Abstract
BACKGROUND
Intrathecal (i.t.) injection of orexin A (OX-A) increases blood pressure and heart rate (HR), but the effects of OX-A on sympathetic and phrenic, nerve activity, and the baroreflex(es), somato-sympathetic and hypoxic chemoreflex(es) are unknown.
EXPERIMENTAL APPROACH
Urethane-anaesthetized, vagotomized and artificially ventilated male Sprague-Dawley rats were examined in this study. The effects of i.t. OX-A (20 nmol 10 µL−1) on cardiorespiratory parameters, and responses to stimulation of the sciatic nerve (electrical), arterial baroreceptors (phenylephrine hydrochloride, 0.01 mg kg−1 i.v.) and peripheral (hypoxia) chemoreceptors were also investigated.
KEY RESULTS
i.t. OX-A caused a prolonged dose-dependent sympathoexcitation, pressor response and tachycardia. The peak effect was observed at 20 nmol with increases in mean arterial pressure, HR and splanchnic sympathetic nerve activity (sSNA) of 32 mmHg, 52 beats per minute and 100% from baseline respectively. OX-A also dose-dependently increased respiratory drive, as indicated by a rise in phrenic nerve amplitude and a fall in phrenic nerve frequency, an increase in neural minute ventilation, a lengthening of the expiratory period, and a shortening of the inspiratory period. All effects of OX-A (20 nmol) were attenuated by the orexin receptor 1 antagonist SB 334867. OX-A significantly reduced both sympathoexcitatory peaks of somato-sympathetic reflex while increasing baroreflex sensitivity. OX-A increased the amplitude of the pressor response and markedly amplified the effect of hypoxia on sSNA.
CONCLUSIONS
Thus, activation of OX receptors in rat spinal cord alters cardiorespiratory function and differentially modulates sympathetic reflexes.
Keywords: Orexin A, sympathetic nerve activity, phrenic nerve discharge, somato-sympathetic reflex, baroreflex, hypoxia
Introduction
Orexin A (OX-A) and orexin B (OX-B), also referred to as hypocretin-1 and -2, are neuropeptides that are cleaved from a common precursor, prepro-orexin. The amino acid sequences of the 33-residue peptide OX-A and the 28-residue peptide OX-B are encoded by a single gene localized on human chromosome 17q21 and share 46% homology (de Lecea et al., 1998; Sakurai et al., 1998). The actions of these peptides are mediated by two G-protein coupled receptors, orexin receptor 1 (OX1) and orexin receptor 2 (OX2). OX-A is 10 times more selective for OX1 than OX-B, while OX-A and OX-B have an equal affinity for OX2 (Sakurai et al., 1998). Activation of OX1 results in the activation of Gαq/11, which induces the elevation of intracellular Ca2+, and the OX2 couples to both Gαq/11 and inhibitory Gαi G proteins (Zhu et al., 2003).
Immunohistochemical and in situ hybridization studies have revealed that OX-containing cell bodies are restricted to the lateral hypothalamus, perifornical area and dorsomedial hypothalamus. OX-containing nerve terminals and receptors, on the other hand, are widely distributed in the hypothalamus, thalamus, cerebral cortex, circumventricular organs, brainstem and spinal cord (Elias et al., 1998; Nambu et al., 1999; Llewellyn-Smith et al., 2003). This distribution of OX-nerve terminals establishes a basis for the contributions by OX to the control of multiple physiological functions, including control of energy homeostasis, feeding behaviour, sleep–wake state, stress response, reward and nociception (Kukkonen et al., 2002; Sakurai, 2007; Tsujino and Sakurai, 2009).
There is a growing body of evidence to suggest that OX is involved in central cardiovascular and respiratory control. Intracerebroventricular (third ventricle) or intracisternomagnal application of OX-A or OX-B augments sympathetic outflow and catecholamine release, and a dose-dependent increase in systemic arterial pressure and heart rate (HR) (Shirasaka et al., 1999; Matsumura et al., 2001; Zhang et al., 2005). On the other hand, prepro-orexin knockout mice with a complete lack of both OX-A and OX-B manifest lower baseline arterial pressure than the wild-type controls (Kayaba et al., 2003). However, the effects of intrathecal OX-A on in vivo splanchnic sympathetic nerve activity (sSNA), phrenic nerve activity (PNA) as well as on sympathetic reflexes are unknown.
Sympathetic preganglionic neurones (SPN), located in the intermediolateral cell column (IML) of the spinal cord, receive inputs from different brain regions and regulate the cardiovascular responses through their projections to the adrenal medulla and sympathetic autonomic ganglia in periphery (Pilowsky and Goodchild, 2002; Guyenet, 2006). Orexinergic fibres and receptors are distributed throughout the spinal cord, including IML. OX fibres have also been found in the dorsal and ventral horn neurones of the spinal cord (van den Pol, 1999; Date et al., 2000; Cluderay et al., 2002). The dense innervation of all spinal cord regions by OX fibres, expression of OX receptors on SPN and the depolarizing action of OX on spinal neurones (van den Top et al., 2003) also suggest that OX is a neuropeptide in the spinal cord. Therefore, it is likely that OX not only regulates cardiovascular function acting as a neurotransmitter in the spinal cord but also modulates sympathetic reflexes.
Because OX-A has a greater selectivity for the OX1, we first evaluated the hypothesis that OX-A, by acting on SPN, might influence spinal sympathetic outflow, as assessed by a change in mean arterial pressure (MAP), HR, sSNA when delivered intrathecally (i.t.). Additionally, the effects of i.t. OX-A on PNA and cardiovascular responses to stimulation of somato-sympathetic, arterial baroreceptor and peripheral chemoreceptor reflexes were examined. Our principal findings are that i.t. OX-A causes a dose-dependent increase in MAP, HR and sSNA, and an increase in phrenic nerve discharge. Adaptive reflexes are differentially affected: barosensitivity is enhanced, the somato-sympathetic reflex is attenuated, and the hypoxic chemoreflex is enhanced for MAP and sSNA. The data reveal that OX-A has pleiotropic effects on cardiorespiratory functions and reflexes that warrant further investigation. Part of this work was presented to the Australian Neuroscience Society (Shahid and Pilowsky, 2010).
Methods
All animal experiments in this study complied with the guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (http://www.nhmrc.gov.au/publications/synopses/eal6syn.htm) and were approved by the Animal Ethics Committee of Macquarie University, Sydney, Australia.
Surgical preparation
Surgical preparation was performed as described previously (Burke et al., 2008; Farnham et al., 2008; Gaede et al., 2009). Briefly, male Sprague-Dawley rats (n= 38, 350–600 g) were anaesthetized with urethane (1.2–1.4 g·kg−1, i.p.). The depth of anaesthesia was assessed approximately every 30 min by monitoring changes in arterial pressure in response to pinching a hind paw; supplemental doses of urethane (20–30 mg, i.v.) were given if blood pressure rose more than 10 mmHg. Animals were placed on a feedback-controlled heating blanket for the duration of the experiment to maintain body temperature between 36°C and 37°C (Harvard Apparatus, Holliston, MA, USA).
The right jugular vein and carotid artery were cannulated with polyethylene tubing [internal diamter (I.D.) = 0.58 mm; outer diameter (O.D. = 0.96 mm] for administration of drugs and fluids, and for measurement of arterial blood pressure (AP) respectively. In some experiments, both femoral veins were cannulated to enable administration of sodium nitroprusside (SNP) or phenylephrine hydrochloride (PE). HR was derived from AP. A tracheal cannula permitted artificial ventilation. Ventilation was adjusted so that phrenic nerve discharge was just above the apnoeic threshold. Nerve recordings were made of PNA and sSNA. The left phrenic nerve was approached dorsally and the left greater splanchnic nerve was dissected using a retro-peritoneal approach. The distal end of the respective nerves were tied with silk thread and cut to permit recording of efferent nerve activity. In an additional subset of animals, the sciatic nerve was isolated, tied and cut. Once the nerves were isolated, they were covered with saline-soaked cotton wool for the duration of the remainder of surgical preparation to prevent desiccation. The neurograms were amplified (×10 000, CWE Inc., Ardmore, PA, USA), band-pass filtered (0.1–2 kHz), sampled at 3 kHz (1401 plus, CED Ltd, Cambridge, UK) and recorded on computer using Spike2 software (v7, CED Ltd, Cambridge, UK).
