Abstract
BACKGROUND AND PURPOSE
The enduring propensity for alcoholics to relapse even following years of abstinence presents a major hurdle for treatment. Here we report a model of relapse following protracted abstinence and investigate the pattern of neuronal activation following cue-induced reinstatement and administration of the orexin1 receptor antagonist SB-334867 in inbred alcohol-preferring rats.
EXPERIMENTAL APPROACH
Rats were trained to self-administer alcohol under operant conditions and divided into two groups: immediate (reinstated immediately following extinction) and delayed (extinguished and then housed for 5 months before reinstatement). Prior to reinstatement, animals were treated with vehicle (immediate n= 11, delayed n= 11) or SB-334867 (20 mg·kg−1 i.p.; immediate n= 6, delayed n= 11). Fos expression was compared between each group and to animals that underwent extinction only.
KEY RESULTS
SB-334867 significantly attenuated cue-induced reinstatement in both groups. Immediate reinstatement increased Fos expression in the nucleus accumbens (NAc), infra-limbic (IL), pre-limbic (PrL), orbitofrontal (OFC) and piriform cortices, the lateral and dorsomedial hypothalamus, central amygdala and basolateral amygdala (BLA), and the bed nucleus of the stria terminalis. Following delayed reinstatement, Fos expression was further elevated in cortical structures. Concurrent with preventing reinstatement, SB-334867 decreased Fos in NAc core, PrL and OFC following immediate reinstatement. Following protracted abstinence, SB-334867 treatment decreased reinstatement-induced Fos in the PrL, OFC and piriform cortices.
CONCLUSIONS AND IMPLICATIONS
Cue-induced alcohol seeking can be triggered following protracted abstinence in rats. The effects of SB-334867 on both behaviour and Fos expression suggest that the orexin system is implicated in cue-induced reinstatement, although some loci may shift following protracted abstinence.
Keywords: cue-induced reinstatement, protracted abstinence, orexin, SB-334867, alcohol
Introduction
One of the most problematic features of alcohol addiction is the long-lasting vulnerability of individuals with alcohol use disorders to craving and relapse even following complete remission of withdrawal symptoms and months or years of abstinence. While stress and a post-dependent dysphoric state brought about through neuroadaptations have been identified as major factors contributing to relapse (Koob, 2009), passive exposure to cues and contexts previously associated with drug availability and use has also been implicated in eliciting craving and relapse (O'Brien et al., 1998). Clinical studies have illustrated the relationship between cue exposure and individual responses (cue reactivity) to alcohol consumption and relapse propensity (Niaura et al., 1988; Rohsenow et al., 1994). Further, cue exposure treatment has demonstrated some success in increasing time to relapse in detoxified alcoholics (Drummond and Glautier, 1994). Consistent with these findings, it is well established that re-exposure to alcohol-associated cues and contexts can precipitate previously extinguished responding for alcohol in animal models (Bienkowski et al., 2004; Dayas et al., 2007).
There is some evidence to suggest that alcohol-predictive cues retain their salience following extended periods of repeated non-reinforced exposure (Ciccocioppo et al., 2001), potentially implicating a role for such mechanisms in the enduring vulnerability of alcoholics to relapse. A number of studies have investigated the neural substrates and mechanisms underlying cue-induced relapse to alcohol-seeking. These studies have identified a number of common regions involved in this response, including areas of the medial prefrontal cortex (mPFC), nucleus accumbens (NAc) core and shell, the cornu ammon regions of the hippocampus, and basolateral (BLA) and central amygdala (CeA) (Zhao et al., 2006; Dayas et al., 2007; 2008; Radwanska et al., 2008). Few studies to date, however, have examined the substrates involved following periods of extended abstinence (Valdez et al., 2002; Adams et al., 2010). It has been documented that abstinence from alcohol results in a number of functional neuronal alterations that may contribute to propensity to relapse, including enhanced corticostriatal synaptic plasticity (Xia et al., 2006), altered hippocampal inhibitory mechanisms and synaptic structure (Faingold et al., 2004), and modification to neurotransmitter and receptor expression and function (Andrade et al., 1992; Vizi et al., 2000; Chandler et al., 2006). It is unknown how these alterations may contribute to the mechanisms involved in relapse. To address this, here we investigated the ability of discrete alcohol-related cues to precipitate relapse not only immediately after extinction, but also when extinction is followed by a period of extended abstinence. Further, we examined the putative neuronal substrates underlying this behaviour. Given that relapse still occurs well after withdrawal symptoms have disappeared, we investigated this in inbred alcohol-preferring rats based upon their propensity to self-administer independent of alcohol dependence. Our strategy also allowed the effect of protracted abstinence on reinstatement to be assessed independent of any effects of withdrawal. Given the pharmacological (Lawrence et al., 2006; Richards et al., 2008) and anatomical (Dayas et al., 2007; Hamlin et al., 2007) evidence for the involvement of orexin (hypocretin) systems in alcohol use and seeking, we also aimed to identify putative anatomic loci where this neuropeptide may regulate relapse-like behaviour. The results of this study demonstrate that specific regions implicated in cue-induced reinstatement of alcohol-seeking show increased activation following protracted abstinence. Further, the role of the orexinergic system in mediating cue-induced reinstatement may involve actions in the prefrontal cortex, although the circuitry through which this occurs may alter following protracted abstinence.
