Abstract
Constipation in children is a common health problem affecting 0.7% to 29.6% children across the world. Exact etiology for developing symptoms is not clear in children and the majority is considered to have functional constipation. Alteration of rectal and pelvic floor function through the brain-gut axis seems to play a crucial role in the etiology. The diagnosis is often a symptom-based clinical process. Recently developed Rome III diagnostic criteria looks promising, both in clinical and research fields. Laboratory investigations such as barium enema, colonoscopy, anorectal manometry and colonic transit studies are rarely indicated except in those who do not respond to standard management. Treatment of childhood constipation involves several facets including education and demystification, toilet training, rational use of laxatives for disimpaction and maintenance and regular follow-up. Surgical options should be considered only when medical therapy fails in long standing constipation. Since most of the management strategies of childhood constipation are not evidence-based, high-quality randomized controlled trials are required to assess the efficacy of currently available or newly emerging therapeutic options. Contrary to the common belief that children outgrow constipation as they grow up, a sizable percentage continue to have symptoms beyond puberty.
Keywords: Children, Constipation, Epidemiology, Management, Pathophysiology
Introduction
Constipation is one of the commonest digestive complaints in children, which has recently grown to quite a proportion in public health problem. Like many other functional disorders, its etiology, pathophysiology and prognosis are ill-understood. This results in strongly-held, believes-driven and self-introduced management strategies, which are blended with the culture of the country, sometimes even harmful to children. However, body of the scientific knowledge has grown both in depth and width during the last decade. This article focuses on current views on definition, epidemiology, clinical features, evaluation and management strategies of constipation in children.
Definitions
Constipation has long been considered a symptom, rather than a disease.1 It is often perceived as infrequent motions or passage of hard stools. Some defined constipation as less than 3 bowel motions per week2 or as difficulty in passing stools.3 Approximately 0.5% of school children have defecation frequency less than 3 per week and 0.3% have fecal incontinence.4 Furthermore, 20% of children also have at least 1 clinical feature of constipation.5 Therefore, it is important to use diagnostic criteria based on multiple symptoms to define constipation.
In 1999, Rome II criteria were developed to diagnose defecation disorders.6 Functional constipation was identified in infants and preschool children and functional fecal retention in older children. Subsequently, Rome II criteria were found to be too restrictive in diagnosing defecation disorders because they did not include cardinal features of constipation (fecal incontinence) as diagnostic criteria and demanded persistence of symptoms for at least 3 months.7,8 Furthermore, division of functional constipation and functional fecal retention has no implications in clinical practice. However, the Rome process established a pathway to formulate universally acceptable diagnostic criteria for childhood defecation disorders through international collaboration.
The pediatric Rome III criteria were released in 2006 (Table 1). Functional constipation was recognized as a separate clinical entity by combining features of functional fecal retention and functional constipation. Furthermore, duration of symptoms was reduced to 8 weeks.9 A recent community-based study comparing Rome II and Rome III criteria shows a 2.5-fold increase in prevalence of functional constipation.10 The more inclusive nature of the Rome III criteria seems to have stemmed from including cardinal clinical features of constipation in the diagnostic criteria and necessity of shorter duration of symptoms.
Table 1.
Pediatric Rome III Criteria for Constipation
aCriteria fulfilled at least once per week for at least 2 mo before diagnosis.
Epidemiology
Global burden of childhood constipation is often underappreciated. This was mainly due to lack of data in this age group. In epidemiological studies, the definitions vary from accepted Rome criteria to proxy reports by parents. A recent systematic review in pediatric age group reported constipation in 0.7% to 29.6%.11 Apart from differences in definitions, the variation in duration of symptoms needed to diagnose constipation, age distribution of the children studied and the method of data collection may also have an influence on the data. Studies that have used standard definitions like Rome II criteria have also showed wide ranges of prevalence.12,13 Therefore, apart from varying definitions, several other factors seem to be responsible for the heterogeneity of epidemiological data, including environmental, socio-cultural and genetic factors.