Rats were secured in a stereotaxic frame, paralysed (pancuronium bromide; 0.8 mg i.v. initially, then 0.4 mg·h−1 i.v.) and artificially ventilated with oxygen-enriched room air. End-tidal CO2 was monitored and maintained between 4.0% and 4.5%. Arterial blood gas was monitored, and the rate and depth of ventilation was adjusted to maintain pH at an optimum range (7.35–7.45, pH 7.4 ± 0.01, PaCO2 40.9 ± 0.8). Animals were infused with 5% glucose in saline (1.0–2.0 mL·h−1) to ensure hydration. Three needle electrodes were placed under the skin at the right and left arm and the right hind limb to record an electrocardiogram. Nerve recordings were made with bipolar silver wire electrodes. The recording electrodes were immersed in a pool of liquid paraffin oil to prevent dehydration and for electrical insulation. After being placed on the recording electrodes, the rat was allowed to stabilize for 30–60 min.
Activation of sympathetic reflexes
Reflexes were evoked as described previously (Miyawaki et al., 2002a; Makeham et al., 2004). Activation of somato-sympathetic reflex was achieved by electrical stimulation (10–25 V, 100, 0.2 ms pulses at 1 Hz) of the sciatic nerve with bipolar electrodes. Sympathetic baroreflex function curves were generated by sequential intravenous injection of SNP (0.01 mg·kg−1) and PE (0.01 mg·kg−1) via two different vein lines. Peripheral chemoreceptors were stimulated with a brief period of hypoxia induced by ventilating the animals with 100% N2 for 12–14 s.
i.t. Drug administration
The occipital crest of the skull was exposed, and the atlanto-occipital membrane was incised. A polyethylene catheter (I.D. = 0.4 mm; O.D. = 0.8 mm) was inserted through this slit into the i.t. space and advanced caudally to the levels of T6–T8. The slit was left open to prevent increases in i.t. pressure caused by the injection of agents or by flushing. The volume of each catheter was measured before insertion (range 6–7 µL), and this volume was then used to flush the catheter. A 25-µL Hamilton syringe was used to inject drugs (OX-A; 100 µM, 500 µM, 1 mM and 2 mM equivalent to 1, 5, 10 and 20 nmol, respectively) or vehicle [10 mM phosphate-buffered saline (PBS); pH 7.4] in a total volume of 10 µL. Injections were made over a 15- to 20-s period. In 11 animals, a selective OX1 antagonist, N-(2-methyl-6-benzoxazolyl)-N-1,5-naphthyridin-4-yl-urea 200 nmol (SB 334867; Tocris Bioscience, Bristol, UK), was injected i.t. 20 min prior to the i.t. injection of OX-A (20 nmol): six with OX-A and five without. Successful catheterization into the i.t. space was confirmed by administering L-glutamate (100 mM, 10 µL) and observing sharp increases in blood pressure (∼20 mmHg), HR [∼30 beats per minute (bpm)] and sSNA (∼30%) (e.g. Hong and Henry, 1992).
The following reflexes were activated before and 40 or 15 min after i.t. injection of OX-A (20 nmol) or PBS, respectively: (i) somato-sympathetic reflex; (ii) baroreflex; and (iii) peripheral chemoreceptor reflex. The spread of injectate was determined by observing the distribution of a 10 µL injection of India ink. Following the death of the animals (3 M KCl, 0.5 mL, i.v.), a laminectomy was performed to verify the location of the catheter tip. To avoid possible confounding effects of drug interactions, each animal received only one treatment of OX-A.
Temporary spinal blockade by microinjection of bupivacaine anaesthetic at the C8 spinal level
The spinal cord was anaesthetized at the C8 level by microinjection of a local anaesthetic, bupivacaine (500 nL, AstraZeneca, Australia) into the middle of each hemi-spinal cord in four animals. These injections were always adequate to cause blood pressure and sympathetic nerve activity to fall to levels equivalent to that seen following spinal transection at the C8 level (Goodchild et al., 2008). Following local anaesthetic injection at C8, OX-A (20 nmol) or PBS was injected i.t.
Data acquisition and analysis
Neurograms were rectified and smoothed (sSNA, 1 s time constant; PNA, 50 ms). Minimum background activity after death was taken as zero sSNA, and this value was subtracted from sSNA before analysis with off-line software (Spike 2 version 6.01). To analyse blood pressure, HR, sSNA, phrenic nerve amplitude (PNamp), phrenic nerve frequency (PNf), neural minute ventilation (=PNamp × PNf), duration of inspiratory burst (TI) and duration of expiratory period (TE), baseline values were obtained by averaging 60 s of data, 5 min prior to drug or PBS injection. Maximum responses were expressed as absolute changes in MAP, HR and PNf, and percent changes in sSNA, PNamp and neural minute ventilation from baseline values. Time course analysis averaged 60 s of data every 5 min from 0 min to 60 or 70 min post-injection for OX-A. To evaluate cardiorespiratory coupling, phrenic-triggered ensemble averages of sSNA were generated from 60-s portions of data. The area under the curve (AUC), less baseline, of sSNA activity during the inspiratory and post-inspiratory phases was determined. sSNA was rectified and smoothed at 1 s and 5 ms time constants to analyse baroreceptor reflex and somato-sympathetic reflex respectively. To analyse reflexes, sSNA was normalized between the activity of sSNA before PBS injection (100%) and the sSNA after death (0%). The sSNA response to sciatic nerve stimulation was analysed using peristimulus waveform averaging. The AUC of the sympathoexcitatory peaks was analysed. The maximum response to stimulation was then expressed as a percentage change from the baseline (control). The response to hypoxia (100% N2 inhalation for 12–14 s) was quantified by comparing the average maximum sSNA during hypoxia compared with a control period during normal hyperoxic ventilation. The percentage changes were calculated according to the formulae below.
Where OXAHR is the response to hypoxia following OX-A, PBSHR is the response to hypoxia following PBS injection. CHR is the response to hypoxia in the absence of any i.t. injection.
Analysis was conducted with GraphPad Prism (version 5.0) (GraphPad, La Jolla, CA, USA). All values are expressed as mean ± standard error. Results are presented as control versus OX-A. Paired t-test was used to analyse peak effects and reflexes. A one-way repeated-measures analysis of variance with Dunnett's post hoc multiple comparison test was used to compare values after OX-A (20 nmol) administration with the baseline value. P < 0.05 was considered significant.
Drugs
OX-A (molecular weight = 3561.2) was obtained from Bachem AG (Bubendorf, Switzerland), SB 334867 from Tocris Bioscience. Urethane, L-glutamate, PE and SNP were purchased from Sigma-Aldrich (St. Louis, MO, USA); pancuronium bromide and bupivacaine from AstraZeneca Pty Ltd (Sydney, NSW, Australia); and PBS (10 mM in 0.9% NaCl) tablets from AMRESCO Inc. (Solon, OH, USA). OX-A was dissolved and further diluted in PBS (10 mM; pH 7.4). SB 334867 was dissolved and further diluted in 10% dimethyl sulphoxide. PBS, PE and SNP were prepared in de-ionized water. Urethane was dissolved in 0.9% NaCl, and L-glutamate was dissolved in PBS.