Methods
Animals
All experiments were performed in accordance with the Prevention of Cruelty to Animals Act, 1986 under the guidelines of the National Health and Medical Research Council Code of Practice for the Care and Use of Animals for Experimental Purposes in Australia. Inbred alcohol-preferring (iP) rats were obtained from the breeding colony at the Howard Florey Institute, University of Melbourne. Parental stock had previously been obtained from Professor T.K. Li (while at Indiana University, Indianapolis). Rats were maintained on a 12 h light-dark cycle with ab libitum access to food and water.
Cue-induced alcohol reinstatement
Adult age-matched male iP rats (n= 42; on average 483 g) were trained to self-administer ethanol (10% v/v) in 20 min operant sessions in sound-attenuated operant chambers (Medical Associates, VT, USA) using a fixed ratio 3 (FR3) administration schedule as previously described (Cowen et al., 2005), conducted 5 days per week. Ethanol availability was conditioned with the presence of an olfactory cue (S+; two drops of vanilla essence placed in a cap directly below the active lever) and a 1 s light stimulus (CS+), located over the active lever, illuminated when the FR3 requirement was met. Both active and inactive lever responses were recorded during each session.
After a period of stable responding for ethanol (35 days), animals were subjected to extinction training sessions 5 days per week, where ethanol, S+ and CS+ were not present, until responding for the active and inactive levers were essentially equal. The day following acquisition of these criteria, a subset of animals was subjected to a reinstatement session where S+ and CS+ were reintroduced into the chamber and responding for both the active and inactive levers recorded (immediate reinstatement). Note that there was no ethanol reward associated with lever-pressing during reinstatement trials. Thirty minutes prior to reinstatement, animals received either vehicle (3% DMSO i.p.) (n= 11) or the orexin1 (OX1) receptor antagonist SB-334867 (20 mg·kg−1 i.p.) (n= 6). This dose has previously been demonstrated to inhibit cue-induced alcohol reinstatement in this breed of rats without affecting responding for water (Lawrence et al., 2006) and is within the range that has previously been demonstrated to selectively reduce OX1 receptor-mediated calcium release (Smart et al., 2001). The remaining 22 rats were housed in their home cages for 5 months prior to being subjected to an identical reinstatement session (delayed reinstatement; vehicle n= 11; SB-334867 n= 11). Three rats were extinguished but not subjected to reinstatement to act as extinction controls for the immunohistochemical study.
Immunohistochemistry
Subsets of rats from each behavioural group were randomly selected for immunohistochemical analysis of Fos expression (extinction only, n= 3; immediate reinstatement, vehicle n= 4; SB-334867 n= 5; delayed reinstatement, vehicle n= 5; SB-334867 n= 4). One hour following the reinstatement session, a time point at which Fos protein levels are around their peak (Herdegen and Leah, 1998), animals were deeply anaesthetized with pentobarbitone (100 mg·kg−1 i.p.) and transcardially perfused with 100 mL 0.1 M phosphate buffered saline (PBS) followed by 400 mL 4% paraformaldehyde (PFA) in 0.1 M PBS. Brains were removed and post-fixed overnight in 4% PFA then cryoprotected for 48 h in 20% sucrose in PBS and rapidly frozen over liquid nitrogen. Serial 40 µm sections were collected and every fourth section processed for Fos immunohistochemistry. Sections were incubated for 48 h in primary antibody (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by biotinylated secondary antibody (1 h, 1:500; anti-rabbit IgG, Vector Laboratories, Burlingame, CA, USA), and streptavidin-horseradish peroxidase complex (1 h, 1:500; Vector Laboratories). The reaction product was visualized with nickel enhanced 3,3'-diaminobenzidine (Sigma-Aldrich, St Louis, MO, USA) in 1% hydrogen peroxide, as previously described (McPherson and Lawrence, 2006). Sections were mounted on slides with 0.5% gelatin and allowed to dry before being counter-stained with neutral red (Sigma-Aldrich) to enable visualization of nuclei of interest, dehydrated and cover slipped. All treatment groups were reacted simultaneously to minimize procedural variation. Control experiments were performed by omitting the primary antibody on a subset of sections; in these experiments, staining failed to occur.
Analysis of Fos expression
Stereological estimates of the total number of Fos positive cells were conducted blind to treatment using the fractionator method (Gundersen et al., 1988). The software package Stereo-Investigator (MicroBrightField, Williston, VT, USA) interfaced with an Olympus BX51 microscope (Center Valley, PA, USA) fitted with a colour video camera (Optronics, Goleta, CA, USA) and motorized x,y,z stage control (Prior, Rockland, MA, USA) was used to conduct estimates of the infra-limbic (IL) and pre-limbic (PrL) cortices (bregma 4.2 mm to 2.52 mm); anterior portion of the piriform cortex (bregma 4.2 mm to 0.36 mm); orbitofrontal cortex (OFC) (bregma 4.2 mm to 2.76 mm) NAc core and shell (bregma 2.76 mm to 0.98 mm); dorsal and ventral bed nucleus of the stria terminalis (BNST) (bregma to −0.36 mm); BLA and CeA, the lateral hypothalamus and dorsal medial hypothalamus (−1.56 mm to −3.36 mm) and ventral tegmental area (VTA) (−4.8 mm to −6.8 mm). Counting frame sizes, areal and section sampling fractions for each of these regions are listed in Table 1. Stereological estimates were conducted based upon findings by Mura and colleagues, demonstrating regional heterogeneity in Fos induction following behavioural testing, highlighting the limitation of obtaining accurate Fos counts when selecting subsets of sections to assay alterations in expression (Mura et al., 2004).
