and exerts neuroprotective effects. Thus, endothelin antagonism in the eye represents a promising approach for pharmacological treatment of glaucoma.
Keywords: glaucoma, endothelin, intraocular pressure, trabecular meshwork, ocular blood flow, retinal ganglion cells, prostaglandin analogues, endothelin receptor blocker
Introduction
Endothelin-1 (ET-1), the most potent vasoconstrictor known to date, is expressed in many organs and tissues and has been shown to play an important role in vascular homeostasis (Yanagisawa et al., 1988) as well as in a variety of pathological processes (Levin, 1995).
In the human eye endothelin is detectable in the posterior part, especially in the choroid, retinal blood vessels, retinal pigment epithelium and optic nerve (Wollensak et al., 1998; Narayan et al., 2004). The precise source of endothelin in the posterior segment of the eye remains unclear. One possible source is the retinal pigment epithelium, which secretes ET-1 towards the basolateral side, suggesting an involvement in the regulation of choroidal blood flow (Narayan et al., 2003, 2004; Dibas et al., 2005a). The ET-1 synthesis and release from optic nerve head astrocytes as seen in cultured human cells may contribute to the signal in the optic nerve (Desai et al., 2004).
In the anterior part of the eye, ET-1 was found in the iris, non-pigmented ciliary epithelium, ciliary muscle, as well as in the trabecular meshwork, endothelial cells lining Schlemm's canal and corneal epithelium (Wollensak et al., 1998; Fernandez-Durango et al., 2003). Apart from its localization in ocular tissues, ET-1 is present in aqueous humour. One possible source of ET-1 in aqueous humour is the non-pigmented ciliary epithelium, because cultured cells synthesize and secrete ET-1 (Lepple-Wienhues et al., 1992; Prasanna et al., 1998). The local synthesis of ET-1 suggests a physiological role in the eye; however, the impact of ET-1 in the healthy eye is not completely understood yet and therefore a subject of intense research.
There is accumulating evidence for a role of ET-1 in the pathogenesis of primary open-angle glaucoma (POAG) (Sugiyama et al., 1995b; Orgul et al., 1996a; Tezel et al., 1997; Yorio et al., 2002; Prasanna et al., 2003). POAG is the most common optic nerve head neuropathy which is associated with a loss of retinal ganglion cells (RGCs) and visual field damage. The main risk factor for causing glaucomatous damage is an elevated intraocular pressure (IOP). Although an elevated IOP is a major risk factor for glaucomatous optic neuropathy, ocular hypertension (increased IOP without glaucomatous damage) and normal tension glaucoma (glaucomatous damage despite normal IOP) indicate that other factors such as an insufficient blood supply due to either increased IOP or reduced ocular blood flow (OBF) play an important role (Flammer et al., 1999, 2002). Today, all therapies applied in glaucoma are focused on lowering of IOP, but there are strong efforts to evaluate the relevance of targeting OBF in glaucoma therapy (Orgul et al., 2005). Both IOP and OBF are affected by ET-1 (Figure 1) which is elevated in aqueous humour of glaucoma patients compared to normal subjects (Noske et al., 1997; Tezel et al., 1997; Koliakos et al., 2004). Also, in animal models of glaucoma increased ET-1 concentrations in aqueous humour were detected (Kallberg et al., 2002; Prasanna et al., 2005). In contrast, no increase in the ET-1 plasma levels were observed in patients with POAG versus controls of similar age (Tezel et al., 1997; Hollo et al., 1998; Henry et al., 2006; Kunimatsu et al., 2006). Another study reported increased plasma ET-1 levels in patients with progressive open-angle glaucoma compared to glaucoma patients with stable visual fields (Emre et al., 2005). Increased ET-1 plasma levels have been found in various diseases (Levin, 1995). In contrast to aqueous humour ET-1, increased plasma ET-1 has no effect on IOP which may be due to the blood-aqueous barrier (Karadag et al., 2009). Increased ET-1 plasma levels were found in patients with multiple sclerosis. This has no effect on brain and retinal circulation when the blood-retina barrier is intact, but the blood flow in the fenestrated choroid and therewith at the ocular nerve head is reduced (Pache et al., 2003; Flammer and Mozaffarieh, 2008). Thus, plasma ET-1 levels do not necessarily reflect local ET-1 concentration in the eye.
Figure 1.
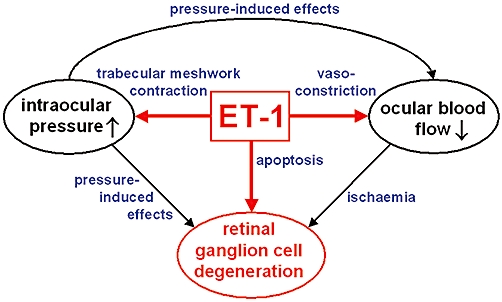
Involvement of endothelin-1 (ET-1) in the pathogenesis of glaucoma. ET-1 causes an increase in intraocular pressure, which directly and via reduced ocular blood flow leads to degeneration of retinal ganglion cells (RGCs). Furthermore, ET-1-induced vasoconstriction generates a decrease in ocular blood flow affecting RGCs. In addition, ET-1 evokes apoptosis of RGCs.
Studies on various animal models have shown that ocular application of ET-1 influences aqueous humour production and outflow (Erickson-Lamy et al., 1991; Taniguchi et al., 1994, 1996; Wiederholt et al., 1995). Several studies with different experimental approaches have established an increase in IOP caused by an elevation of ET-1 in aqueous humour (Granstam et al., 1991; Okada et al., 1995; Sugiyama et al., 1995a; Hollo et al., 2000). It was further observed that intravitreal injection of endothelin decreased retinal and optic nerve head blood flow (Granstam et al., 1992; Sugiyama et al., 2009) which causes ischemic conditions leading to injury of RGCs and pathological changes of the optic nerve head (Haefliger et al., 1993; Sugiyama et al., 1996). Reduced optic nerve blood flow caused by exogenous application of ET-1 resulted in RGC death in the absence of elevated IOP (Orgul et al., 1996b; Chauhan et al., 2004). This may be caused by ischemic conditions and also by a direct apoptotic effect of ET-1 as shown in a study of Krishnamoorthy et al. (Krishnamoorthy et al., 2008). This study revealed that ET-1 treatment directly mediates apoptosis of virally transformed rat RGCs.
Moreover, it has been shown that increased ET-1 levels in glaucoma is involved in astrocyte proliferation that occurs in glaucomatous optic neuropathy in human and also in animals with experimentally increased IOP (Hernandez, 2000). Additionally, an effect of ET-1 on the regulation of anterograde axonal transport in the optic nerve was described (Stokely et al., 2002). Possibly, ET-1 is indirectly linked to the loss of RGCs in glaucoma by these effects.
In accordance with these findings, it was demonstrated that intravitreal injection or perfusion of ET-1 into eyes of different animal models such as monkeys, rabbits and rats generate optic neuropathy similar to glaucomatous optic nerve head damage including axon loss (Cioffi et al., 1995; Orgul et al., 1996a,b; Cioffi and Sullivan, 1999; Oku et al., 1999).
In conclusion, ET-1 exerts effects on aqueous humour dynamics in the anterior chamber, on optic nerve head blood circulation, and on viability of RGCs. Consequently, the ocular effects of endothelin may provide a possible target for the pharmacological treatment of glaucoma which includes lowering of IOP and consequently the risk for glaucomatous damage and diminishing the probability of formation or progression of glaucomatous optic neuropathy. The sites and mechanisms of ET-1 antagonism described in the following are illustrated in Figure 2 and drugs with anti-endothelin effects are listed in Table 1.
Figure 2.
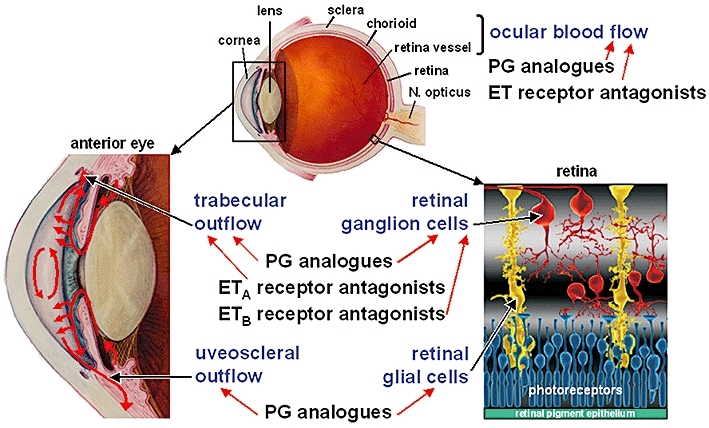
Sites and mechanisms of endothelin-1 (ET-1) antagonism in the eye. The ET-1 effects on ocular blood flow, trabecular outflow and viability of retinal ganglion cells could be inhibited by prostaglandin (PG) analogues, especially by prostaglandin F receptor agonists, and by endothelin receptor antagonists. In addition, PG analogues exert positive effects on uveoscleral outflow and retinal glial cells [retina part modified from Lu et al., 2006; Copyright (2010) National Academy of Sciences, USA].