Results
Effects of i.t. OX-A on resting cardio-respiratory parameters
To test the hypothesis that exogenous application of OX-A to the spinal cord modulates cardiorespiratory responses, OX-A (1, 5, 10 and 20 nmol) was administered i.t., and the effects on MAP, HR, sSNA, PNf, PNamp, neural minute ventilation, TE and TI were evaluated (n= 34). OX-A evoked a dose-dependent and significant increase in MAP, HR and sSNA (Figure 1A, B). The maximum elevation occurred after injection of 20 nmol OX-A, and the highest levels attained were 32 ± 5 mmHg (MAP, n= 6, P < 0.01), 52 ± 6 bpm (HR, n= 6, P < 0.001) and 100 ± 9% (sSNA, n= 6, P < 0.001) from the baseline (Figure 1A, B). Following administration of OX-A (20 nmol, i.t.), the peak pressor effect was reached after approximately 20 min (Figure 1A, C). Blood pressure was monitored for about 70 min, and remained elevated (Figure 1C). Both HR and sSNA increased progressively after 20 nmol OX-A injection, reaching a maximum change of 35 bpm and 97%, respectively, at the end of the recording period (Figure 1A, C). Injection of PBS (vehicle) into the spinal cord was without effect on MAP (5 ± 1 mmHg, n= 6), HR (5 ± 1 bpm, n= 6) or sSNA (8 ± 1%, n= 6) above basal values (Figure 1A–C).
Figure 1.
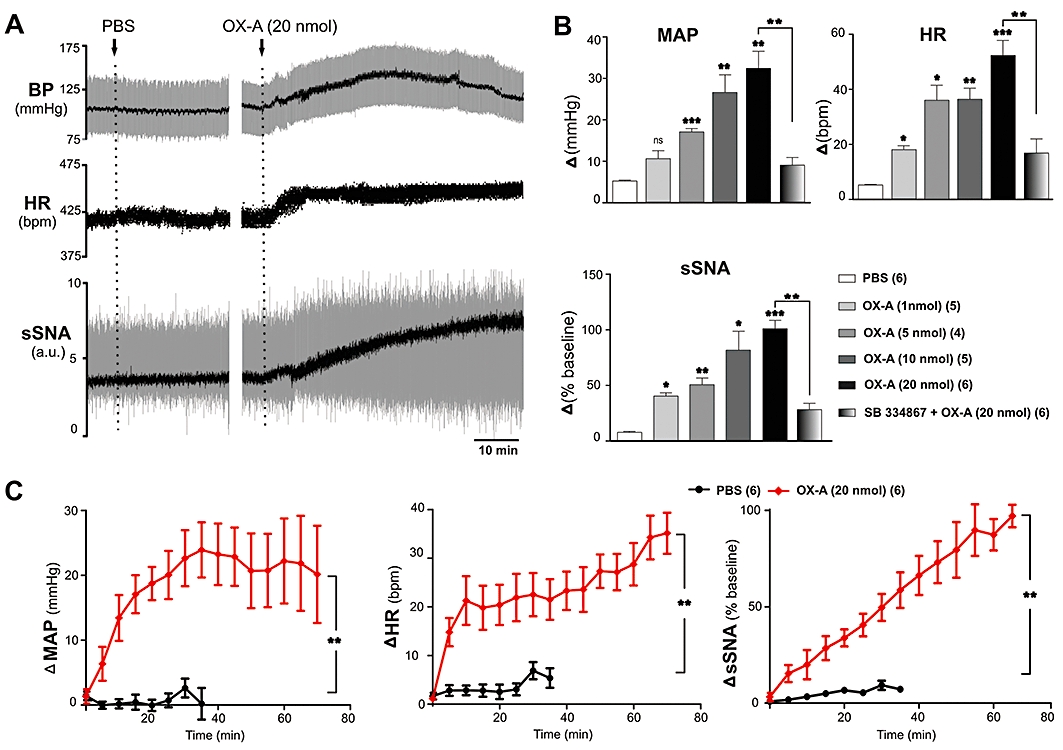
Effect of intrathecal injection of orexin A (OX-A) on mean arterial pressure (MAP), heart rate (HR) and splanchnic sympathetic nerve activity (sSNA). (A) Representative trace of data from a recording of blood pressure (BP), HR and sSNA (arbitrary unit, a.u.) before and after injection of phosphate-buffered saline (PBS) or OX-A (20 nmol). Rectified and integrated sSNA (black) is superimposed over raw sSNA (grey). MAP (black) is superimposed over BP (grey). (B) Comparison of peak cardiovascular effects produced by PBS, OX-A (1, 5, 10 and 20 nmol) or SB334867 (200 nmol) + OX-A (20 nmol). Peak effects are shown as absolute (BP, HR) or percentage (sSNA) change from their respective basal values. (C) Grouped time course effects of PBS (black) or OX-A (20 nmol) (red) on MAP, HR and sSNA. Values are expressed as mean ± standard error. Number of animals are shown in parentheses. bpm, beats per minute; ns, non-significant; ***P < 0.001, **P < 0.01, *P < 0.05 compared with PBS [except SB 334867 (200 nmol) + OX-A (20 nmol) that was compared with OX-A (20 nmol)].
Injection of OX-A (1, 5, 10 and 20 nmol) evoked a dose-dependent increase in PNamp, and neural minute ventilation and a decrease in PNf (Figures 2A, B and 3A). The maximum decrease in PNf of 18 ± 2 bursts·min−1 (n= 6, P < 0.01) was elicited by OX-A (20 nmol). OX-A (20 nmol) also caused a peak increase in PNamp of 62 ± 9% (n= 6, P < 0.01) and neural minute ventilation of 53.47 ± 12% (n= 6, P < 0.05) from the baseline (Figure 3A). Following OX-A (20 nmol) administration, PNf decreased, PNamp and neural minute ventilation increased gradually reaching peak levels after about 30 min (Figure 2A, B). There is a clear dose–response relationship between the concentration of OX-A and increasing PNamp (Figure 3A). A dose–response relationship is also seen between the concentration of OX-A and the fall in PNf (Figure 3A). However, the maximum effect on PNamp is seen at 20 nmol, while the effect on PNf is maximal at 5 nmol; this means that beyond 5 nmol, there is an increasing effect on phrenic neural minute ventilation. OX-A (20 nmol) caused a lengthening of TE and a shortening of TI, reaching a peak level of 0.75 ± 0.1 s (n= 5, P < 0.05) and −0.122 ± 0.03 s (n= 3, P < 0.05) respectively (Figures 2A, B and 3B). No significant changes in PNf, PNamp, neural minute ventilation, TE or TI were observed after injection of PBS (vehicle) (Figures 2A, B and 3A, B).
Figure 2.
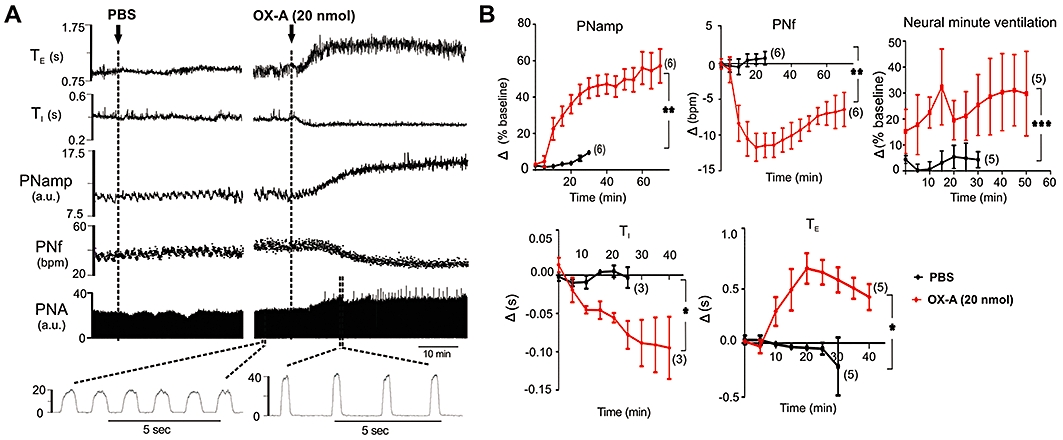
Effect of intrathecal injection of orexin A (OX-A)on phrenic nerve activity (PNA). (A) Representative trace of data from a recordings of rectified PNA (arbitrary unit, a.u.), phrenic nerve frequency (PNf), phrenic nerve amplitude (PNamp), inspiratory period (TI) and expiratory period (TE) before and after injection of phosphate-buffered saline (PBS) or OX-A. (B) Grouped time course effects of PBS (black) or OX-A (20 nmol) (red) on PNamp, PNf, neural minute ventilation, TI and TE. Following injection of OX-A there is an increase in PNamp associated with a bradypnoea that is due to both an increase in TE and a decrease in TI. There is an overall increase in neural minute ventilation over the period of the response. Values are expressed as mean ± standard error. Number of animals is shown in parentheses. bpm, beats per minute; ns, non-significant; ***P < 0.001, **P < 0.01, *P < 0.05 compared with PBS.