Table 1.
Section sampling and areal sampling fractions for stereological estimation of number of Fos-positive neurones in the regions investigated
| Region | SSF | ASF |
|---|---|---|
| mPFC | 1/8 | 3/5 |
| Piriform | 1/8 | 12/25 |
| OFC | 1/8 | 4/15 |
| NAc core | 1/8 | 3/5 |
| NAc shell | 1/8 | 2/3 |
| BLA | 1/8 | 1/2 |
| LH | 1/8 | 4/5 |
| DMH | 1/8 | 4/5 |
| dBNST | 1/4 | 3/5 |
| vBNST | 1/4 | 3/5 |
| CeA | 1/8 | 5/12 |
mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; NAc, nucleus accumbens; BLA, basolateral amygdalae; LH, lateral hypothalamus; DMH, dorsomedial hypothalamus; BNST, bed nucleus of the stria terminalis; CeA, central amygdala.
Statistical analysis
Differences in lever responding were assessed using a mixed-factorial analysis of variance (anova) with Fisher's least significant difference post hoc comparisons for treatment (SB-334867 vs. vehicle) and reinstatement session (immediate vs. delayed) and within-subject comparisons between extinction and reinstatement sessions. Differences between the number of Fos-positive neurones was assessed using a three-way anova with planned comparisons to test effects of treatment (SB-334867 vs. vehicle), reinstatement session (immediate vs. delayed) and brain region. A separate two-way anova with planned comparisons was also conducted to examine the effect of reinstatement (extinction vs. vehicle) on each of the regions investigated for each of the separate reinstatement sessions. All data sets were subjected to a Shapiro–Wilks test for normality before assigning appropriate statistical tests. All Fos data were normalized by a square root transformation to ensure normal distribution. Significance was set at P < 0.05 for all tests.
Results
Cue-induced alcohol reinstatement: effects of protracted abstinence and the OX1 antagonist SB-334867
Following acquisition of alcohol self-administration, the mean number of active lever presses over the last 3 days was 106 ± 6, with a mean alcohol (10% v/v) consumption of 6.9 ± 0.4 mL·kg−1 per session. The mean number of active lever presses at extinction was 5 ± 1, achieved over a period of 31 days. The re-introduction of S+ and CS+, during reinstatement sessions, both immediately [F(1,35)= 29.927, P < 0.001] and following protracted abstinence [F(1,35)= 28.992, P < 0.001], significantly restored active lever responding in vehicle-treated rats (Figure 1). SB-334867 significantly attenuated responding for the active lever in both immediate [F(1,35)= 14.048, P < 0.001] and delayed reinstatement groups [F(1,35)= 20.611 P < 0.001, Figure 1A]. No difference was observed for any treatment between immediate and delayed reinstatement groups. No effect of treatment or protracted abstinence was observed on inactive lever responding [F(3,35)= 1.858, P > 0.05; F(1,35)= 0.116, P > 0.05; Figure 1B].
Figure 1.
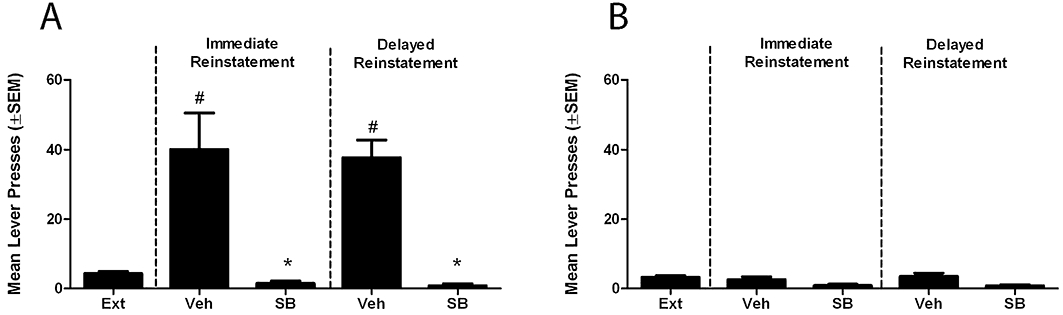
Cue-induced reinstatement and the effect of SB-334867 immediately post-extinction and following protracted abstinence. (A) Re-exposure to S+ and CS+ significantly increased responding for the active lever in both immediate and delayed reinstatement sessions when compared with extinction. SB significantly reduced reinstatement in both sessions. (B) No effect of SB or protracted abstinence was observed on inactive lever responses. #P < 0.001, compared with extinction; *P < 0.001, compared with vehicle, mixed factorial analysis of variance with planned comparisons. Ext, extinction; Veh, vehicle; SB, SB-334867.
Pattern of neuronal Fos expression following cue-induced reinstatement
Immediate reinstatement
Two-way anova revealed a significant effect of reinstatement [F(1,67)= 365.540, P < 0.0001] and brain region [F(10,67)= 17.713, P < 0.0001], and a significant interaction between the two [F(10,67)= 9.540, P < 0.0001]. Planned comparisons revealed that the number of Fos-positive neurones was significantly increased in all regions investigated with the exception of the VTA (Tables 2 and 3).
Table 2.