Until recently, it was believed that constipation is a disease of the developed world, but studies from Asia have reported equally high prevalence of constipation. A survey in Sri Lanka using Rome III criteria reported constipation in 10.6% of 10-16 years old.14 Similarly, prevalence of constipation in Japan was 18.5%.15
Gender specific prevalence of constipation also varies between studies. Some studies have reported no difference in prevalence of constipation between girls and boys,3,12,16,17 while others found significantly higher prevalence in girls.13 Another study found a clear negative correlation between prevalence of constipation and age.18
The available data indicate that constipation is on the rise. A recent analysis of longitudinal data in the USA beginning from 1979 showed nearly 4-fold increase in rates of constipation during the last decade. There was a surge in both outpatient clinic visits and hospitalizations due to constipation between 1992 and 2004, with more than a doubling of rates in diagnosing constipation from outpatient clinics and nearly 4-fold increase in rates of hospital discharge under the diagnosis of constipation. Furthermore, children under 15 years had the highest number of clinic visits for constipation.19 In addition, 5.4 million prescriptions were filled for constipation in the USA in 2004. All these facts prove that constipation is a growing health problem among children worldwide.
Risk Factors
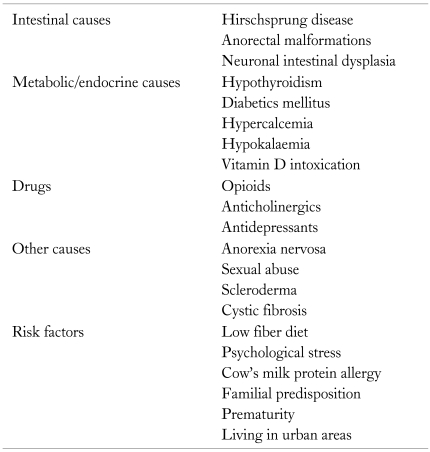
Several risk factors have been identified in association with pediatric constipation. The main risk factors for constipation are listed in Table 2.
Table 2.
Causes and Risk Factors of Constipation in Children
Low consumption of dietary fiber has long been considered as one of the leading risk factors. Undigested fibers in the colon are thought to increase the colonic transit and increase stool output. Lee et al20 found that kindergarten children with constipation took significantly low median dietary fiber than non constipated children. Furthermore, fruits and total plant food intake were significantly lower in the constipated group.20 Two other studies among older children also noted that children with constipation consume significantly less amount of dietary fiber than controls.21,22 Available studies from Asia also show that fiber consumption in Asian countries such as Hong Kong20,23 and Maldives24 is lower than the recommended values.
Few studies have demonstrated its relationship with psychological factors. Inan et al25 has shown that physical or psychological trauma and personal health problems were associated with constipation in school-aged children. Furthermore, they have found that abnormal oral habits (which were considered as an indirect measure of psychological stress) also showed a significant association with constipation.25 A study from Sri Lanka involving school children of 10-16 years old noted that school-related stressful events such as separation from best friend, bullying at school, failure of exam and family-related events such as severe illness of family member, parents' job loss and frequent punishment by parents were predisposing them to develop constipation.26 Furthermore, children living in a war-affected area had high prevalence of constipation compared to non war-affected areas.26 Psychological factors including emotional stress are likely to modulate colonic and rectal functions, through the brain gut axis, leading to constipation.
Cow's milk protein allergy is considered as a risk factor for constipation. Several studies have reported reduction of constipation by elimination of cow's milk from diet.27,28 However further studies are needed to confirm this association and to introduce cow's milk-free diet to infants and children with constipation. Other demonstrated risk factors are extreme low birth weight,29 positive family history18,21 and living in urban areas.18,30 High consumption of junk foods with low fiber content and sedentary life style might have contributed to higher prevalence of constipation reported in children living in urban areas.
Pathophysiology
The pathophysiology of constipation in children is multi-factorial and is associated with interactions of many risk factors. Many organic diseases cause constipation (Table 2). However, the majority of constipation patients secondary to organic conditions usually have other clinical features suggestive of the relevant underlying organic disease. Organic diseases presenting as isolated constipation are rather uncommon.
Over 90% of children with this condition have functional constipation.31 Borowitz et al32 reported painful defecation as the commonest factor for constipation. If there is pain during defecation, children usually withhold stools. During the withholding, rectal mucosa absorbs water from the fecal mass, which becomes harder and larger as the time passes and ultimately defecation becomes difficult. Therefore, when the desire to pass stools comes, children adopt retentive posture, hide from parents till the urge pass off. Passage of this fecal mass is painful and sometimes results in anal fissures which further aggravate pain and precipitate stool withholding. This sets up a vicious cycle of stool retention. Accumulation of stools in rectum causes gradual dilatation leading to megarectum resulting in loss of rectal sensation and urge for defecation. It had been shown that children with megarectum have high sensory threshold for rectal sensation.33,34
Other intestinal pathologies leading to chronic constipation surprisingly have not received much attention. Several studies have demonstrated slow colonic transit in 25%-69% children with constipation.35-37 Furthermore, those with slow transit constipation had more severe symptoms including night time soiling.35 Laparoscopic biopsies of the colon have shown deficiency of neurotransmitters such as substance P in some children.38,39 Furthermore it was shown that number of antegrade pressure waves in the colon was significantly decreased in children with slow transit constipation.40
Clinical Features
The commonest symptoms of constipation are reduced stool frequency and passage of hard stools. The other symptoms include fecal soiling, passage of large volume stools, painful defecation and characteristic "retentive posturing." Straining at defecation, abdominal pain, anorexia, vomiting and bleeding per rectum are other associated features, although they are excluded from the diagnostic criteria.16,18 Similarly constipation was the commonest cause of acute abdominal pain presenting to emergency department or primary care clinics.41 The physical examination shows palpable fecal masses in the abdomen and fecal impaction in the rectum.