Figure 3.
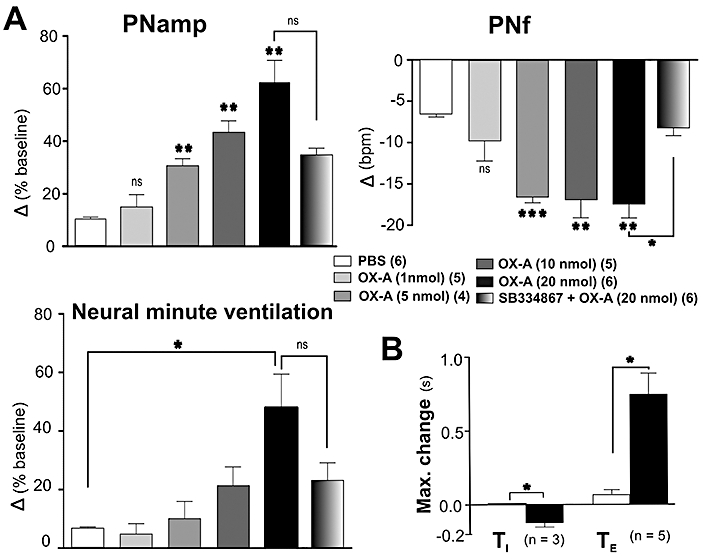
Effect of intrathecal injection of orexin A (OX-A)on phrenic nerve activity (PNA). (A) Comparison of peak effects produced by phosphate-buffered saline (PBS), OX-A (1, 5, 10 and 20 nmol) or SB 334867 (200 nmol) + OX-A (20 nmol) on PNamp, PNf and neural minute ventilation. Peak effects are shown as absolute or percentage change from respective basal values. (B) Grouped data illustrating the effects of PBS and OX-A (20 nmol) on TI and TE. Values are expressed as mean ± standard error. Number of animals is shown in parentheses. bpm, beats per minute; ns, non-significant; ***P < 0.001, **P < 0.01, *P < 0.05 compared with PBS [except SB 334867 (200 nmol) + OX-A (20 nmol) that was compared with OX-A (20 nmol)].
In the absence of OX-A, SB 334867 (200 nmol) did not affect MAP, HR, sSNA, PNamp, PNf or neural minute ventilation (n= 5) (data not shown). On the other hand, the cardiorespiratory effects of OX-A (20 nmol) were significantly reduced by prior i.t. injection of SB 334867 [MAP: 9 ± 2 vs. 32 ± 5 mmHg, P < 0.01; HR: 16 ± 6 vs. 52 ± 6 bpm, P < 0.01; sSNA: 28 ± 6 vs. 100 ± 9%, P < 0.01; PNamp: 34 ± 3 vs. 62 ± 8%, not significant (ns); PNf: −8 ± 1 vs. −18 ± 2 bursts·min−1, P < 0.05; neural minute ventilation: 23 ± 6 vs. 53 ± 12%, ns, of baseline; n= 6, Figures 1B and 3A].
Peri-phrenic averaging of the sympathetic nerve activity reveals an inspiratory (I) and post-inspiratory (P-I) peak of sSNA (Figure 4A). The amplitude of P-I peak of sSNA was significantly increased by i.t. injection of 20 nmol OX-A (153 ± 41% vs. 13 ± 7% of PBS; P < 0.05; n= 5, Figure 4A, C) over baseline (control). OX-A showed a gradual increase in P-I peak over time (Figure 4B). In contrast, no significant change in the amplitude of I peak was observed (43 ± 9% vs. 27 ± 3% of PBS; ns; n= 5, Figure 4A, C).
Figure 4.
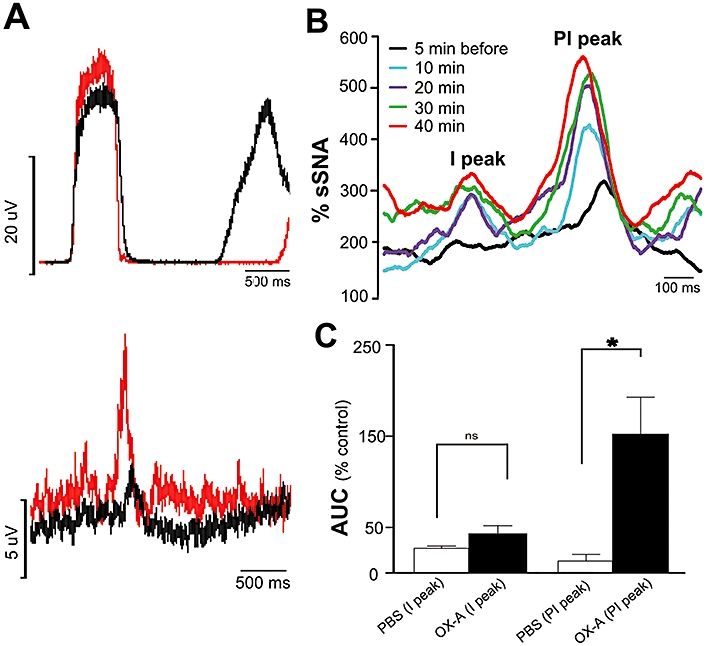
Effect of orexin A (OX-A) on phrenic nerve discharge-related rhythmicity of sSNA. (A) Phrenic-triggered average of sSNA before (black) and after (red) intrathecal injection of OX-A (20 nmol). (B) Time course effect of OX-A (20 nmol) on inspiratory (I) and post-inspiratory (PI) related activity of sSNA. (C) Grouped data illustrating the effects of OX-A (20 nmol, n= 5) on I and PI peaks of sSNA. Note that there is a marked increase in PI following OX-A injection. Values are expressed as mean ± standard error. ns, non-significant; *P < 0.05 compared with phosphate-buffered saline (PBS). Both PBS and OX-A values were normalized to the control period prior to injections.
Effects of bupivacaine anaesthesia at the C8 spinal level on OX-A activity
C8 anaesthesia caused a fall in MAP (from 95 ± 7 to 59 ± 3 mmHg), HR (from 459 ± 8 to 408 ± 19 bpm) and sSNA (−45 ± 12%) without any change in PNA (n= 3, Figure 5B). Following C8 transection, i.t. OX-A (20 nmol) caused a greater increase in MAP, HR and sSNA, but for a shorter period when compared with intact animals (MAP: 74 ± 7 vs. 32 ± 5 mmHg, P < 0.05; HR: 51 ± 10 vs. 52 ± 6 beats·min−1, ns; sSNA: 292 ± 26 vs. 100 ± 9%, P < 0.05, of the baseline, Figure 5A–C). Conversely, the effects of OX-A on PNA were abolished when compared with the response prior to C8 anaesthesia (PNamp: 10 ± 4 vs. 62 ± 8%, P < 0.05; PNf: 0.7 ± 1 vs. −18 ± 2 bursts·min−1, P < 0.05, of the baseline; Figure 5A–C). PBS injection after C8 anaesthesia induced no change in MAP, HR, sSNA or PNA (n= 1; data not shown).
Figure 5.