Stereological cell counts of Fos-positive cells induced by cue-induced reinstatement and the effect of SB-334867
| Immediate reinstatement | Delayed reinstatement | ||||
|---|---|---|---|---|---|
| Region | Extinction | Vehicle | SB-334867 | Vehicle | SB-334867 |
| PrL | 6.2 ± 1.6 | 63.8 ± 10.3# | 37.5 ± 8.1* | 111.8 ± 21.8#† | 82.3 ± 14.9*† |
| IL | 2.5 ± 0.6 | 28.1 ± 9.6# | 26.2 ± 1.4 | 53.5 ± 9.6#† | 41.9 ± 10.5† |
| Piriform | 15.5 ± 2.8 | 66.5 ± 3.5# | 47.6 ± 9.4 | 102.5 ± 10.6#† | 71.6 ± 5.6*† |
| OFC | 17.4 ± 1.9 | 108.6 ± 9.5# | 57.9 ± 6.2* | 149.5 ± 20.5#† | 98.4 ± 26.8*† |
| NAc core | 9.7 ± 2.9 | 53.3 ± 10.0# | 27.8 ± 3.3* | 63.0 ± 5.9# | 60.0 ± 7.3† |
| NAc shell | 7.5 ± 0.5 | 40.9 ± 4.0# | 28.3 ± 4.1 | 52.9 ± 2.9# | 43.5 ± 5.5 |
| BLA | 10.3 ± 2.4 | 38.6 ± 5.1# | 21.7 ± 2.0 | 50.5 ± 7.7# | 34.7 ± 6.9 |
| LH | 3.4 ± 0.1 | 31.0 ± 4.4# | 20.0 ± 1.9 | 42.4 ± 5.5# | 25.8 ± 4.2 |
| DMH | 3.6 ± 0.3 | 35.6 ± 4.4# | 22.6 ± 4.6 | 33.6 ± 6.6# | 29.1 ± 4.4 |
| dBNST | 3.6 ± 0.3 | 27.4 ± 1.0# | 29.8 ± 3.5 | 33.9 ± 6.6# | 33.1 ± 1.6 |
| vBNST | 5.4 ± 3.0 | 29.3 ± 1.6# | 27.4 ± 2.8 | 40.8 ± 1.9# | 37.2 ± 7.7 |
| CeA | 10.1 ± 3.3 | 38.2 ± 1.4# | 60.5 ± 3.3 | 55.1 ± 4.9# | 67.3 ± 9.1 |
| VTA | 2.4 ± 0.2 | 9.1 ± 0.1 | 10.6 ± 2.4 | 11.6 ± 2.5 | 12.4 ± 1.3 |
Counts are expressed as square root of mean ± SEM.
PrL, pre-limbic cortex; IL, infra-limbic cortex;; OFC, orbitofrontal cortex; NAc, nucleus accumbens; BLA, basolateral amygdalae; LH, lateral hypothalamus; DMH, dorsomedial hypothalamus; BNST, bed nucleus of the stria terminalis; CeA, central amygdalae; VTA, ventral tegmental area.
P < 0.05 (vs. extinction), two-way analysis of variance (anova) with planned comparisons,
P < 0.05 (vs. vehicle);
P < 0.05 (vs. immediate reinstatement), three-way anova with planned comparisons.
Table 3.
Percentage change in Fos-positive cells due to the effect of protracted abstinence or SB-334867 treatment in brain regions where a significant effect of either abstinence or drug treatment was observed
| Effect of protracted abstinence | Effect of SB-334867 treatment | ||
|---|---|---|---|
| Vehicle (%) | Immediate (%) | Delayed (%) | |
| PrL | +75* | −42* | −26* |
| IL | +90* | −7 | −22 |
| Piriform | +54* | −29 | −30* |
| OFC | +38* | −47* | −34* |
| NAc core | +18 | −48* | −5 |
Denotes a significant effect of each treatment as illustrated in Table 2.
PrL, pre-limbic cortex; IL, infra-limbic cortex;; OFC, orbitofrontal cortex; NAc, nucleus accumbens
Delayed reinstatement
In animals undergoing reinstatement following a period of protracted abstinence, two-way anova demonstrated a significant effect of reinstatement (F1,76= 239.108, P < 0.0001), brain region (F10,76= 10.780, P < 0.0001) and a significant interaction between the two (F10,76= 7.317, P < 0.0001). Planned comparisons revealed that the number of Fos-positive neurones was significantly increased in all regions investigated with the exception of the VTA (Tables 2 and 3).
Alterations in the pattern of neuronal Fos expression by SB-334867 and protracted abstinence
Three-way anova of Fos expression in animals undergoing either immediate or delayed reinstatement and SB-334867 or vehicle treatment revealed a significant effect of treatment [F(1,177)= 30.096. P < 0.0001], reinstatement session [F(1,177)= 61.092, P < 0.0001] and brain region [F(10,177)= 37.231, P < 0.0001]. Significant interactions were observed between treatment × region [F(10,177)= 4.320, P < 0.0001] and session × region [F(10,177)= 3.061, P < 0.0001].
Effect of SB-334867 treatment
During immediate reinstatement, the SB-334867 treatment significantly decreased the number of Fos-positive neurones in the PrL and orbitofrontal cortices and NAc Core when compared with vehicle-treated animals (P < 0.05, planned comparisons) (Figure 2, Tables 2 and 3). SB-334867 treatment following protracted abstinence significantly reduced the number of Fos-positive neurones in the PrL, orbitofrontal and piriform cortices when compared with vehicle-treated animals (P < 0.05, planned comparisons) (Figure 2, Tables 2 and 3).
Figure 2.