Evaluation
A detail history and thorough physical examination are the cornerstones in assessing a child with chronic constipation. These 2 steps would reveal the possible etiology and associated complications in the majority. Investigations are only needed in those who show clinical features of organic diseases and children do not respond to initial medical management.
Clinical History
History of meconium passage
Neonates pass meconium within first 48 hours. Delayed passage of meconium raises the possibility of short segment Hirschsprung disease and anorectal malformations.
Time of onset
A majority develop constipation around 2-4 years of age.42 Significant intestinal pathologies such as anorectal malformations and neuronal intestinal dysplasia are common in children presenting with constipation very early in life. In some patients, the onset of symptoms is related to major stressful life events such as birth of a sibling or parental job loss.
Bowel habits and defecation behaviors
The majority of children with constipation have infrequent passage of stools. Hard and large caliber stools that can clog the toilet may lead to passage of blood with stools. Adaptation of withholding posture should be specifically questioned because sometimes parents interpret this as a genuine attempt to pass stools. Children stand on tip toes and often hold on to furniture till the desire for defecation is passed. Sweating and facial redness are also noted in this period. Leaking stools into the underwear without realizing indicates severe constipation.
Associated symptoms
Although non-specific, the presence of abdominal pain, nausea, and vomiting are associated with constipation. Most parents would complain the child has loss of appetite and fail to gain weight. History of urinary incontinence is also a feature.43 Endocrine diseases which may cause constipation, such as diabetes mellitus would have features such as polyuria, polydipsia and weight loss. Furthermore, children with hypothyroidism may present with lethargy, poor school performances and weight gain.
Drugs
It is vital to take a history on current medications when assessing for constipation as certain drugs lead to constipation as adverse effects (Table 2).
Psychology
A detail history of psychological state is another important part in the assessment. It may reveal features of anorexia nervosa, depression and anxiety.
Risk factors
All other possible risk factors for the development of constipation are discussed above. General medical history, social details and the developmental history are also integral components in the assessment.
Physical Examination
Physical growth
Measurement of height and weight and comparison with the age appropriate centile charts gives idea about the physical growth. Hypothyroidism and other organic disorders may present as short stature or failure to thrive.
General examination
Young children with constipation often cling to their parents and look frightened during the consultation. Smell of the faeces due to incontinence and general demeanor of the child are also important to note. Children with anorectal malformation and hypercalcaemia may sometimes show associated features. Young girls with anorexa nervosa often would show features of weight loss. Presence of scars, lipomas and haemangiomas on the lower spine would suggest the possibility of spinal dysraphism and underlying neurological abnormalities.
Abdominal examination
The main aim of the abdominal examination is to assess the presence of palpable fecal mass. Usually it is found in the left iliac fossa or supra pubic region.
Perianal inspection and digital examination of the rectum
Inspection of the perianal region shows position of the anus, fissures, tags and inflammation. Children who had experienced chronic sexual abuse would show characteristic features. Repaired anorectal malformations would show surgical scars around the anus. Digital rectal examination assesses the anal tone and detects the presence of fecal mass. It is noted that the frequency of digital examination of rectum is unacceptably low in children with constipation.44
Neurological assessment
This will reveal neurological abnormalities in the lower spinal cord which may present as constipation.
Investigations
Laboratory investigations are rarely indicated in childhood constipation except in those with evidence of organic diseases from history and examination and in those who do not respond to adequate medical management. Otherwise, investigations are unlikely to reveal any additional information for the management.