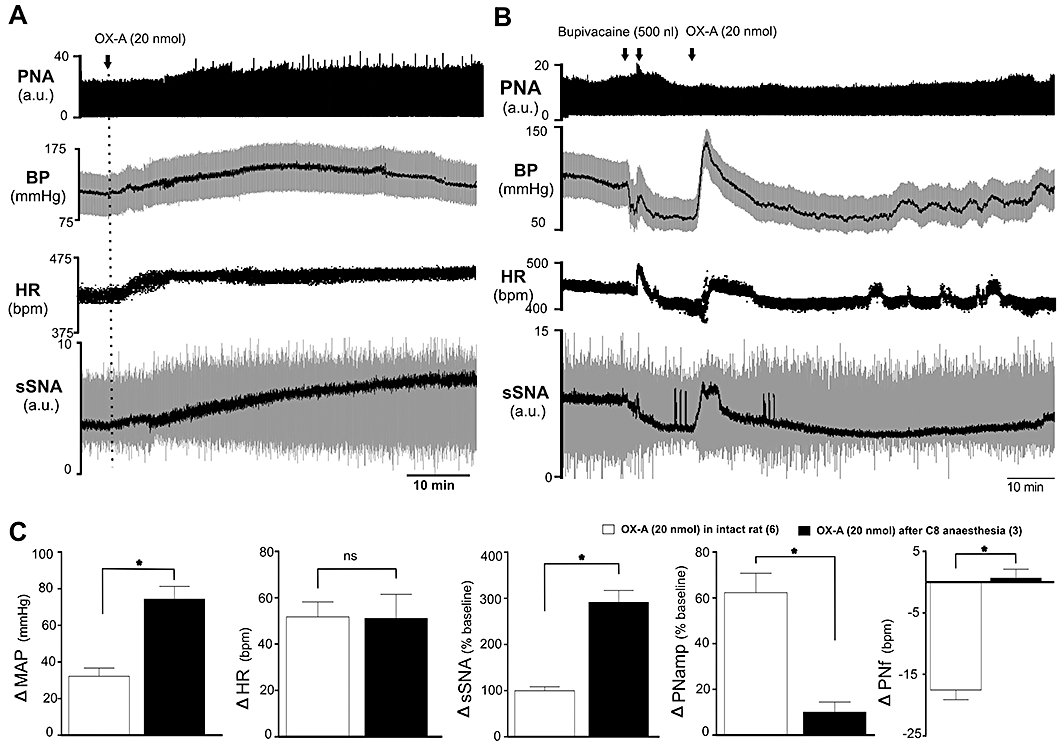
Effect of intrathecal orexin A (OX-A; 20 nmol) on mean arterial pressure (MAP), heart rate (HR), splanchnic sympathetic nerve activity (sSNA) and phrenic nerve activity (PNA) in an intact rat (A) and a C8 anaesthetized rat (B). (A) Representative trace of data from a recording of PNA, blood pressure (BP), HR and sSNA (arbitrary unit, a.u.) in intact rat. Integrated sSNA (black) is superimposed over raw sSNA (grey). MAP (black) is superimposed over blood pressure (BP) (grey). (B) Representative trace of data from a recording of PNA, BP, HR and sSNA in a C8 anaesthetized rat. (C) Comparison of peak cardio-respiratory effects produced by OX-A (20 nmol) in intact (n= 6) and C8 anaesthetized rat (n= 3). Peak effects are shown as absolute or percentage change from respective basal values. Values are expressed as mean ± standard error. Note that OX-A causes a greater increase in MAP, HR and sSNA for a shorter period when compared with the response prior to C8 anaesthesia. The effect on PNA was abolished. Number of animals is shown in parentheses. bpm, beats·per minute (HR) or bursts·per minute (PNf); ns, non-significant; *P < 0.05 compared with OX-response in intact animal.
Effects of OX-A on somato-sympathetic reflex
The average sSNA response to intermittent stimulation of the sciatic nerve was evaluated (somato-sympathetic reflex) before and after i.t. injection of PBS (vehicle) or OX-A (20 nmol). In five animals, intermittent stimulation of the sciatic nerve resulted in two characteristic excitatory peaks in sSNA with latencies of 93 ± 3 ms and 188 ± 2 ms (n= 5, Figure 6A). The latencies were not significantly altered by PBS (91 ± 3 ms and 186 ± 1 ms, n= 5, ns) or by OX-A (89 ± 2 ms and 191 ± 4 ms, n= 5, ns) injection. OX-A significantly increased the basal sSNA to 221 ± 22% (n= 5, P < 0.05) as compared with PBS (100 ± 7%). OX-A markedly attenuated the amplitude of both excitatory peaks. The first and second sympatho-excitatory peaks were attenuated by 25 ± 10% and 72 ± 13% of the baseline (n= 5, P < 0.05, Figure 6A), as compared with the effect seen following PBS injection (Figure 6A).
Figure 6.
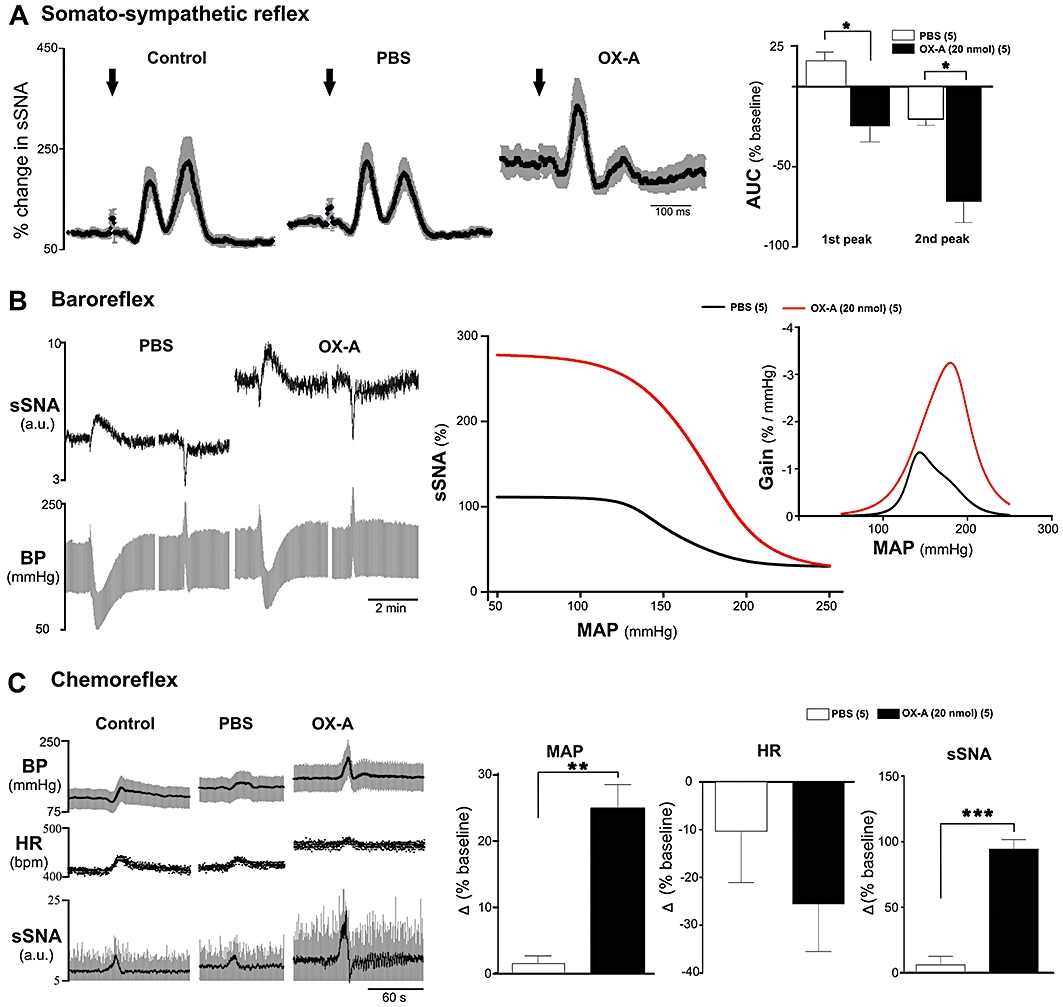
Effect of intrathecal injection of orexin A (OX-A) on somato-sympathetic, baroreceptor and peripheral chemoreceptor reflex. (A) Effect on somato-sympathetic reflex evoked by sciatic nerve (SN) stimulation. Left 3 traces represent grouped effect of SN-evoked stimulation of sSNA at control period and after injection of phosphate-buffered saline and OX-A. Data are mean (black) ± standard error (grey). Arrows indicate the time of stimulation. Trace at right represents group data illustrating the heights of 1st and 2nd sympathoexcitatory peaks. (B) Effect on baroreceptor reflex evoked by intravenous injection of SNP and PE. Left trace represents experimental recording of the effect of changes in BP on sSNA due to SNP or PE after phosphate-buffered saline (PBS) and OX-A injection. Middle trace shows average sympathetic baroreflex functional curves generated for data after PBS (black) or OX-A (red) injection. Trace at right represents baroreflex gain for sSNA (error bars are omitted for clarity). (C) Effect of peripheral chemoreceptor reflex activated by brief hypoxia with 100% N2 for 12–14 s. Left trace shows experimental recording of hypoxic episodes at control period and after PBS or OX-A injection. 3 traces of right represent comparison of peak effects on MAP, HR and sSNA after intrathecal injection of PBS and OX-A in response to brief hypoxia. Values are expressed as mean ± standard error. Number of animals is shown in parentheses. bpm, beats per minute; ns, non-significant; **P < 0.01, *P < 0.05 compared with PBS.