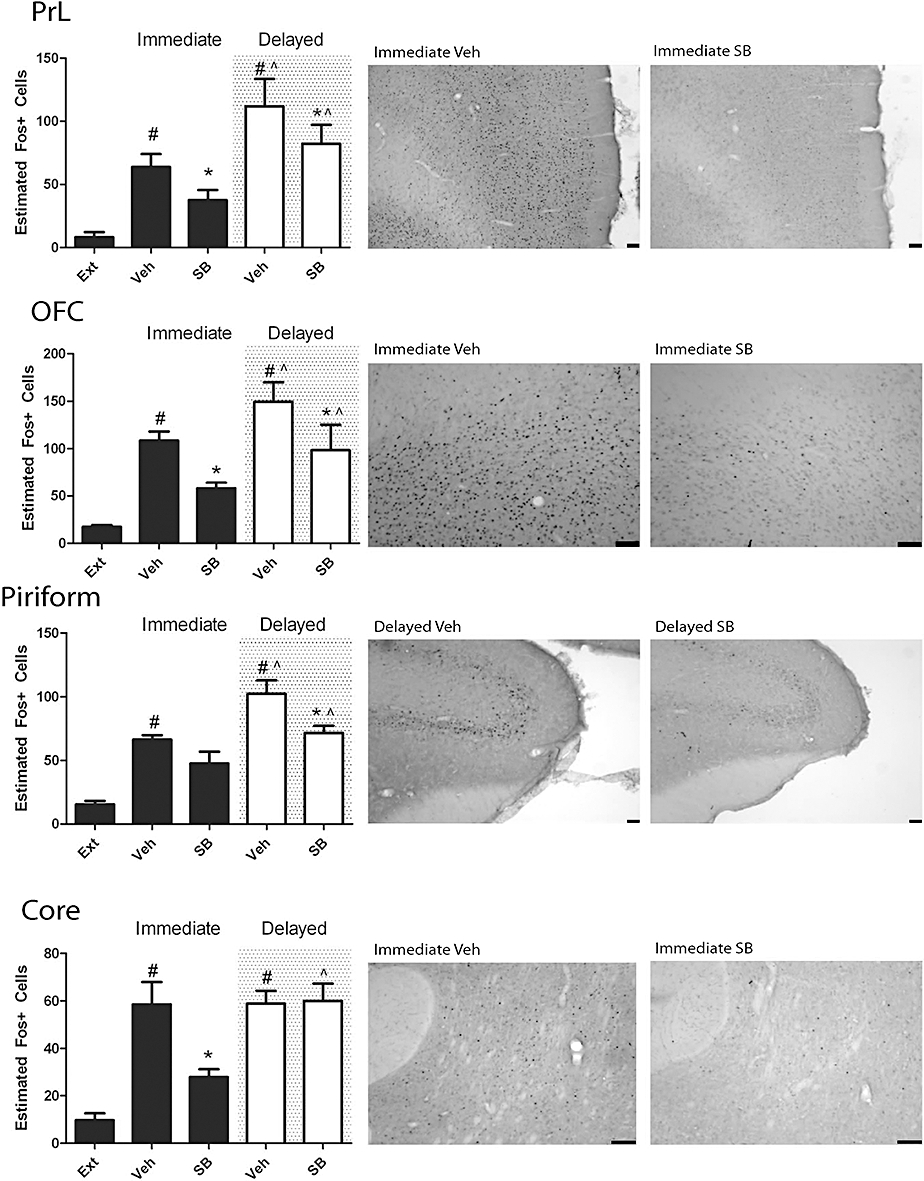
Graphs and representative photomicrographs illustrating the effect of reinstatement and SB-334867 on Fos expression in the pre-limbic (PrL), orbitofrontal (OFC), piriform cortex and nucleus accumbens core. Reinstatement significantly increased the number of Fos-positive neurones in the PrL, OFC, piriform cortex and nucleus accumbens core in both the immediate and delayed reinstatement groups. In the PrL and OFC, SB-334867 significantly reduced this increase in the immediate and delayed group, while the effect was only observed in the immediate reinstatement group in the accumbens core, and the delayed reinstatement group in the piriform cortex. *P < 0.05 versus vehicle; ∧P < 0.05 versus immediate reinstatement, three-way analysis of variance with planned comparisons. #P < 0.05 versus extinction, two-way anova with planned comparisons. Ext, extinction; Veh, vehicle; SB, SB-334867. All scale bars = 100 µm.
Effect of protracted abstinence
Planned comparisons investigating the effect of protracted abstinence found that the number of Fos-positive neurones increased in the IL, PrL, orbitofrontal and piriform cortices in vehicle-treated animals compared with immediate reinstaters (P < 0.05, planned comparisons). In SB-334867-treated animals, Fos expression was significantly increased following delayed reinstatement when compared with Fos expression in SB-334867-treated animals undergoing immediate reinstatement in the IL, orbitofrontal and piriform cortices and NAc core (P < 0.05, planned comparisons) (Figure 2, Tables 2 and 3).