Table 1.
Drugs with anti-endothelin effects as a possible approach for glaucoma therapy
| Human studies | Animal studies | ||||
|---|---|---|---|---|---|
| Drug classes | IOP | Blood flow | IOP | Blood flow | RGC |
| ET receptor blocker | |||||
| ETA blocker | |||||
| BQ-123 | Ø | ↑ | ↓ | ↑ | |
| ETB blocker | |||||
| BQ-788 | + | ||||
| ETA/B blocker | |||||
| Bosentan | Ø | ↑ | |||
| PG F analogues | |||||
| Travoprost | ↓ | ↑ | ↓ | ↑ | |
| Latanoprost | ↓ | ↑ | ↓ | ↑ | + |
| Bimatoprost | ↓ | ↑ | ↓ | ||
| Tafluprost | ↓ | ↓ | ↑ | + | |
| Unoprostone | ↓ | ↑ | ↓ | ↑ | + |
(↑) increase, (↓) decrease, (+) positive effect, (Ø) no effect.
ET, endothelin, IOP, intraocular pressure; PG, prostaglandin, RGC, retinal ganglion cell.
Endothelin effect on intraocular pressure
The IOP is regulated by the balance of aqueous humour production in the non-pigmented ciliary epithelium and the aqueous humour outflow. Aqueous humour leaves the eye in part via the uveoscleral and mainly via the trabecular outflow route. An increase in IOP is caused by a decrease in aqueous humour outflow from the eye. Aqueous humour has to pass the trabecular meshwork, a sponge-like tissue with contractile properties, before entering the venous system via Schlemm's canal and collector channels. Contraction of the trabecular meshwork leads to decreased aqueous humour outflow and increased IOP, whereas relaxation exerts the converse effect (Wiederholt et al., 1995, 2000).
Different effects of endothelins on IOP are described in various animal models. MacCumber et al. found a decrease in IOP after injection of ET-1 or ET-3 in rabbit eyes which was not due to an increased aqueous outflow (MacCumber et al., 1991). The authors assumed a decreased production of aqueous humour as the most likely mechanism for the IOP reduction.
In contrast, other studies on rabbit eyes presented a dose-dependent rise in IOP after intracameral injection of ET-1, ET-2 or ET-3 (Granstam et al., 1991) or a biphasic IOP response, an early transient IOP rise followed by a subsequent prolonged decrease after intravitreal injection of ET-1 (Sugiyama et al., 1995a).
In the monkey eye, intracameral injection of ET-1 increased the outflow facility which is expected to be, at least in part, mediated through an ET-1 effect on the ciliary muscle (Erickson-Lamy et al., 1991).
In the bovine eye, it was shown that ET-1 induced a reduction of the aqueous humour outflow (Wiederholt et al., 1995). This effect is caused by the ET-1-mediated contraction of trabecular meshwork (Choritz et al., 2005; Rosenthal et al., 2005; Thieme et al., 2006). According to this model, it is assumed that drugs which inhibit the ET-1-induced contraction in the trabecular meshwork are suitable for lowering IOP. The ET-1-induced contraction could be blocked by inhibition of myosin light chain kinase (MLCK) or Rho kinase (ROCK) (Rosenthal et al., 2005; Renieri et al., 2008). Studies on animal models showed that inhibition of MLCK (Tian et al., 2000; Honjo et al., 2002) or ROCK (Honjo et al., 2001; Rao et al., 2001; Tokushige et al., 2007) resulted in an increase in trabecular outflow facility and reduction in IOP.
Although MLCK and ROCK inhibitors, ML-7, M-9 or Y27632, lower the IOP, they are not suitable for glaucoma drug therapy because of side effects on accommodation and pupil size, due to an influence on the parasympathetically innervated muscles in the anterior chamber of the eye. A specific endothelin antagonism on the trabecular meshwork is described for endothelin receptor blockers and prostaglandin (PG) analogues.
Endothelin receptor antagonists
The effect of ET-1 is mediated by two G-protein-coupled receptors, endothelin receptor A (ETA receptor) and endothelin receptor B (ETB receptor). The ETA receptor predominates in vasculature smooth-muscle cells and other smooth-muscle organs whereas ETB receptor is additionally found on other tissues including brain and many epithelia [for review, see (Davenport, 2002; Pinet, 2004)]. Both receptors induce contraction when expressed in smooth muscle cells (Clozel and Gray, 1995; Lüscher and Wenzel, 1995).
For both receptors specific and mixed antagonists have been developed (Figure 3). The competitive antagonists ambrisentan and bosentan have been proved to be promising therapeutic agents.
Figure 3.
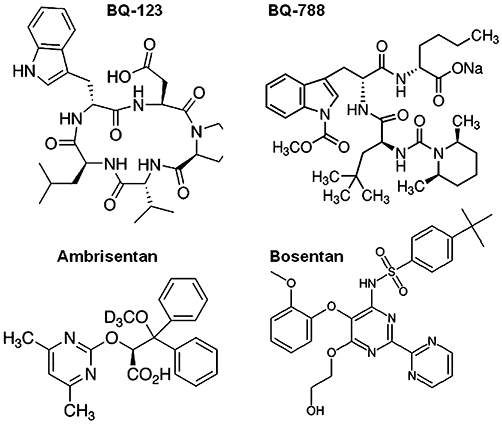
Endothelin receptor antagonists. ETA receptor antagonists: BQ-123 (peptide), ambrisentan (non-peptide); ETB receptor antagonist: BQ-788 (peptide); mixed antagonist: bosentan (non-peptide).
Most antagonists are either ETA receptor selective such as BQ-123, BQ-485 and ambrisentan, or mixed antagonists, such as bosentan. Only a limited number of ETB receptor selective antagonists such as BQ-788 have been developed so far. The endothelin receptor antagonists can be divided into two groups, peptide (BQ-123, BQ-485, BQ-788) and non-peptide antagonists (ambrisentan and bosentan). However, the peptide compounds do not penetrate the blood-brain barrier when given systemically (Benigni and Remuzzi, 1999). Hence, several non-peptide antagonists have been developed, but no non-peptide ETB receptor antagonist is available so far. Unlike peptide antagonists, many non-peptide receptor-selective antagonists have oral bioavailability and some may cross the blood-brain barrier (Benigni et al., 2004).
Endothelin receptor antagonists are approved for the management of pulmonary arterial hypertension. The mixed antagonist bosentan was the first drug of this class, now specific ETA receptor antagonists, ambrisentan and sitaxsentan, are available (Rubin and Roux, 2002; Kingman et al., 2009). The most significant adverse effects associated with the use of these drugs are major birth defects, thus they are contraindicated in pregnant women. Furthermore, a dose-dependent incidence of liver toxicity, headache, nasopharyngitis and peripheral oedema is described. These strong side effects may prevent the development of endothelin receptor antagonists for glaucoma therapy.
Effect of endothelin receptor inhibition on intraocular pressure
The ET-1 effect on IOP seems be mediated predominantly via the ETA receptor as contractility studies on the trabecular meshwork and different animal studies reveal.
Although both endothelin receptors are expressed in the trabecular meshwork, ET-1 induces contraction of this tissue predominantly by activation of ETA receptor, as shown in studies with specific endothelin receptor antagonists (Choritz et al., 2005) (Figure 4). Because this contraction contributes to the increase in IOP in glaucoma, an inhibition by specific ETA receptor antagonists seems to be a target for IOP-lowering drugs.
Figure 4.