Effects of OX-A on baroreflex
In five animals, the changes in sSNA were plotted against the changes in MAP evoked by i.v. injection of SNP and PE. The changes in AP following PE injection were of nearly equal magnitude (71 ± 7 mmHg following PBS injection, and 65 ± 8 mmHg following OX-A injection). In fact, the blood pressure increase following PE after OX-A injection was, if anything, slightly smaller than the increase that followed PBS injection. OX-A (20 nmol, i.t.) significantly enhanced the reflex sympathoexcitatory and inhibitory responses evoked by these equipotent doses of SNP and PE (Figure 6B). OX-A significantly increased the upper plateau, range of sSNA and maximum gain of the sSNA without significantly altering lower plateau, the threshold level, midpoint, the saturation levels of MAP and the operating range as compared with PBS (Table 1).
Table 1.
Parameters describing baroreflex control of sSNA after intrathecal injection of PBS or OX-A (20 nmol)
| Lower plateau (%) | Upper plateau (%) | Mid-point (mmHg) | Max. gain (%/mmHg) | Range of SNA (%) | Threshold level (mmHg) | Saturation level (mmHg) | Operating range (mmHg) | |
|---|---|---|---|---|---|---|---|---|
| PBS | 29.7 ± 8.7 | 111.2 ± 2.3 | 157.9 ± 7.0 | −1.8 ± 0.4 | 81.6 ± 9.4 | 118.0 ± 6.6 | 197.8 ± 12.9 | 79.7 ± 14.9 |
| OX-A (20 nmol) | 24.6 ± 16.9 (ns) | 279.1 ± 38.0 (P < 0.01) | 175.2 ± 6.2 (ns) | −3.6 ± 0.6 (P < 0.05) | 254.6 ± 48 (P < 0.01) | 121.4 ± 10.4 (ns) | 228.9 ± 12.1 (ns) | 107.5 ± 18.8 (ns) |
Values are means ± standard error (n= 5). Maximum (Max.) gain is the slope of the sigmoid curve of best fit at the MAP corresponding to steepest part of the curve.
ns, non-significant; OX-A, orexin A; PBS, phosphate-buffered saline; SNA, sympathetic nerve activity.
Effects of OX-A on peripheral chemoreflex
Activation of peripheral chemoreceptors with brief hypoxia evoked an increase in MAP, sSNA and HR (Figure 6C). Peak effects occurred near the end of stimulus and recovered rapidly to baseline. Injection of OX-A (20 nmol) into the spinal cord significantly increased the pressor response which was 25 ± 4% greater than control, compared with the PBS response which was only 1 ± 1% greater than control (n= 5, P < 0.01, Figure 6C). The sympathoexcitatory effect was also markedly increased by OX-A compared with the response following PBS (94 ± 7% vs. 6 ± 7%, n= 5, P < 0.001, Figure 6C). The effect on HR was unchanged by OX-A (−25 ± 10 vs. −10 ± 11%, n= 5, ns, Figure 6C). OX-A increased peak PNf, but had no effect on the peak amplitude of PNamp and neural minute ventilation during hypoxia [PNf: 21 ± 5 vs. 1 ± 2%, P < 0.05; peak PNamp: −35 ± 11 vs. −21 ± 12%, ns; neural minute ventilation: 7 ± 5 vs. −4 ± 7%, ns, of control baseline (n= 5)].
Discussion
This study is the first to investigate the in vivo effects of i.t. OX-A on sympathetic outflow, phrenic burst discharge and sympathetic physiological reflexes. The major findings are that i.t. injection of OX-A in the 1–20 nmol range (i) elicits sympathetically mediated hypertension and tachycardia, confirming an earlier report (Antunes et al., 2001); (ii) modulates respiratory drive by increasing PNamp and decreasing PNf; (iii) reduces both sympathoexcitatory peaks in response to sciatic nerve stimulation; (iv) increases sympathetic baroreflex sensitivity; and (v) potentiates the pressor responses and sympathoexcitation to hypoxia.
SPN integrate the central nervous system output to vasomotor pathways and chromaffin cells (Gilbey, 1997). L-Glutamate, or a related amino acid, is likely to be the major excitatory neurotransmitter to these neurones (Pilowsky and Goodchild, 2002; Pilowsky et al., 2009). Extensive evidence supports the view that the excitatory cardiovascular effects in the spinal cord are modulated by a number of neuropeptides (Pilowsky et al., 2009), including vasopressin (Riphagen and Pittman, 1989), angiotensin (Yashpal et al., 1989), substance P (Yashpal et al., 1987) and pituitary adenylate cyclase activating polypeptide (PACAP) (Farnham et al., 2008).
OX-A fibres and nerve terminals are widely distributed throughout the spinal cord in rat, mouse and human (Cutler et al., 1999; van den Pol, 1999; Date et al., 2000). OX-A directly, and reversibly, depolarizes SPN by activation of pertussis toxin-sensitive G-proteins and closure of a K+ conductance via a protein kinase A-dependent pathway (Antunes et al., 2001; van den Top et al., 2003). Pretreatment with α1 and β-adrenoceptor antagonists attenuates the pressor and tachycardiac response to i.t. OX-A. Intravenous OX-A does not affect blood pressure or HR (Chen et al., 2000; Antunes et al., 2001). These findings support the idea that the pressor and tachycardiac effects induced by i.t. OX-A are mediated centrally. In addition, mRNA for OX1 and OX2 are expressed on the majority of SPN and neurones in the dorsal and ventral horns of the spinal cord (Cluderay et al., 2002; van den Top et al., 2003; Guan et al., 2004). OX1 is selective for OX-A and OX2 interacts with both OX-A and OX-B (Sakurai et al., 1998). As a selective OX1 antagonist was unable to abolish the responses to OX-A completely, it seems likely that the excitatory effects of OX-A observed in our study may be due to both OX1 and OX2.
An unanticipated finding of this study is that PNA increased after OX-A injection at T6–T8 spinal levels. The respiratory effects of i.t. OX-A do not appear to be due to either retrograde diffusion of the peptide into supra-spinal structures or diffusion to C3–C5 level, because (i) C8 anaesthesia abolished the effects of OX-A on PNA; and (ii) India ink injected i.t. at the end of the experiments only spread as far rostral as T2. Moreover, in our previous study, we reported that transection at the C1 level leaves the effects of i.t. bicuculline infusion unaltered (Goodchild et al., 2000; 2008;) and i.t. administration of drugs appear not to spread more than a few segments away from the site of administration (Yaksh and Rudy, 1976) and are able to penetrate around 2 mm (Yamada et al., 1984). Furthermore, it is known that in an adult cat, SPN have dendrites that reach the dorsal pial surface of the spinal cord (Pilowsky et al., 1994); in the neonatal rat, dendrites of SPN are reported to project towards the dorsolateral funiculus (Shen and Dun, 1990), but it is not yet known if they reach the sub-pial area in the adult. Paralysis, vagotomy and artificial ventilation of the animals rule out the possibility that OX-A acts directly on intercostal and abdominal motor neurones, thereby limiting lung inflation leading to an increase in phrenic nerve output. The mechanisms underlying the increase in PNamp, decrease in PNf and increase in neural minute ventilation following i.t. OX-A injection are currently unknown. Conceivably, activation of spinal OX receptors might affect sensory and intraspinal pathways, thereby modulating spinal pattern generators. On the other hand, the decrease in PNf is likely to be mediated by spino-bulbar connections modulating the respiratory rhythm generator.