Discussion
Here we demonstrate that re-exposure to alcohol-predictive cues following an extended period of abstinence is sufficient to reinstate previously extinguished operant responding for alcohol, demonstrating that rats, like humans, exhibit enduring vulnerability to relapse-like behaviour. Patterns of Fos expression associated with cue-induced reinstatement provide support for the involvement of a cortico-striatal circuit, as has been previously suggested (Kalivas and Volkow, 2005; Koob and Volkow, 2010). Following protracted abstinence, the overall pattern of neural activation was similar, although a number of structures exhibited significantly greater Fos expression. Importantly, responding on the active lever was similar between the immediate and delayed reinstatement groups, suggesting that the elevated Fos expression associated with protracted abstinence may reflect an altered response to reward-related cues. In addition, we show that reinstatement can be disrupted by the administration of the OX1 receptor antagonist SB-334867, suggesting that the orexin system remains involved in the integration of the salience of cues following protracted abstinence. Intriguingly, the putative anatomic loci where SB-334867 may act (directly or indirectly) to regulate relapse-like alcohol-seeking apparently shifts from the orbitofrontal/PrL cortex and accumbens core following immediate reinstatement to primarily a cortical locus following delayed reinstatement. These findings collectively suggest that the circuitry through which orexin impacts upon alcohol-seeking driven by exposure to cues may change over time.
Propensity of alcohol-predictive cues to induce reinstatement is maintained over a period of protracted abstinence and involves the orexinergic system
The present data support previous findings regarding the role of alcohol predictive cues in eliciting relapse (e.g. Bienkowski et al., 2004) and demonstrate that these cues retain their salience even following 5 months of abstinence. The latter fact extends the current knowledge base, and provides evidence that rats exhibit an enduring relapse propensity for a considerable part of their lifespan. It is interesting that no ‘incubation of craving’ effect was observed in the current study, given that responding on the active lever was similar between the immediate and delayed reinstatement groups. This finding does not necessarily discount an increase in responding during intermediate time points not examined. This phenomenon has been observed previously for heroin, where reinstatement assessed following 1 day, 1, 3 or 6 months of withdrawal followed an inverted U-shaped curve, with higher responding after 1 and 3 months than after 1 day or 6 months of withdrawal (Shalev et al., 2001). It should be noted that the animals used in the current study were not alcohol dependent and therefore did not necessarily experience florid withdrawal. Given this, dependency and subsequent withdrawal may be required for the incubation of craving and may explain the apparent lack of this phenomenon in the current study. It is however cogent to note that this study was designed to address the reaction to cue re-exposure following an extended abstinence; in this context, the data demonstrate an enduring ability of cues to precipitate relapse-like responding directed at the active lever.
The current data also support a role for the orexinergic system in this phenomenon, given that the OX1 receptor antagonist SB-334867 was capable of preventing cue-induced reinstatement following protracted abstinence. Previous work has implicated the orexinergic system in cue- and stress-induced reinstatement of alcohol seeking (Lawrence et al., 2006; Richards et al., 2008). The present data suggest that despite extinction and long-term abstinence, the orexinergic system is seemingly still involved in the integration of the salience of cues previously linked to the availability of a positive reinforcer.
Pattern of neural activation following reinstatement of alcohol-seeking by discrete cues
Brain structures implicated in relapse have been suggested from human imaging studies. A study in alcoholics showed increased cue-induced activation of the striatum, anterior cingulate and mPFC. Interestingly, this activation was more pronounced in subsequent relapsers compared with alcoholics who remained abstinent during a 3 month follow up. Cue-induced activation of the mPFC, but not the severity of alcohol craving, was associated with the subsequent amount of alcohol intake upon relapse. Consequently, the authors suggested that cue-induced activation of parts of an attention network may attribute incentive salience to alcohol cues that trigger relapse among alcoholics (Grusser et al., 2004). The current study found that exposure to discrete alcohol predictive cues significantly increased Fos expression in the IL, PrL, orbitofrontal and piriform cortices, NAc core and shell, basolateral and central amygdala, lateral and dorsomedial hypothalamus and the BNST. These regions are largely consistent with findings from previous studies investigating the pattern of Fos activation induced by reinstatement of alcohol seeking by exposure to discriminative cues (Zhao et al., 2006; Dayas et al., 2007) and contexts (Hamlin et al., 2007), although partially distinct activation patterns are observed between cue and context-driven reinstatement (Chaudhri et al., 2010). There is also significant overlap between the regions identified in the current study and those implicated in cue and context-driven reinstatement to other drugs of abuse (e.g. Hamlin et al., 2008; Kufahl et al., 2009), suggesting a potentially common network through which drug predictive cues may act to drive relapse.
Fos activation following reinstatement of alcohol-seeking is enhanced following protracted abstinence
The majority of studies investigating relapse to drug seeking have been conducted almost immediately after the operant task is extinguished. Because human relapse is an enduring problem (Epstein et al., 2006), we attempted to model this aspect more closely, by extinguishing rats and then subjecting them to 5 months of abstinence prior to precipitating relapse. Within this context, it is important to note that extinction is an approach few alcoholic patients undertake when trying to achieve abstinence, and represents a limitation of the model used in the current study. Regardless of the approach taken to obtain abstinence, however, relapse often occurs weeks to months following treatment, not immediately on the day following treatment or initiation of abstinence. Given this, the current study aimed to address if the neural correlates involved in relapse following a period of protracted abstinence differ from those that are involved in immediate relapse.