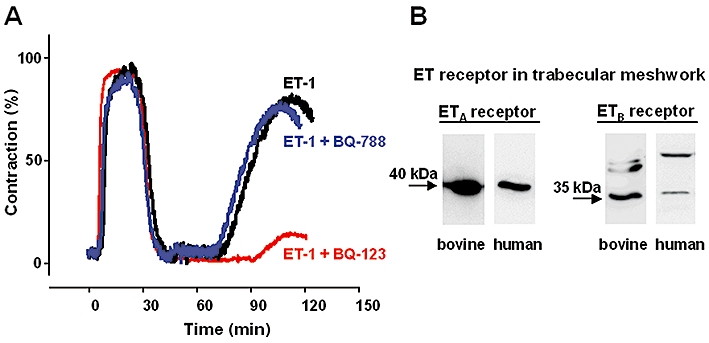
Effect of endothelin receptor blockers on endothelin-1 (ET-1)-induced contraction in trabecular meshwork. (A) Original recording of isometric force in bovine trabecular meshwork which was a well-established model for glaucoma research. After a carbachol (10−6 mol·L−1)-induced peak which was set to 100% contraction obtainable, the contraction was elicited by ET-1 (10−8 mol·L−1) or ET-1 after pre-incubation with either the ETA receptor antagonist BQ-123 or the ETB receptor antagonist BQ-788 (both 10−6 mol·L−1). The following ET-1-induced contraction in the presence of BQ-123 was strongly reduced, whereas BQ-788 has no effect. (B) Expression of endothelin receptors verified with Western blot analysis in cultured cells of bovine and human trabecular meshwork (modified from Choritz et al., 2005; Copyright S. Karger AG, Basel; Rosenthal et al., 2007).
Convincing evidence for this assumption is provided by several studies on the rabbit model. In this model, argon laser treatment of one eye causes an increase in ET-1 concentration in the aqueous humour and an increase in IOP in comparison to the contralateral eye without laser treatment (Hollo et al., 2000, 209). Pretreatment of the eye with the ETA receptor antagonist BQ-485 protected against the laser treatment-induced increase in IOP, but did not influence the laser-induced ET-1 release. This suggests that ETA receptor antagonists may prevent the effect of ET-1 on IOP (Hollo et al., 2002).
The above assumption is confirmed by another study that reveals an increased IOP after intraocular injection of the serine protease chymase, which converts big endothelin to endothelin. This chymase-induced ocular hypertension was inhibited by the ETA receptor antagonist BQ-123 (Haque et al., 1996; Konno et al., 2005).
A further study showed that intravitreally administered ET-1 induced a biphasic IOP response, an early transient IOP rise followed by a subsequent prolonged decrease (Sugiyama et al., 1995a). The transient rise in IOP was probably due to contraction of the trabecular meshwork, whereas the prolonged IOP reduction was caused by an indirect ET-1 effect. It came out that intraocular ET-1 injection activates the release of endogenous PGs into aqueous humour which in turn antagonize the ET-1 effect on IOP. The ETA receptor selective antagonists 97–139 and BQ-123 had no effect on IOP when used alone but significantly inhibited both, the IOP rise and the IOP reduction caused by ET-1. These results indicate that both, the IOP response and the elevation of PGs in aqueous humour following ET-1 injection, are at least partially mediated by ETA receptors.
Taken together, animal studies revealed that the ocular hypertensive effect of ET-1 can be blocked by intraocularly administered antagonists of the ETA receptor. In contrast, patient studies which analyse the effects of orally or intravenously applied endothelin receptor antagonists (bosentan or BQ-123) on OBF showed no effect on IOP neither in glaucoma patients nor in healthy subjects (Fuchsjager-Mayrl et al., 2003; Polak et al., 2003; Resch et al., 2009). Possibly, an intraocular administration of endothelin receptor antagonists would exert IOP-lowering effects in glaucoma patients as seen in animal models. Until now, it is not tested whether local application of ET receptor antagonists could affect the IOP and also, no endothelin receptor antagonist for topical application is available.
Effect of endothelin receptor inhibition on ocular blood flow
In addition to the endothelin receptors on the trabecular meshwork, ETA receptor and ETB receptor were detected in the vascular smooth muscle of choroidal and retinal vessels, with the former being predominant (Stitt et al., 1996). Several studies indicate that the endothelin system is involved in the processes that lead to reduced OBF in glaucoma. In rabbits intravitreal injection of ET-1 caused a decrease in OBF (Sugiyama et al., 1995b, 2009).
Also in healthy volunteers intravenous injection of ET-1 induced a decrease in optic nerve head, choroidal and retinal blood flow (Polak et al., 2001, 2003). These effects were significantly inhibited when the ETA receptor inhibitor BQ-123 was co-administered. Since application of BQ-123 alone did not affect optic nerve head, choroidal, and retinal blood flow, ET-1 does not seem to contribute substantially to the regulation of basal vascular tone in these tissues. Another study presented, that ET-1 contributes to the hyperoxia-induced retinal vasoconstriction in humans and this effect is attenuated by BQ-123 (Dallinger et al., 2000).
Additionally, administration of the ETA receptor/ETA receptor blocker bosentan increased choroidal and optic nerve head blood flow in patients with POAG and sex- and age-matched healthy volunteers. The effect of bosentan on OBF parameters was comparable between the two groups (Resch et al., 2009).
However, endothelin receptor antagonists improve OBF and may be taken into consideration as a new approach for the treatment of glaucoma.
Effect on retinal ganglion cells
Additionally to the indirect damaging effects of ET-1 on RGCs caused by an increased IOP and decreased OBF, ET-1 also exerts a direct apoptotic effect on RGCs. This effect is mediated exclusively via the ETB receptor, although both receptors are expressed in RGCs (MacCumber and D'Anna, 1994). Ocular ET-1 administration in rats has been shown to cause apoptosis of RGCs which was markedly attenuated in ETB receptor-deficient rats, suggesting a key role for ETB receptor in apoptosis of RGCs. In virally transformed rat RGCs (RGC-5 cells), ET-1 treatment produced apoptotic changes which were associated with ETB receptor activation and accompanied by a significant up-regulation of ETB receptor expression. Pretreatment of the cells with the ETB receptor antagonist BQ788 attenuated ET-1-mediated apoptosis (Krishnamoorthy et al., 2008).
Additionally, an effect of ET-1 on the regulation of anterograde axonal transport in the optic nerve was described which seems to be mediated by activation of the ETB receptor (Stokely et al., 2002). Intravitreous injection of ET-1 in rats caused a biphasic change of anterograde axonal transport, after an initial, rapid enhancement a prolonged reduction of anterograde axonal transport into the optic nerve occurs. This dysregulation of axonal transport might contribute to RGC loss. These findings indicate positive effects of ETB receptor inhibition on the survival of RGCs.
Furthermore, ET-1 might be indirectly linked to the loss of RGCs by astroglial proliferation which is associated with glaucomatous optic nerve neuropathy. ET-1 induces astroglial proliferation in cultured human optic nerve head astrocytes through ETA/B receptor activation (Prasanna et al., 2002). This effect could be blocked by a mixed receptor antagonist.
Taken together, the neuroprotective effect of mixed ET receptor antagonists or specific ETB receptor antagonists may be used in glaucoma therapy for preventing or delaying RGC loss.
Prostaglandin analogues
Today, prostaglandin F2α (PGF2α) analogues (Figure 5) are the first-line drugs in the medical treatment of glaucoma and ocular hypertension (Al-Jazzaf et al., 2003; Hylton and Robin, 2003; Perry et al., 2003; Nguyen, 2004) because these agents are the most effective drugs for lowering IOP.
Figure 5.
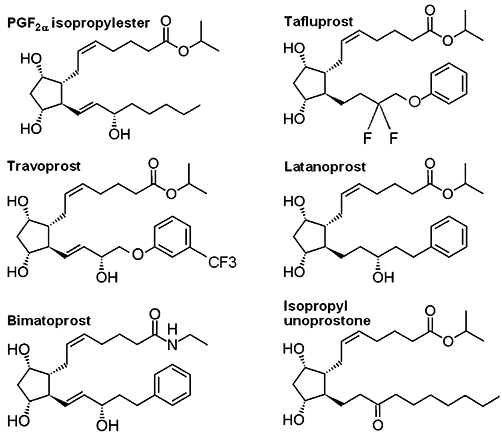
Prostaglandin F receptor agonists. Chemical structure of the prostaglandin analogues travoprost, latanoprost, and bimatoprost and the docosanoid isopropyl unoprostone which are applied in glaucoma therapy in comparison to prostaglandin F2α (PGF2α) isopropylester.