The very long-lasting increase in PNA and sympathetic nerve activity (SNA) following i.t. OX-A seen here mimics the long-term facilitation (LTF) of PNA and SNA induced by acute intermittent hypoxia (AIH) (Xing and Pilowsky, 2010). A possible role for OX-A in AIH/LTF is supported by the finding that OX neurone ablated mice have a reduced propensity to develop AIH/LTF (Toyama et al., 2009).
SNA activity displays a rhythmic fluctuation in relation to PNA (Adrian et al., 1932; Miyawaki et al., 2002b) that is dependent on the level of arterial CO2. The significant increase in post-inspiratory peak, as well as change in baseline, activity of sSNA by i.t. OX-A may be due to the depolarization and increase in sensitivity of SPN (Date et al., 2000; Antunes et al., 2001; van den Top et al., 2003). A large part of the enhancement of SNA seen here was clearly related to a marked increase in phrenic discharge-related activity in SNA.
Lamina I of spinal cord receives inputs from myelinated and unmyelinated nociceptors and transfers the information to brainstem including rostral ventrolateral medulla (RVLM) and higher regions of the brain (Sato and Schmidt, 1973; McMullan et al., 2008). The somato-sympathetic response, integrated in the RVLM, is relayed to SPN (Stornetta et al., 1989; Miyawaki et al., 2002a; Makeham et al., 2005). The present study demonstrates that the activation of OX receptors in the spinal cord inhibited both the first and second sympatho-excitatory peaks of the somato-sympathetic reflex induced by sciatic nerve stimulation. This reflex inhibition was unrelated to OX-A-induced sympathoexcitation. The distribution of OX fibres as well as receptors in dorsal horn, including superficial lamina (van den Pol, 1999; Date et al., 2000; Grudt et al., 2002) and in dorsal root ganglionic cells (Bingham et al., 2001) suggest that OX-A might modulate somato-sympahetic reflex at the spinal level. Activation of spinal γ-aminobutyric acid –‘A’ subtype receptors is hypothesized to decrease nociceptive traffic in the spinothalamic tract. OX receptors have been found to increase pre-synaptic release of GABA in the lateral hypothalamus (van den Pol et al., 1998). If this is also the case in spinal cord, i.t. OX-A-induced inhibition of somato-sympathetic reflex might be due to the modulation of sensory information from primary afferents by increasing the release of GABA. However, the precise mechanism is yet to be established.
RVLM neurones that are inhibited by baroreceptors project to SPN and provide the major descending excitatory input responsible for baroreflex activity (Pilowsky and Goodchild, 2002; Pilowsky et al., 2009). Two pathways may mediate baroreceptor-induced sympathoinhibition: (i) inhibition of tonically active descending excitatory pathway, which is believed to be the principal mode of action (Pilowsky and Goodchild, 2002); and (ii) a direct spinal inhibitory mechanism (Wang et al., 2008). The present study reveals an increase in the upper plateau, range and maximum gain of the baroreflex function curves obtained from sSNA, indicating that i.t. OX-A significantly increases baroreflex sensitivity. OX-A may modulate either the disfacilitation or the direct inhibition at the spinal level to enhance the baroreflex sensitivity.
Hypoxia causes a rapid and reversible excitation of reticulospinal sympathoexcitatory RVLM neurones that monosynaptically excite SPN. i.t. Kynurenate blocks the sympathetic excitation elicited by hypoxia, revealing the involvement of glutamatergic transmission (Sun and Reis, 1994). i.t. OX-A increases the amplitude of the pressor response and markedly amplifies the chemoreflex effect on sSNA. PNf was increased, but PNamp was not affected, and neural minute ventilation was unchanged following OX-A.
In conclusion, the present study reveals a direct excitatory effect of OX-A on SPN that is presumably mediated by the activation of OX1 and OX2 in the rat spinal cord and a potent modulation of respiratory drive to SPN. Our results also show that OX-A differentially modulates behavioural reflexes. Collectively, these data help to elucidate the role of the OX-A system in the regulation of autonomic nervous system and physiological reflexes.
Acknowledgments
Work in the Authors' laboratory is supported by grants from the National Health and Medical Research Council of Australia (457080, 457069, 604002), Macquarie University and the Garnett Passe and Rodney Williams Memorial Foundation. I.Z.S. and A.A.R. are supported by a Macquarie Research Excellence Scholarship. The authors are grateful to Drs Qi-Jian Sun, Peter Burke, Simon McMullan and Ms Melissa Inglott for helpful discussions.
Glossary
Abbreviations
- AIH
acute intermittent hypoxia
- AUC
area under the curve
- ECG
electrocardiogram
- HR
heart rate
- ICM
intracisternomagna
- ICV
intracerebroventricular
- IML
intermediolateral cell column; i.t., intrathecal
- LTF
long term facilitation
- MAP
mean arterial pressure
- OX
orexin
- OX-A
orexin A
- OX-B
orexin B
- OX1
orexin receptor 1
- OX2
orexin receptor 2
- PE
phenylephrine hydrochloride
- PNA
phrenic nerve activity
- PNamp
phrenic nerve amplitude
- PNf
phrenic nerve frequency
- RVLM
rostral ventrolateral medulla
- SAP
systemic arterial pressure; SB 334867 (N-(2-methyl-6-benzoxazolyl)-N-1,5-naphthyridin-4-yl-urea
- SNP
sodium nitroprusside
- SPN
sympathetic preganglionic neurones
- sSNA
splanchnic sympathetic nerve activity
- TE
expiratory period
- TI
inspiratory period
Conflict of interest
The authors have no conflicts of interest to declare.