The reinstatement of alcohol seeking following protracted abstinence induced Fos expression in the same regions as observed following immediate reinstatement, although the amount of activation was significantly increased in the IL, PrL, orbitofrontal and piriform cortices compared with that found in cortical regions of immediate reinstaters. Withdrawal has been demonstrated to result in a number of alterations within these regions, some of which may contribute to an increased reactivity to reward associated cues. For example, ΔFosB is increased in the OFC during alcohol withdrawal, which has been suggested to relate to increased cue reactivity (Doran et al., 2007; Tetley et al., 2010). As discussed previously, the animals in the current study were not dependent and therefore may not have experienced florid withdrawal. Nevertheless, neural activation upon cue exposure was still enhanced following extinction and abstinence compared with reinstatement immediately after extinction. What this increased cortical Fos expression relates to in terms of behavioural outcomes still remains to be determined, given that the current study found no difference in alcohol-seeking between the two reinstatement time points. These data may reflect altered neural responses to the cue presentation and its integration, rather than any alteration in activation related to behavioural output. In other words, the central processing of cues and their salience may alter during extended periods of abstinence, yet once integrated, result in a similar behavioural response. In keeping with this idea, the pattern of activation during recent and remote memory retrieval relating to fear conditioning demonstrates increased activation of cortical regions following remote retrieval which has been suggested to play a role in the integration and processing of these memories (Frankland et al., 2004).
Alterations in the pattern of Fos activation by the OX1 receptor antagonist SB-334867 – evidence for orexinergic loci of action in cue-induced reinstatement of alcohol seeking
A role for the neuropeptide orexin A in mediating stress, cue- and context-induced relapse to drug seeking has been established for alcohol, other drugs of abuse and natural rewards (Boutrel et al., 2005; Harris et al., 2005; Lawrence et al., 2006; Richards et al., 2008). Orexin A containing neurones are solely located in the hypothalamus; however, their projections and OX1 receptors are widely distributed throughout the brain (Peyron et al., 1998; Marcus et al., 2001; Baldo et al., 2003). The precise regions of the brain involved in mediating the effects of orexin on drug seeking remain to be elucidated (Lawrence, 2010), although the insular cortex appears to be one region where orexins may integrate drug rewards (Hollander et al., 2008).
This is the first study to our knowledge that has investigated the pattern of Fos expression following inhibition of alcohol seeking by the OX1 receptor antagonist SB-334867. SB-334867 decreased Fos expression associated with reinstatement in the PrL and orbitofrontal cortices and the NAc core in the immediate reinstatement group. All these regions are implicated in the reinstatement of reward seeking (Lasseter et al., 2009; Rocha and Kalivas, 2010), receive projections from orexinergic neurones and contain OX1 receptors, although to differing degrees (Peyron et al., 1998; Marcus et al., 2001; Baldo et al., 2003). The NAc core is an exception, being essentially devoid of orexinergic projections and OX1 receptors (Marcus et al., 2001; Baldo et al., 2003), suggesting a secondary effect of SB-334867 on the reinstatement-induced Fos expression in this structure.
A cortico-amygdala-striatal pathway has been implicated in the reinstatement of alcohol seeking, with the PrL cortex and NAc core critically involved in mediating this behaviour (Backstrom and Hyytia, 2007; Chaudhri et al., 2008). Of these regions, the PrL and orbitofrontal cortices are possibly best anatomically arranged to be directly affected by changes in orexinergic signalling. Both the PrL and orbitofrontal cortices send direct projections to the NAc core (McFarland et al., 2003). Moreover, pyramidal cells in the prefrontal cortex receive orexin A innervation (Peyron et al., 1998), express OX1 receptors (Marcus et al., 2001) and orexin A enhances the excitability of layer 5 pyramidal cells from rat prefrontal cortex that is sensitive to antagonism by SB-334867 (Xia et al., 2005; Li et al., 2009). The decrease in Fos expression observed in the NAc core following treatment with SB-334867 may be an indirect consequence of decreased activation of the PrL cortex. Indeed, previous data have demonstrated the ability for the inactivation of the prefrontal cortex to reduce activation of the NAc core (Ishikawa et al., 2008).
There is some evidence to suggest that orexin may effect drug seeking via its action in the VTA (Harris et al., 2005; Wang et al., 2009). The current study, however, failed to find an effect of SB-334867 on Fos expression in this region. It should be noted that cue-induced reinstatement was not associated with elevated Fos expression in the VTA, similar to previous findings using the renewal paradigm (Hamlin et al., 2007). Consequently, the ability to detect a drug effect in the VTA may be limited under the current experimental conditions. Direct microinjection studies are required to confirm the anatomical loci where OX1 receptor antagonism attenuates alcohol seeking.
Following protracted abstinence, reinstatement-induced Fos expression was reduced by SB-334867 treatment in the PrL, orbitofrontal and piriform cortices, but not in the accumbens core. This suggests that SB-334867 may act through different mechanisms to inhibit reinstatement of alcohol seeking, depending upon the timing of the reinstatement relative to extinction training. Importantly, the differential effects of SB-334867 on Fos expression observed between immediate and delayed reinstatement argue against these changes occurring as the result of non-specific effects of SB-334867, and support the relationship between reduced alcohol-seeking behaviour and Fos expression. While it is theoretically possible that the reduction in Fos is observed due to a reduction in motor behaviour associated with reduced drug seeking, the differential patterns observed between immediate and delayed reinstatement groups treated with SB-334867 again argue against this, because their motor outputs are similar.
Conclusions
In summary, the results of this study for the first time demonstrate that specific brain nuclei implicated in cue-induced reinstatement of alcohol seeking show enhanced Fos expression following extinction and protracted abstinence compared with immediately after extinction, despite alcohol seeking being comparable. Further, the role of the orexinergic system in mediating discrete cue-induced reinstatement may occur through actions in the prefrontal cortex, although the mechanism through which this occurs may vary after an extended period of abstinence.