The PGF2α analogues latanoprost, travoprost and bimatoprost are approved for glaucoma therapy in the USA and Europe, tafluprost is approved in Germany since 2008 and in Switzerland since 2010. Travoprost, latanoprost, and tafluprost are ester prodrugs of PGF2α. Bimatoprost is the amide prodrug of 17-phenyl-PGF2α and has been classified as a prostamide. The docosanoid unoprostone that is often included in the group of PG analogues is approved for glaucoma therapy in Japan (unoprostone isopropyl ester, Rescula®).
Several studies performed on animal models revealed that these drugs attenuate the ET-1-induced smooth muscle contraction (Thieme et al., 2001, 2006). It is assumed that this effect contributes to the reduction of IOP and the improvement of OBF during treatment with PG analogues. Additionally, neuroprotective effects against ET-1-induced neuronal injury are described (Munemasa et al., 2008).
Effect of prostaglandin F analogues on intraocular pressure
Prostaglandin analogues provoke a strong reduction of IOP which is caused by an enhancement of both, the uveoscleral and trabecular outflow of aqueous humour. The increase of uveoscleral outflow is due to an increased matrix-metalloproteinase (MMP) production in tissues of the uveoscleral outflow pathway, such as ciliary muscle (Weinreb et al., 1997, 2002; Gaton et al., 2001) and sclera (Kim et al., 2001; Weinreb et al., 2004). Additionally, an increased trabecular outflow was observed in patients with ocular hypertension or POAG (Toris et al., 2007) and in human anterior segments in organ culture (Wan et al., 2007) which might be due to histological changes in the trabecular outflow pathway (Bahler et al., 2008).
Furthermore, in animal studies the ET-1-induced contraction of the trabecular meshwork was found to be reduced by unoprostone, PGF2α and fluprostenol (fluprostenol-Isopropylester = travoprost) (Figure 6) (Thieme et al., 2001, 2006). This effect is mediated via the prostaglandin F receptor (FP receptor). The IOP-lowering effect of PG analogues used in glaucoma therapy is also mediated via the FP receptor as studies on FP receptor knock-out mice revealed. In FP receptor knock-out mice application of latanoprost, travoprost, bimatoprost or unoprostone had no effect on IOP (Ota et al., 2005).
Figure 6.
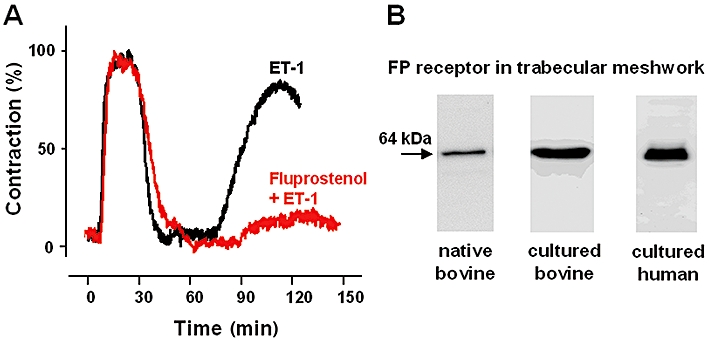
Effect of fluprostenol on endothelin-1 (ET-1)-induced contraction in trabecular meshwork. (A) Original recording of isometric force in bovine trabecular meshwork which serves as a well-established model for glaucoma research. After a carbachol (10−6 mol·L−1)-induced peak which was set to 100% contraction obtainable, the contraction was elicited by ET-1 (10−8 mol·L−1) or ET-1 after pre-incubation with fluprostenol (10−6 mol·L−1). The ET-1-induced contraction was strongly diminished in presence of the prostaglandin F receptor (FP receptor) agonist fluprostenol. (B) Expression of FP receptors verified with Western blot analysis in native bovine trabecular meshwork and cultured cells of bovine and human trabecular meshwork (modified from Thieme et al., 2006).
In contrast, the contraction elicited by muscarinic agonists is not affected by the PG analogues. This is in accordance with the finding that glaucoma treatment with PG analogues is not associated with side effects on accommodation and pupil size. It is probable that the anti-endothelin effect on trabecular meshwork contractility accounts for the IOP-lowering effect of PG analogues for two reasons. First, the strong reduction of IOP could not be explained by an exclusive increase in the comparatively low uveoscleral outflow (Nilsson, 1997); and second, the rapid IOP reduction within hours after application of the drugs could not be explained by ECM degradation. Therefore, endothelin-antagonism on trabecular meshwork contractility decisively contributes to the IOP-lowering effect of PG analogues.
Improvement of ocular blood flow by prostaglandin F analogues
Several studies on glaucoma patients showed an increase in OBF after treatment with PG analogues which was assumed to correlate with the IOP-lowering effect of the drugs (Sponsel et al., 2002a,b; Erkin et al., 2004). Application of bimatoprost and travoprost resulted in an improvement in the central retinal artery blood flow in newly diagnosed open-angle glaucoma patients (Alagoz et al., 2008). An improved OBF after treatment with latanoprost was also found in patients with POAG, normal tension glaucoma and ocular hypertension (Vetrugno et al., 1998; McKibbin and Menage, 1999; Georgopoulos et al., 2002; Sponsel et al., 2002b; Erkin et al., 2004; Gherghel et al., 2008). Also, unoprostone caused an increase in OBF, a comparison of latanoprost and unoprostone revealed, that latanoprost once daily produced a OBF increase nearly twofold greater than those obtained with unoprostone twice daily (Sponsel et al., 2002a). In this study, latanoprost caused a larger IOP reduction than unoprostone. A direct anti-endothelin effect of unoprostone on OBF was verified in a study with healthy individuals. In this placebo-controlled study, it was shown that intravenous injection of ET-1 decreased choroidal blood flow. This effect was significantly blunted when topical unoprostone was coadministered (Polska et al., 2002).
Different animal studies revealed a direct, IOP-independent effect of PG analogues on blood flow. In rabbits ET-1 decreased optic nerve head blood flow. Pretreatment with intravitreal injection of unoprostone did not affect IOP, but partly inhibited the blood flow-decreasing effect of ET-1 (Sugiyama and Azuma, 1995). Another study showed that the ET-1-induced decrease in OBF was almost completely prevented by tafluprost and significantly inhibited by latanoprost and travoprost. These drugs are shown to relax the ciliary artery contraction induced by ET-1 (Kurashima et al., 2010). Moreover, the vasoconstrictive effect of ET-1 on perfused porcine retinal arterioles was slightly inhibited by PGF2α, a pronounced inhibition was induced by unoprostone (Yu et al., 2001).
Assuming a similar mode of action for all PG analogues, the improvement of OBF after treatment with these drugs is due to an IOP-dependent effect and additionally to a direct anti-endothelin effect on vascular smooth muscle.
In summary, the improvement of OBF induced by application of PG analogues could be beneficial for glaucoma patients suffering from impaired ocular perfusion, either due to vasoconstriction caused by ET-1 or to abnormal vascular autoregulation.
Neuroprotective effect of prostaglandin F analogues
In addition to the indirect neuroprotective effects of PG analogues due to an increased OBF, some direct neuroprotective effects of these drugs are described in animal studies, data on humans are not available so far. A morphometric study in the rat showed that intravitreal injection of ET-1 led to cell loss in the RGC layer. This is accompanied by a decrease in neurofilament protein in the optic nerve. Simultaneous injection of the unoprostone metabolite M1 attenuated RGC loss and the decrease in neurofilament protein induced by ET-1 compared with ET-1 injection alone. These results suggest that unoprostone exerts neuroprotective effects against ET-1-induced neuronal injury (Munemasa et al., 2008). A direct anti-apoptotic effect of latanoprost and tafluprost on rat RGCs in vivo and in vitro was also described (Kudo et al., 2006; Kanamori et al., 2009).
Furthermore, an anti-apoptotic effect of latanoprost (Nakanishi et al., 2006) and the unoprostone metabolite M1 (Mukuno et al., 2004) on rat retinal glial cells which ensure the maintaining of retinal homeostasis and trophic support for the neurons was observed. In contrast, in an experimental rat model with increased IOP and resulting gliosis in the retina, latanoprost attenuates the retinal glial reaction and may afford neuroprotection to the ganglion cells by this effect (Vidal et al., 2010).