Supplemental material
References
- Adrian ED, Bronk DW, Phillips G. Discharges in mammalian sympathetic nerves. J Physiol (Lond) 1932;74:115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes VR, Cristina Brailoiu G, Kwok EH, Scruggs P, Dun ANJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol. 2001;281:R1801–R1807. doi: 10.1152/ajpregu.2001.281.6.R1801. [DOI] [PubMed] [Google Scholar]
- Bingham S, Davey PT, Babbs AJ, Irving EA, Sammons MJ, Wyles M, et al. Orexin-A, an hypothalamic peptide with analgesic properties. Pain. 2001;92:81–90. doi: 10.1016/s0304-3959(00)00470-x. [DOI] [PubMed] [Google Scholar]
- Burke PG, Li Q, Costin ML, McMullan S, Pilowsky PM, Goodchild AK. Somatostatin 2A receptor-expressing presympathetic neurons in the rostral ventrolateral medulla maintain blood pressure. Hypertension. 2008;52:1127–1133. doi: 10.1161/HYPERTENSIONAHA.108.118224. [DOI] [PubMed] [Google Scholar]
- Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol. 2000;278:R692–R697. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Morris R, Sheridhar V, Wattam TAK, Holmes S, Patel S, et al. Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides. 1999;20:1455–1470. doi: 10.1016/s0196-9781(99)00157-6. [DOI] [PubMed] [Google Scholar]
- Date Y, Mondal MS, Matsukura S, Nakazato M. Distribution of orexin-A and orexin-B (hypocretins) in the rat spinal cord. Neurosci Lett. 2000;288:87–90. doi: 10.1016/s0304-3940(00)01195-2. [DOI] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- Farnham MM, Li Q, Goodchild AK, Pilowsky PM. PACAP is expressed in sympathoexcitatory bulbospinal C1 neurons of the brain stem and increases sympathetic nerve activity in vivo. Am J Physiol. 2008;294:R1304–R1311. doi: 10.1152/ajpregu.00753.2007. [DOI] [PubMed] [Google Scholar]
- Gaede AH, Lung MS, Pilowsky PM. Catestatin attenuates the effects of intrathecal nicotine and isoproterenol. Brain Res. 2009;1305:86–95. doi: 10.1016/j.brainres.2009.09.088. [DOI] [PubMed] [Google Scholar]
- Gilbey MP. Fundamental aspects of the control of sympathetic preganglionic neuronal discharge. In: Jordan D, editor. Central Nervous Control of Autonomic Function. Vol. 11. Singapore: Harwood Academic Publishers; 1997. pp. 1–28. [Google Scholar]
- Goodchild AK, Van Deurzen BTM, Sun Q-J, Chalmers J, Pilowsky PM. Spinal GABAA receptors do not mediate the sympathetic baroreceptor reflex in the rat. Am J Physiol. 2000;279:R320–R331. doi: 10.1152/ajpregu.2000.279.1.R320. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Van Deurzen BTM, Hildreth CM, Pilowsky PM. Control of sympathetic, respiratory and somatomotor outflow by an intraspinal pattern generator. Clin Exp Pharmacol Physiol. 2008;35:447–453. doi: 10.1111/j.1440-1681.2008.04913.x. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, van den Pol AN, Perl ER. Hypocretin-2 (orexin-B) modulation of superficial dorsal horn activity in rat. J Physiol (Lond) 2002;538:517–525. doi: 10.1113/jphysiol.2001.013120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JL, Wang QP, Hori T, Takenoya F, Kageyama H, Shioda S. Ultrastructure of orexin-1 receptor immunoreactivities in the spinal cord dorsal horn. Peptides. 2004;25:1307–1311. doi: 10.1016/j.peptides.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hong Y, Henry JL. Glutamate, NMDA and NMDA receptor antagonists: cardiovascular effects of intrathecal administration in the rat. Brain Res. 1992;569:38–45. doi: 10.1016/0006-8993(92)90366-h. [DOI] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol. 2003;285:R581–R593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Holmqvist T, Ammoun S, Akerman KE. Functions of the orexinergic/hypocretinergic system. Am J Physiol. 2002;283:C1567–C1591. doi: 10.1152/ajpcell.00055.2002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X-B, Foye PE, Danielson PE, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Martin CL, Marcus JN, Yanagisawa M, Minson JB, Scammell TE. Orexin-immunoreactive inputs to rat sympathetic preganglionic neurons. Neurosci Lett. 2003;351:115–119. doi: 10.1016/s0304-3940(03)00770-5. [DOI] [PubMed] [Google Scholar]
- McMullan S, Pathmanandavel K, Pilowsky PM, Goodchild AK. Somatic nerve stimulation evokes qualitatively different somatosympathetic responses in the cervical and splanchnic sympathetic nerves in the rat. Brain Res. 2008;1217:139–147. doi: 10.1016/j.brainres.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Makeham JM, Goodchild AK, Costin NS, Pilowsky PM. Hypercapnia selectively attenuates the somato-sympathetic reflex. Respir Physiol Neurobiol. 2004;140:133–143. doi: 10.1016/j.resp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Makeham JM, Goodchild AK, Pilowsky PM. NK1 receptor activation in rat rostral ventrolateral medulla selectively attenuates somato-sympathetic reflex while antagonism attenuates sympathetic chemoreflex. Am J Physiol Physiol. 2005;288:R1707–R1715. doi: 10.1152/ajpregu.00537.2004. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T, Abe I. Central orexin-A augments sympathoadrenal outflow in conscious rabbits. Hypertension. 2001;37:1382–1387. doi: 10.1161/01.hyp.37.6.1382. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Goodchild AK, Pilowsky PM. Activation of mu-opioid receptors in rat ventrolateral medulla selectively blocks baroreceptor reflexes while activation of delta opioid receptors blocks somato-sympathetic reflexes. Neuroscience. 2002a;109:133–144. doi: 10.1016/s0306-4522(01)00439-0. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Goodchild AK, Pilowsky PM. Evidence for a tonic GABA-ergic inhibition of excitatory respiratory-related afferents to presympathetic neurons in the rostral ventrolateral medulla. Brain Res. 2002b;924:56–62. doi: 10.1016/s0006-8993(01)03025-6. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002;20:1675–1688. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Pilowsky P, Llewellyn-Smith IJ, Arnolda L, Minson J, Chalmers J. Intracellular recording from sympathetic preganglionic neurons in cat lumbar spinal cord. Brain Res. 1994;656:319–328. doi: 10.1016/0006-8993(94)91476-1. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Lung MSY, Spirovski D, McMullan S. Differential regulation of the central neural cardiorespiratory system by metabotropic neurotransmitters. Phil Trans R Soc B. 2009;364:2537–2552. doi: 10.1098/rstb.2009.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Gao X-B, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riphagen CL, Pittman QJ. Mechanisms underlying the cardiovascular responses to intrathecal vasopressin administration in rats. Can J Physiol Pharmacol. 1989;67:269–275. doi: 10.1139/y89-044. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sato A, Schmidt RF. Somatosympathetic reflexes: afferent fibers, central pathways, discharge characteristics. Physiol Rev. 1973;53:916–947. doi: 10.1152/physrev.1973.53.4.916. [DOI] [PubMed] [Google Scholar]
- Shahid IZ, Pilowsky PM. 2010. Intrathecal orexin A increases sympathetic outflow and respiratory drive and modulates physiological reflexes Proceedings of the Australian Neuroscience Society, Sydney, Australia, Pos-Tue-088.
- Shen E, Dun NJ. Neonate rat sympathetic preganglionic neurons intracellularly labelled with lucifer yellow in thin spinal cord slices. J Auton Nerv Syst. 1990;29:247–254. doi: 10.1016/0165-1838(90)90151-8. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277:R1780–R1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Morrison SF, Ruggiero DA, Reis DJ. Neurons of rostral ventrolateral medulla mediate somatic pressor reflex. Am J Physiol. 1989;256:R448–R462. doi: 10.1152/ajpregu.1989.256.2.R448. [DOI] [PubMed] [Google Scholar]
- Sun MK, Reis DJ. Intrathecal kynurenate but not benextramine blocks hypoxic sympathoexcitation in chemodenervated anesthetized rats. J Auton Nerv Syst. 1994;47:141–150. doi: 10.1016/0165-1838(94)90075-2. [DOI] [PubMed] [Google Scholar]
- van den Top M, Nolan MF, Lee K, Richardson PJ, Buijs RM, Davies CH, et al. Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J Physiol. 2003;549:809–821. doi: 10.1113/jphysiol.2002.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama S, Sakurai T, Tatsumi K, Kuwaki T. Attenuated phrenic long-term facilitation in orexin neuron-ablated mice. Respir Physiol Neurobiol. 2009;168:295–302. doi: 10.1016/j.resp.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- Wang L, Spary E, Deuchars J, Deuchars SA. Tonic GABAergic inhibition of sympathetic preganglionic neurons: a novel substrate for sympathetic control. J Neurosci. 2008;28:12445–12452. doi: 10.1523/JNEUROSCI.2951-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Pilowsky PM. Acute intermittent hypoxia in rat in vivo elicits a robust increase in tonic sympathetic nerve activity that is independent of respiratory drive. J Physiol (Lond) 2010;588:3075–3088. doi: 10.1113/jphysiol.2010.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yamada KA, McAllen RM, Loewy AD. GABA antagonists applied to the ventral surface of the medulla oblongata block the baroreceptor reflex. Brain Res. 1984;297:175–180. doi: 10.1016/0006-8993(84)90556-0. [DOI] [PubMed] [Google Scholar]
- Yashpal K, Gauthier S, Henry JL. Substance P given intrathecally at the spinal T9 level increases arterial pressure and heart rate in the rat. J Auton Nerv Syst. 1987;18:93–103. doi: 10.1016/0165-1838(87)90096-8. [DOI] [PubMed] [Google Scholar]
- Yashpal K, Gauthier S, Henry JL. Angiotensin II stimulates sympathetic output by a direct spinal action. Neuropeptides. 1989;14:21–29. doi: 10.1016/0143-4179(89)90030-9. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett. 2005;385:131–136. doi: 10.1016/j.neulet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y, et al. Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci. 2003;92:259–266. doi: 10.1254/jphs.92.259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