Acknowledgments
These studies were funded by the National Health & Medical Research Council of Australia (project grant 508976) of which AJL is a Senior Fellow (454303); the Pratt and Besen Foundations and the Victorian Government's Operational Infrastructure Support Program. We thank Professor Brian Oldfield for the provision of SB-334867.
Glossary
Abbreviations
- BLA
basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- CeA
central amygdala
- CS+
conditioned stimulus
- DMH
dorsomedial hypothalamus
- IL
infra-limbic cortex
- iP
Indiana alcohol preferring rats
- LH
lateral hypothalamus
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- OFC
orbitofrontal cortex
- OX1
orexin1 receptor
- PrL
pre-limbic cortex
- S+
stimulus
- VTA
ventral tegmental area
Conflicts of interest
None.
Supplemental material
References
- Adams CL, Short JL, Lawrence AJ. Cue-conditioned alcohol seeking in rats following abstinence: involvement of metabotropic glutamate 5 receptors. Br J Pharmacol. 2010;159:534–542. doi: 10.1111/j.1476-5381.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade JP, Fernando PM, Madeira MD, Paula-Barbosa MM, Cadete-Leite A, Zimmer J. Effects of chronic alcohol consumption and withdrawal on the somatostatin-immunoreactive neurons of the rat hippocampal dentate hilus. Hippocampus. 1992;2:65–71. doi: 10.1002/hipo.450020109. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, et al. Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol. 2004;14:355–360. doi: 10.1016/j.euroneuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ, Carpenter-Hyland E, Hendricson AW, Maldve RE, Morrisett RA, Zhou FC, et al. Structural and functional modifications in glutamateric synapses following prolonged ethanol exposure. Alcohol Clin Exp Res. 2006;30:368–376. doi: 10.1097/01.ALC.0000167959.84516.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci. 2008;28:2288–2298. doi: 10.1111/j.1460-9568.2008.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Weiss F. Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcohol Clin Exp Res. 2001;25:1414–1419. doi: 10.1097/00000374-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Adams C, Kraehenbuehl T, Vengeliene V, Lawrence AJ. The acute anti-craving effect of acamprosate in alcohol-preferring rats is associated with modulation of the mesolimbic dopamine system. Addict Biol. 2005;10:233–242. doi: 10.1080/13556210500223132. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D. Effect of impulsivity on craving and behavioral reactivity to smoking cues. Psychopharmacology. 2007;194:279–288. doi: 10.1007/s00213-007-0832-x. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Glautier S. A controlled trial of cue exposure treatment in alcohol dependence. J Consult Clin Psychol. 1994;62:809–817. doi: 10.1037//0022-006x.62.4.809. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL, Knapp DJ, Chester JA, Gonzalez LP. Integrative neurobiology of the alcohol withdrawal syndrome – from anxiety to seizures. Alcohol Clin Exp Res. 2004;28:268–278. doi: 10.1097/01.alc.0000113421.41962.8d. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci USA. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci. 2008;28:5088–5098. doi: 10.1523/JNEUROSCI.0253-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, et al. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:1370–1381. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ. Regulation of alcohol-seeking by orexin (hypocretin) Neurons Brain Res. 2010;1314:124–129. doi: 10.1016/j.brainres.2009.07.072. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Chen F, Ye J, Chen X, Yan J, Li Y, et al. The modulation of orexin A on HCN currents of pyramidal neurons in mouse prelimbic cortex. Cereb Cortex. 2009;20:1756–1767. doi: 10.1093/cercor/bhp241. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson CS, Lawrence AJ. Exposure to amphetamine in rats during periadolescence establishes behavioural and extrastriatal neural sensitization in adulthood. Int J Neuropsychopharmacol. 2006;9:377–392. doi: 10.1017/S1461145705005845. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Mura A, Murphy CA, Feldon J, Jongen-Relo AL. The use of stereological counting methods to assess immediate early gene immunoreactivity. Brain Res. 2004;1009:120–128. doi: 10.1016/j.brainres.2004.02.054. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K, Wrobel E, Korkosz A, Rogowski A, Kostowski W, Bienkowski P, et al. Alcohol relapse induced by discrete cues activates components of AP-1 transcription factor and ERK pathway in the rat basolateral and central amygdala. Neuropsychopharmacology. 2008;33:1835–1846. doi: 10.1038/sj.npp.1301567. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, et al. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol. 1994;62:620–626. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, et al. SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley AC, Brunstrom JM, Griffiths PL. The role of sensitivity to reward and impulsivity in food-cue reactivity. Eat Behav. 2010;11:138–143. doi: 10.1016/j.eatbeh.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vizi S, Palfi A, Gulya K. Multiple calmodulin genes exhibit systematically differential responses to chronic ethanol treatment and withdrawal in several regions of the rat brain. Brain Res Mol Brain Res. 2000;83:63–71. doi: 10.1016/s0169-328x(00)00185-6. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chen X, Song C, Ye J, Yu Z, Hu Z. Postsynaptic excitation of prefrontal cortical pyramidal neurons by hypocretin-1/orexin A through the inhibition of potassium currents. J Neurosci Res. 2005;82:729–736. doi: 10.1002/jnr.20667. [DOI] [PubMed] [Google Scholar]
- Xia JX, Li J, Zhou R, Zhang XH, Ge YB, Ru Yuan X. Alterations of rat corticostriatal synaptic plasticity after chronic ethanol exposure and withdrawal. Alcohol Clin Exp Res. 2006;30:819–824. doi: 10.1111/j.1530-0277.2006.00095.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