Inhibition of endothelin synthesis
A quite different way to antagonize the endothelin-induced glaucomatous damage in the eye would be the inhibition of endothelin synthesis. ET-1 is produced from its biologically almost inactive precursor Big ET-1 (38 amino acids) by a membrane-bound Zn-dependent metalloprotease, endothelin-converting enzyme (ECE). The ECE is expressed in the blood vessels of the retina, optic nerve and choroids (Wollensak et al., 2002; Dibas et al., 2005b). Additionally, ECE activity was found in ciliary epithelium and retinal pigment epithelium (Prasanna et al., 1999; Dibas et al., 2005a). Plasma membrane ECE activity could be inhibited by phosphoramidon (potent inhibitor of ECE), thiorphan (metalloprotease inhibitor) and phenanthroline (inhibitor of zinc-dependent proteases).
The ECE activity may emerge as a possible target in preventing ET-1-induced increase in IOP and ET-1-induced damage of RGCs and the optic nerve.
General conclusions
The potent vasoconstrictor ET-1 has been found to be increased in the aqueous humour of glaucoma patients compared to control and is suspected to be involved in the pathogenesis of the disease by effects on IOP, OBF and RGCs. An antagonism of endothelin effects provides a promising approach for medical treatment of glaucoma, by three mechanisms: (i) lowering the IOP due to relaxation of the trabecular meshwork; (ii) promotion of blood flow caused by IOP-dependent and -independent effects on ocular vessels; and (iii) increasing the survival of RGCs.
Hence, antagonism of endothelin signalling through both, pressure-dependent and -independent pathways, stands for a promising therapeutic principle in the pharmacological treatment of glaucoma.
Acknowledgments
We express our gratitude to Professor Michael Wiederholt, Berlin, for inspiring and guiding our work for many years.
Glossary
Abbreviations
- ECE
endothelin-converting enzyme
- ET-1
endothelin-1
- ETA
receptor, endothelin receptor A
- ETB
receptor, endothelin receptor B
- FP
receptor, prostaglandin F receptor
- IOP
intraocular pressure
- MLCK
myosin light chain kinase
- MMP
matrix-metalloproteinase
- OBF
ocular blood flow
- PG
prostaglandin
- ROCK
Rho kinase
- RGC
retinal ganglion cell
Conflict of interest
The authors declare no conflict of interest.
Supplemental material
References
- Alagoz G, Gurel K, Bayer A, Serin D, Celebi S, Kukner S. A comparative study of bimatoprost and travoprost: effect on intraocular pressure and ocular circulation in newly diagnosed glaucoma patients. Ophthalmologica. 2008;222:88–95. doi: 10.1159/000112624. [DOI] [PubMed] [Google Scholar]
- Al-Jazzaf AM, DeSantis L, Netland PA. Travoprost: a potent ocular hypotensive agent. Drugs Today. 2003;39:61–74. doi: 10.1358/dot.2003.39.1.799432. [DOI] [PubMed] [Google Scholar]
- Bahler CK, Howell KG, Hann CR, Fautsch MP, Johnson DH. Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am J Ophthalmol. 2008;145:114–119. doi: 10.1016/j.ajo.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A, Remuzzi G. Endothelin antagonists. Lancet. 1999;353:133–138. doi: 10.1016/S0140-6736(98)09423-9. [DOI] [PubMed] [Google Scholar]
- Benigni A, Perico N, Remuzzi G. The potential of endothelin antagonism as a therapeutic approach. Expert Opin Investig Drugs. 2004;13:1419–1435. doi: 10.1517/13543784.13.11.1419. [DOI] [PubMed] [Google Scholar]
- Chauhan BC, LeVatte TL, Jollimore CA, Yu PK, Reitsamer HA, Kelly ME, et al. Model of endothelin-1-induced chronic optic neuropathy in rat. Invest Ophthalmol Vis Sci. 2004;45:144–152. doi: 10.1167/iovs.03-0687. [DOI] [PubMed] [Google Scholar]
- Choritz L, Rosenthal R, Fromm M, Foerster MH, Thieme H. Pharmacological and functional characterization of endothelin receptors in bovine trabecular meshwork and ciliary muscle. Ophthalmic Res. 2005;37:179–187. doi: 10.1159/000086471. [DOI] [PubMed] [Google Scholar]
- Cioffi GA, Sullivan P. The effect of chronic ischemia on the primate optic nerve. Eur J Ophthalmol. 1999;9:S34–S36. doi: 10.1177/112067219900901S12. [DOI] [PubMed] [Google Scholar]
- Cioffi GA, Orgul S, Onda E, Bacon DR, Van Buskirk EM. An in vivo model of chronic optic nerve ischemia: the dose-dependent effects of endothelin-1 on the optic nerve microvasculature. Curr Eye Res. 1995;14:1147–1153. doi: 10.3109/02713689508995821. [DOI] [PubMed] [Google Scholar]
- Clozel M, Gray GA. Are there different ETB receptors mediating constriction and relaxation? J Cardiovasc Pharmacol. 1995;26:262–264. [PubMed] [Google Scholar]
- Dallinger S, Dorner GT, Wenzel R, Graselli U, Findl O, Eichler HG, et al. Endothelin-1 contributes to hyperoxia-induced vasoconstriction in the human retina. Invest Ophthalmol Vis Sci. 2000;41:864–869. [PubMed] [Google Scholar]
- Davenport AP. International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol Rev. 2002;54:219–226. doi: 10.1124/pr.54.2.219. [DOI] [PubMed] [Google Scholar]
- Desai D, He S, Yorio T, Krishnamoorthy RR, Prasanna G. Hypoxia augments TNF-alpha-mediated endothelin-1 release and cell proliferation in human optic nerve head astrocytes. Biochem Biophys Res Commun. 2004;318:642–648. doi: 10.1016/j.bbrc.2004.04.073. [DOI] [PubMed] [Google Scholar]
- Dibas A, Prasanna G, Yorio T. Characterization of endothelin-converting enzyme activities in ARPE-19 cells, a human retinal pigmented epithelial cell line. J Ocul Pharmacol Ther. 2005a;21:196–204. doi: 10.1089/jop.2005.21.196. [DOI] [PubMed] [Google Scholar]
- Dibas A, Prasanna G, Yorio T. Localization of endothelin-converting enzyme in bovine optic nerve and retina. J Ocul Pharmacol Ther. 2005b;21:288–297. doi: 10.1089/jop.2005.21.288. [DOI] [PubMed] [Google Scholar]
- Emre M, Orgul S, Haufschild T, Shaw SG, Flammer J. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br J Ophthalmol. 2005;89:60–63. doi: 10.1136/bjo.2004.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson-Lamy K, Korbmacher C, Schuman JS, Nathanson JA. Effect of endothelin on outflow facility and accommodation in the monkey eye in vivo. Invest Ophthalmol Vis Sci. 1991;32:492–495. [PubMed] [Google Scholar]
- Erkin EF, Tarhan S, Kayikcioglu OR, Deveci H, Guler C, Goktan C. Effects of betaxolol and latanoprost on ocular blood flow and visual fields in patients with primary open-angle glaucoma. Eur J Ophthalmol. 2004;14:211–219. doi: 10.1177/112067210401400305. [DOI] [PubMed] [Google Scholar]
- Fernandez-Durango R, Rollin R, Mediero A, Roldan-Pallares M, Garcia Feijo J, Garcia Sanchez J, et al. Localization of endothelin-1 mRNA expression and immunoreactivity in the anterior segment of human eye: expression of ETA and ETB receptors. Mol Vis. 2003;9:103–109. [PubMed] [Google Scholar]
- Flammer J, Mozaffarieh M. Autoregulation, a balancing act between supply and demand. Can J Ophthalmol. 2008;43:317–321. doi: 10.3129/i08-056. [DOI] [PubMed] [Google Scholar]
- Flammer J, Haefliger IO, Orgul S, Resink T. Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma. 1999;8:212–219. [PubMed] [Google Scholar]
- Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Fuchsjager-Mayrl G, Luksch A, Malec M, Polska E, Wolzt M, Schmetterer L. Role of endothelin-1 in choroidal blood flow regulation during isometric exercise in healthy humans. Invest Ophthalmol Vis Sci. 2003;44:728–733. doi: 10.1167/iovs.02-0372. [DOI] [PubMed] [Google Scholar]
- Gaton DD, Sagara T, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN. Increased matrix metalloproteinases 1, 2, and 3 in the monkey uveoscleral outflow pathway after topical prostaglandin F(2 alpha)-isopropyl ester treatment. Arch Ophthalmol. 2001;119:1165–1170. doi: 10.1001/archopht.119.8.1165. [DOI] [PubMed] [Google Scholar]
- Georgopoulos GT, Diestelhorst M, Fisher R, Ruokonen P, Krieglstein GK. The short-term effect of latanoprost on intraocular pressure and pulsatile ocular blood flow. Acta Ophthalmol Scand. 2002;80:54–58. doi: 10.1034/j.1600-0420.2002.800111.x. [DOI] [PubMed] [Google Scholar]
- Gherghel D, Hosking SL, Cunliffe IA, Armstrong RA. First-line therapy with latanoprost 0.005% results in improved ocular circulation in newly diagnosed primary open-angle glaucoma patients: a prospective, 6-month, open-label study. Eye (Lond) 2008;22:363–369. doi: 10.1038/sj.eye.6702639. [DOI] [PubMed] [Google Scholar]
- Granstam E, Wang L, Bill A. Effects of endothelins (ET-1, ET-2 and ET-3) in the rabbit eye; role of prostaglandins. Eur J Pharmacol. 1991;194:217–223. doi: 10.1016/0014-2999(91)90108-3. [DOI] [PubMed] [Google Scholar]
- Granstam E, Wang L, Bill A. Ocular effects of endothelin-1 in the cat. Curr Eye Res. 1992;11:325–332. doi: 10.3109/02713689209001786. [DOI] [PubMed] [Google Scholar]
- Haefliger IO, Flammer J, Luscher TF. Heterogeneity of endothelium-dependent regulation in ophthalmic and ciliary arteries. Invest Ophthalmol Vis Sci. 1993;34:1722–1730. [PubMed] [Google Scholar]
- Haque MS, Sugiyama K, Taniguchi T, Kitazawa Y. Effects of BQ-123, an ETA recepter-selective antagonist, on changes of intraocular pressure, blood-aqueous barrier and aqueous prostaglandin concentrations caused by endothelin-1 in rabbit. Jpn J Ophthalmol. 1996;40:26–32. [PubMed] [Google Scholar]
- Henry E, Newby DE, Webb DJ, Hadoke PW, O'Brien CJ. Altered endothelin-1 vasoreactivity in patients with untreated normal-pressure glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2528–2532. doi: 10.1167/iovs.05-0240. [DOI] [PubMed] [Google Scholar]
- Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19:297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Hollo G, Lakatos P, Farkas K. Cold pressor test and plasma endothelin-1 concentration in primary open-angle and capsular glaucoma. J Glaucoma. 1998;7:105–110. [PubMed] [Google Scholar]
- Hollo G, Lakatos P, Vargha P. Immediate increase in aqueous humour endothelin 1 concentration and intra-ocular pressure after argon laser trabeculoplasty in the rabbit. Ophthalmologica. 2000;214:292–295. doi: 10.1159/000027507. [DOI] [PubMed] [Google Scholar]
- Hollo G, Kothy P, Lakatos P, Vargha P. Endothelin-A receptor antagonist BQ-485 protects against intraocular pressure spike induced by laser trabeculoplasty in the rabbit. Ophthalmologica. 2002;216:459–462. doi: 10.1159/000067547. [DOI] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- Honjo M, Inatani M, Kido N, Sawamura T, Yue BY, Honda Y, et al. A myosin light chain kinase inhibitor, ML-9, lowers the intraocular pressure in rabbit eyes. Exp Eye Res. 2002;75:135–142. doi: 10.1006/exer.2002.2009. [DOI] [PubMed] [Google Scholar]
- Hylton C, Robin AL. Update on prostaglandin analogs. Curr Opin Ophthalmol. 2003;14:65–69. doi: 10.1097/00055735-200304000-00001. [DOI] [PubMed] [Google Scholar]
- Kallberg ME, Brooks DE, Garcia-Sanchez GA, Komaromy AM, Szabo NJ, Tian L. Endothelin 1 levels in the aqueous humor of dogs with glaucoma. J Glaucoma. 2002;11:105–109. doi: 10.1097/00061198-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Naka M, Fukuda M, Nakamura M, Negi A. Tafluprost protects rat retinal ganglion cells from apoptosis in vitro and in vivo. Graefes Arch Clin Exp Ophthalmol. 2009;247:1353–1360. doi: 10.1007/s00417-009-1122-6. [DOI] [PubMed] [Google Scholar]
- Karadag R, Yagci R, Aydin B, Kanbay M, Erdurmus M, Keskin UC, et al. Effects of erytropoietin treatment and hemodialysis on the serum endothelin level and intraocular pressure of hemodialysis patients. Int Ophthalmol. 2009;29:385–388. doi: 10.1007/s10792-008-9253-z. [DOI] [PubMed] [Google Scholar]
- Kim JW, Lindsey JD, Wang N, Weinreb RN. Increased human scleral permeability with prostaglandin exposure. Invest Ophthalmol Vis Sci. 2001;42:1514–1521. [PubMed] [Google Scholar]
- Kingman M, Ruggiero R, Torres F. Ambrisentan, an endothelin receptor type A-selective endothelin receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2009;10:1847–1858. doi: 10.1517/14656560903061275. [DOI] [PubMed] [Google Scholar]
- Koliakos GG, Konstas AG, Schlotzer-Schrehardt U, Hollo G, Mitova D, Kovatchev D, et al. Endothelin-1 concentration is increased in the aqueous humour of patients with exfoliation syndrome. Br J Ophthalmol. 2004;88:523–527. doi: 10.1136/bjo.2003.028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno T, Maruichi M, Takai S, Oku H, Sugiyama T, Uchibori T, et al. Effect of chymase on intraocular pressure in rabbits. Eur J Pharmacol. 2005;524:132–137. doi: 10.1016/j.ejphar.2005.09.048. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy RR, Rao VR, Dauphin R, Prasanna G, Johnson C, Yorio T. Role of the ETB receptor in retinal ganglion cell death in glaucoma. Can J Physiol Pharmacol. 2008;86:380–393. doi: 10.1139/Y08-040. [DOI] [PubMed] [Google Scholar]
- Kudo H, Nakazawa T, Shimura M, Takahashi H, Fuse N, Kashiwagi K, et al. Neuroprotective effect of latanoprost on rat retinal ganglion cells. Graefes Arch Clin Exp Ophthalmol. 2006;244:1003–1009. doi: 10.1007/s00417-005-0215-0. [DOI] [PubMed] [Google Scholar]
- Kunimatsu S, Mayama C, Tomidokoro A, Araie M. Plasma endothelin-1 level in Japanese normal tension glaucoma patients. Curr Eye Res. 2006;31:727–731. doi: 10.1080/02713680600837382. [DOI] [PubMed] [Google Scholar]
- Kurashima H, Watabe H, Sato N, Abe S, Ishida N, Yoshitomi T. Effects of prostaglandin F2a analogues on endothelin-1-induced impairmentof rabbit ocular blood fl ow: comparison among ta fl uprost, travoprost,and latanoprost. Exp Eye Res. 2010 doi: 10.1016/j.exer.2010.09.004. DOI: 10.1016/j.exer.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Becker M, Stahl F, Berweck S, Hensen J, Noske W, et al. Endothelin-like immunoreactivity in the aqueous humour and in conditioned medium from cultured ciliary epithelial cells. Curr Eye Res. 1992;11:1041–1046. doi: 10.3109/02713689209015075. [DOI] [PubMed] [Google Scholar]
- Levin ER. Endothelins. N Engl J Med. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- Lu YB, Franze K, Seifert G, Steinhauser C, Kirchhoff F, Wolburg H, et al. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci U S A. 2006;103:17759–17764. doi: 10.1073/pnas.0606150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher TF, Wenzel RR. Endothelin and endothelin antagonists: pharmacology and clinical implications. Agents Actions Suppl. 1995;45:237–253. doi: 10.1007/978-3-0348-7346-8_34. [DOI] [PubMed] [Google Scholar]
- MacCumber MW, D'Anna SA. Endothelin receptor-binding subtypes in the human retina and choroid. Arch Ophthalmol. 1994;112:1231–1235. doi: 10.1001/archopht.1994.01090210119024. [DOI] [PubMed] [Google Scholar]
- MacCumber MW, Jampel HD, Snyder SH. Ocular effects of the endothelins. Abundant peptides in the eye. Arch Ophthalmol. 1991;109:705–709. doi: 10.1001/archopht.1991.01080050121041. [DOI] [PubMed] [Google Scholar]
- McKibbin M, Menage MJ. The effect of once-daily latanoprost on intraocular pressure and pulsatile ocular blood flow in normal tension glaucoma. Eye (Lond) 1999;13:31–34. doi: 10.1038/eye.1999.6. [DOI] [PubMed] [Google Scholar]
- Mukuno H, Nakamura M, Kanamori A, Nagai A, Negi A, Seigel G. Unoprostone isopropyl rescues retinal progenitor cells from apoptosis in vitro. Curr Eye Res. 2004;29:457–464. doi: 10.1080/02713680490889465. [DOI] [PubMed] [Google Scholar]
- Munemasa Y, Kitaoka Y, Hayashi Y, Takeda H, Fujino H, Ohtani-Kaneko R, et al. Effects of unoprostone on phosphorylated extracellular signal-regulated kinase expression in endothelin-1-induced retinal and optic nerve damage. Vis Neurosci. 2008;25:197–208. doi: 10.1017/S095252380808053X. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Nakamura M, Mukuno H, Kanamori A, Seigel GM, Negi A. Latanoprost rescues retinal neuro-glial cells from apoptosis by inhibiting caspase-3, which is mediated by p44/p42 mitogen-activated protein kinase. Exp Eye Res. 2006;83:1108–1117. doi: 10.1016/j.exer.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Narayan S, Prasanna G, Krishnamoorthy RR, Zhang X, Yorio T. Endothelin-1 synthesis and secretion in human retinal pigment epithelial cells (ARPE-19): differential regulation by cholinergics and TNF-alpha. Invest Ophthalmol Vis Sci. 2003;44:4885–4894. doi: 10.1167/iovs.03-0387. [DOI] [PubMed] [Google Scholar]
- Narayan S, Brun AM, Yorio T. Endothelin-1 distribution and basolateral secretion in the retinal pigment epithelium. Exp Eye Res. 2004;79:11–19. doi: 10.1016/j.exer.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Nguyen QH. The role of prostaglandin analogues in the treatment of glaucoma in the 21st century. Int Ophthalmol Clin. 2004;44:15–27. doi: 10.1097/00004397-200404420-00004. [DOI] [PubMed] [Google Scholar]
- Nilsson SF. The uveoscleral outflow routes. Eye. 1997;11:149–154. doi: 10.1038/eye.1997.43. [DOI] [PubMed] [Google Scholar]
- Noske W, Hensen J, Wiederholt M. Endothelin-like immunoreactivity in aqueous humor of patients with primary open-angle glaucoma and cataract. Graefes Arch Clin Exp Ophthalmol. 1997;235:551–552. doi: 10.1007/BF00947082. [DOI] [PubMed] [Google Scholar]
- Okada K, Sugiyama K, Haque MS, Taniguchi T, Kitazawa Y. The effects of endothelin-1 on intraocular pressure and pupillary diameter in rabbits. Jpn J Ophthalmol. 1995;39:233–241. [PubMed] [Google Scholar]
- Oku H, Sugiyama T, Kojima S, Watanabe T, Azuma I. Experimental optic cup enlargement caused by endothelin-1-induced chronic optic nerve head ischemia. Surv Ophthalmol. 1999;44:S74–S84. doi: 10.1016/s0039-6257(99)00068-5. [DOI] [PubMed] [Google Scholar]
- Orgul S, Cioffi GA, Bacon DR, Van Buskirk EM. An endothelin-1-induced model of chronic optic nerve ischemia in rhesus monkeys. J Glaucoma. 1996a;5:135–138. [PubMed] [Google Scholar]
- Orgul S, Cioffi GA, Wilson DJ, Bacon DR, Van Buskirk EM. An endothelin-1 induced model of optic nerve ischemia in the rabbit. Invest Ophthalmol Vis Sci. 1996b;37:1860–1869. [PubMed] [Google Scholar]
- Orgul S, Zawinka C, Gugleta K, Flammer J. Therapeutic strategies for normal-tension glaucoma. Ophthalmologica. 2005;219:317–323. doi: 10.1159/000088372. [DOI] [PubMed] [Google Scholar]
- Ota T, Aihara M, Narumiya S, Araie M. The effects of prostaglandin analogues on IOP in prostanoid FP-receptor-deficient mice. Invest Ophthalmol Vis Sci. 2005;46:4159–4163. doi: 10.1167/iovs.05-0494. [DOI] [PubMed] [Google Scholar]
- Pache M, Kaiser HJ, Akhalbedashvili N, Lienert C, Dubler B, Kappos L, et al. Extraocular blood flow and endothelin-1 plasma levels in patients with multiple sclerosis. Eur Neurol. 2003;49:164–168. doi: 10.1159/000069085. [DOI] [PubMed] [Google Scholar]
- Perry CM, McGavin JK, Culy CR, Ibbotson T. Latanoprost : an update of its use in glaucoma and ocular hypertension. Drugs Aging. 2003;20:597–630. doi: 10.2165/00002512-200320080-00005. [DOI] [PubMed] [Google Scholar]
- Pinet F. What is the role of endothelin system? Med Sci (Paris) 2004;20:339–345. doi: 10.1051/medsci/2004203339. [DOI] [PubMed] [Google Scholar]
- Polak K, Petternel V, Luksch A, Krohn J, Findl O, Polska E, et al. Effect of endothelin and BQ123 on ocular blood flow parameters in healthy subjects. Invest Ophthalmol Vis Sci. 2001;42:2949–2956. [PubMed] [Google Scholar]
- Polak K, Luksch A, Frank B, Jandrasits K, Polska E, Schmetterer L. Regulation of human retinal blood flow by endothelin-1. Exp Eye Res. 2003;76:633–640. doi: 10.1016/s0014-4835(02)00312-3. [DOI] [PubMed] [Google Scholar]
- Polska E, Doelemeyer A, Luksch A, Ehrlich P, Kaehler N, Percicot CL, et al. Partial antagonism of endothelin 1-induced vasoconstriction in the human choroid by topical unoprostone isopropyl. Arch Ophthalmol. 2002;120:348–352. doi: 10.1001/archopht.120.3.348. [DOI] [PubMed] [Google Scholar]
- Prasanna G, Dibas A, Tao W, White K, Yorio T. Regulation of endothelin-1 in human non-pigmented ciliary epithelial cells by tumor necrosis factor-alpha. Exp Eye Res. 1998;66:9–18. doi: 10.1006/exer.1997.0407. [DOI] [PubMed] [Google Scholar]
- Prasanna G, Dibas A, Finkley A, Yorio T. Identification of endothelin converting enzyme-1 in human non-pigmented ciliary epithelial cells. Exp Eye Res. 1999;69:175–183. doi: 10.1006/exer.1999.0691. [DOI] [PubMed] [Google Scholar]
- Prasanna G, Krishnamoorthy R, Clark AF, Wordinger RJ, Yorio T. Human optic nerve head astrocytes as a target for endothelin-1. Invest Ophthalmol Vis Sci. 2002;43:2704–2713. [PubMed] [Google Scholar]
- Prasanna G, Narayan S, Krishnamoorthy RR, Yorio T. Eyeing endothelins: a cellular perspective. Mol Cell Biochem. 2003;253:71–88. doi: 10.1023/a:1026005418874. [DOI] [PubMed] [Google Scholar]
- Prasanna G, Hulet C, Desai D, Krishnamoorthy RR, Narayan S, Brun AM, et al. Effect of elevated intraocular pressure on endothelin-1 in a rat model of glaucoma. Pharmacol Res. 2005;51:41–50. doi: 10.1016/j.phrs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–1037. [PubMed] [Google Scholar]
- Renieri G, Choritz L, Rosenthal R, Meissner S, Pfeiffer N, Thieme H. Effects of endothelin-1 on calcium-independent contraction of bovine trabecular meshwork. Graefes Arch Clin Exp Ophthalmol. 2008;246:1107–1115. doi: 10.1007/s00417-008-0817-4. [DOI] [PubMed] [Google Scholar]
- Resch H, Karl K, Weigert G, Wolzt M, Hommer A, Schmetterer L, et al. Effect of dual endothelin receptor blockade on ocular blood flow in patients with glaucoma and healthy subjects. Invest Ophthalmol Vis Sci. 2009;50:358–363. doi: 10.1167/iovs.08-2460. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Choritz L, Schlott S, Bechrakis NE, Jaroszewski J, Wiederholt M, et al. Effects of ML-7 and Y-27632 on carbachol- and endothelin-1-induced contraction of bovine trabecular meshwork. Exp Eye Res. 2005;80:837–845. doi: 10.1016/j.exer.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Choritz L, Zorn R, Münzer G, Fromm M, Pfeiffer N, et al. Endothelin receptor B in trabecular meshwork. Exp Eye Res. 2007;85:482–491. doi: 10.1016/j.exer.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Roux S. Bosentan: a dual endothelin receptor antagonist. Expert Opin Investig Drugs. 2002;11:991–1002. doi: 10.1517/13543784.11.7.991. [DOI] [PubMed] [Google Scholar]
- Sponsel WE, Paris G, Trigo Y, Pena M. Comparative effects of latanoprost (Xalatan) and unoprostone (Rescula) in patients with open-angle glaucoma and suspected glaucoma. Am J Ophthalmol. 2002a;134:552–559. doi: 10.1016/s0002-9394(02)01643-4. [DOI] [PubMed] [Google Scholar]
- Sponsel WE, Paris G, Trigo Y, Pena M, Weber A, Sanford K, et al. Latanoprost and brimonidine: therapeutic and physiologic assessment before and after oral nonsteroidal anti-inflammatory therapy. Am J Ophthalmol. 2002b;133:11–18. doi: 10.1016/s0002-9394(01)01286-7. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Chakravarthy U, Gardiner TA, Archer DB. Endothelin-like immunoreactivity and receptor binding in the choroid and retina. Curr Eye Res. 1996;15:111–117. doi: 10.3109/02713689609017618. [DOI] [PubMed] [Google Scholar]
- Stokely ME, Brady ST, Yorio T. Effects of endothelin-1 on components of anterograde axonal transport in optic nerve. Invest Ophthalmol Vis Sci. 2002;43:3223–3230. [PubMed] [Google Scholar]
- Sugiyama T, Azuma I. Effect of UF-021 on optic nerve head circulation in rabbits. Jpn J Ophthalmol. 1995;39:124–129. [PubMed] [Google Scholar]
- Sugiyama K, Haque MS, Okada K, Taniguchi T, Kitazawa Y. Intraocular pressure response to intravitreal injection of endothelin-1 and the mediatory role of ETA receptor, ETB receptor, and cyclooxygenase products in rabbits. Curr Eye Res. 1995a;14:479–486. doi: 10.3109/02713689509003759. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Moriya S, Oku H, Azuma I. Association of endothelin-1 with normal tension glaucoma: clinical and fundamental studies. Surv Ophthalmol. 1995b;39:49–56. doi: 10.1016/s0039-6257(05)80073-6. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Utsumi T, Azuma I, Fujii H. Measurement of optic nerve head circulation: comparison of laser speckle and hydrogen clearance methods. Jpn J Ophthalmol. 1996;40:339–343. [PubMed] [Google Scholar]
- Sugiyama T, Mashima Y, Yoshioka Y, Oku H, Ikeda T. Effect of unoprostone on topographic and blood flow changes in the ischemic optic nerve head of rabbits. Arch Ophthalmol. 2009;127:454–459. doi: 10.1001/archophthalmol.2008.606. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Okada K, Haque MS, Sugiyama K, Kitazawa Y. Effects of endothelin-1 on intraocular pressure and aqueous humor dynamics in the rabbit eye. Curr Eye Res. 1994;13:461–464. doi: 10.3109/02713689408999874. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Haque MS, Sugiyama K, Okada K, Nakai Y, Kitazawa Y. Effects of endothelin A and B receptors on aqueous humor dynamics in the rabbit eye. J Ocul Pharmacol Ther. 1996;12:123–130. doi: 10.1089/jop.1996.12.123. [DOI] [PubMed] [Google Scholar]
- Tezel G, Kass MA, Kolker AE, Becker B, Wax MB. Plasma and aqueous humor endothelin levels in primary open-angle glaucoma. J Glaucoma. 1997;6:83–89. [PubMed] [Google Scholar]
- Thieme H, Stumpff F, Ottlecz A, Percicot CL, Lambrou GN, Wiederholt M. Mechanisms of action of unoprostone on trabecular meshwork contractility. Invest Ophthalmol Vis Sci. 2001;42:3193–3201. [PubMed] [Google Scholar]
- Thieme H, Schimmat C, Münzer G, Boxberger M, Fromm M, Pfeiffer N, et al. Endothelin antagonism: effects of FP receptor agonists prostaglandin F2alpha and fluprostenol on trabecular meshwork contractility. Invest Ophthalmol Vis Sci. 2006;47:938–945. doi: 10.1167/iovs.05-0527. [DOI] [PubMed] [Google Scholar]
- Tian B, Brumback LC, Kaufman PL. ML-7, chelerythrine and phorbol ester increase outflow facility in the monkey eye. Exp Eye Res. 2000;71:551–566. doi: 10.1006/exer.2000.0919. [DOI] [PubMed] [Google Scholar]
- Tokushige H, Inatani M, Nemoto S, Sakaki H, Katayama K, Uehata M, et al. Effects of topical administration of y-39983, a selective rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest Ophthalmol Vis Sci. 2007;48:3216–3222. doi: 10.1167/iovs.05-1617. [DOI] [PubMed] [Google Scholar]
- Toris CB, Zhan G, Fan S, Dickerson JE, Landry TA, Bergamini MV, et al. Effects of travoprost on aqueous humor dynamics in patients with elevated intraocular pressure. J Glaucoma. 2007;16:189–195. doi: 10.1097/IJG.0b013e31802fc6d3. [DOI] [PubMed] [Google Scholar]
- Vetrugno M, Cantatore F, Gigante G, Cardia L. Latanoprost 0.005% in POAG: effects on IOP and ocular blood flow. Acta Ophthalmol Scand Suppl. 1998;227:40–41. doi: 10.1111/j.1600-0420.1998.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Vidal L, Diaz F, Villena A, Moreno M, Campos JG, Perez de Vargas I. Reaction of Muller cells in an experimental rat model of increased intraocular pressure following timolol, latanoprost and brimonidine. Brain Res Bull. 2010;82:18–24. doi: 10.1016/j.brainresbull.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Wan Z, Woodward DF, Cornell CL, Fliri HG, Martos JL, Pettit SN, et al. Bimatoprost, prostamide activity, and conventional drainage. Invest Ophthalmol Vis Sci. 2007;48:4107–4115. doi: 10.1167/iovs.07-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Kashiwagi K, Kashiwagi F, Tsukahara S, Lindsey JD. Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Invest Ophthalmol Vis Sci. 1997;38:2772–2780. [PubMed] [Google Scholar]
- Weinreb RN, Toris CB, Gabelt BT, Lindsey JD, Kaufman PL. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002;47:53–64. doi: 10.1016/s0039-6257(02)00306-5. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Lindsey JD, Marchenko G, Marchenko N, Angert M, Strongin A. Prostaglandin FP agonists alter metalloproteinase gene expression in sclera. Invest Ophthalmol Vis Sci. 2004;45:4368–4377. doi: 10.1167/iovs.04-0413. [DOI] [PubMed] [Google Scholar]
- Wiederholt M, Bielka S, Schweig F, Lütjen-Drecoll E, Lepple-Wienhues A. Regulation of outflow rate and resistance in the perfused anterior segment of the bovine eye. Exp Eye Res. 1995;61:223–234. doi: 10.1016/s0014-4835(05)80042-9. [DOI] [PubMed] [Google Scholar]
- Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Ret Eye Res. 2000;19:271–295. doi: 10.1016/s1350-9462(99)00015-4. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Schaefer HE, Ihling C. An immunohistochemical study of endothelin-1 in the human eye. Curr Eye Res. 1998;17:541–545. doi: 10.1076/ceyr.17.5.541.5187. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Löffler B, Beyermann B, Ihling C. An immunohistochemical study of endothelin-1 converting enzyme in the human eye. Curr Eye Res. 2002;24:6–11. doi: 10.1076/ceyr.24.1.6.5431. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yorio T, Krishnamoorthy R, Prasanna G. Endothelin: is it a contributor to glaucoma pathophysiology? J Glaucoma. 2002;11:259–270. doi: 10.1097/00061198-200206000-00016. [DOI] [PubMed] [Google Scholar]
- Yu DY, Su EN, Cringle SJ, Schoch C, Percicot CP, Lambrou GN. Comparison of the vasoactive effects of the docosanoid unoprostone and selected prostanoids on isolated perfused retinal arterioles. Invest Ophthalmol Vis Sci. 2001;42:1499–1504. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