Plain abdominal X-ray
Plain abdominal radiograph is performed to identify the degree of fecal loading in the colon and rectum.45-47 It is considered to be useful in children who are not willing to undergo a rectal examination due to pain and fear.5 However a systematic review shows that interpretation of the radiological findings is difficult, inconsistent and there is a poor correlation between clinical and radiological diagnosis.48 The scoring systems for fecal loading are reported to have wide inter-observer and intra-observer variability, poor diagnostic accuracy, poor reproducibility and depend on the experience of the scorer.49,50 Therefore, plain abdominal radiograph has a very limited value in clinical assessment of constipation.
Colonic transit studies
The transit time of the colon is studied using radio-opaque markers51-53 and radionuclear scintigraphy.54 The calculated total and segmental transit times allow to differentiate constipation due to delayed segmental and pan-colonic transit from constipation with normal transit.51,55,56
Several previous studies have reported delayed colonic transit times (segmental or total) in children with constipation.36-38,49,57 de Lorijin and co-workers57 reported delayed transit on rectosigmoid (48%) followed by descending and ascending colon (21%-22%). Another study showed slow transit constipation in 60% of the children with constipation and of them, 13% had pelvic floor dysfunction.38 Children with slow transit constipation have lower defecation frequency and higher prevalence of day and night time soiling, painful defecation and palpable rectal or abdominal masses.35,37,58 Therefore, colonic transit studies are beneficial in children with chronic treatment-resistant constipation to determine colonic transit abnormalities.
Anorectal manometry
Anorectal manometry is a collection of several tests that measure pressure changes in the rectum and the anal canal. It is often combined with surface electrode electromyography of the external anal sphincter and puborectalis muscle.36 They provide details on rectal sensation, state of recto-anal inhibitory reflex, tone of anal sphincter and defecation dynamics.
Some studies have shown an increased threshold for rectal sensation in constipated children especially those with megarectum.33,34 However probably the most important benefit of anorectal manometry in children with constipation is to exclude Hirschsprung disease. Generally, presence of recto-anal inhibitory reflex excludes Hirschsprung disease. However several studies have noted variable sensitivity, specificity, positive and negative predictive values in the diagnosis of Hirschsprung disease.59-64 Furthermore, false positive results may occur due to immaturity of ganglion cells (in premature babies) and artefacts.61 Therefore in cases with strong clinical suspicion of Hirschsprung disease, it is imperative to perform a suction biopsy to confirm or exclude the diagnosis.
Defecation dynamics are tested using anorectal manometry with integrated electromyogram of the external anal sphincter and puborectalis muscle. It is defined abnormal if there is increased manometric and myoelectrical activities in the sphincter complex during bearing down.34 Pelvic floor dyssynergia was noted among constipated children in several studies.65-67 Previous studies have shown that children with constipation have abnormally high resting anal tone.65-67 In contrast, another study failed to show a difference in anal tone between constipated children and controls.34
Colonic manometry
Colonic manometry measures the intracolonic pressure using a multichannel manometry probe. It is useful in patients with intractable constipation. Children with functional constipation show normal colonic motor activity (presence of high amplitude propagating contractions and gastro-colonic response to meal). Children with rare colonic muscle disorders demonstrate absent or weak colonic contractions. The gastro-colonic response is absent in colonic neuropathy.68
By analyzing 375 colonic manometries, Villarreal et al69 found colonic neuropathy in 130 and colonic myopathy in 15 and signified the diagnostic validity of colonic manometry in intractable constipation. Another study noted 30% of 173 children to have colonic neuromuscular diseases.70 Colonic manometry via appendicostomy has shown abnormal high amplitude contractions, increased retrograde propagating sequences and lack of increase in amplitude of propagating sequences normally induced by meals and waking.40
Therefore, it is an important investigation in children with chronic treatment-resistant constipation, who do not respond to maximum doses of combined laxative therapy. Significant manometric abnormalities in such clinical situations make the clinician to think about other management options such as antegrade colonic enemas or surgical interventions.
Other investigations
Fecoflowmetry evaluates pressure changes in the rectum and anal canal during infusion of saline and also evacuation rates of saline from the rectum using uroflowmeter. Previous study has shown abnormalities in pressure curves and fecoflowmetry curves in children with chronic constipation.71 Pelvic ultrasonograpy has been used to measure the diameter of the rectum in children with chronic constipation. These studies have shown larger rectal diameter in children with constipation compared to controls.72,73 Anal endosonography has also revealed abnormalities in the sphincter complex in children with chronic constipation.74 However these investigations need further validation before using in routine evaluation of affected children.
Management
Management of constipation encompasses several facets. However, only few randomized controlled trials are available to assess therapeutic options currently being used in treatment. In addition, little is known of their optimum therapeutic dosages and long-term side effects. Therefore, management of childhood constipation is mainly based on individual experience.
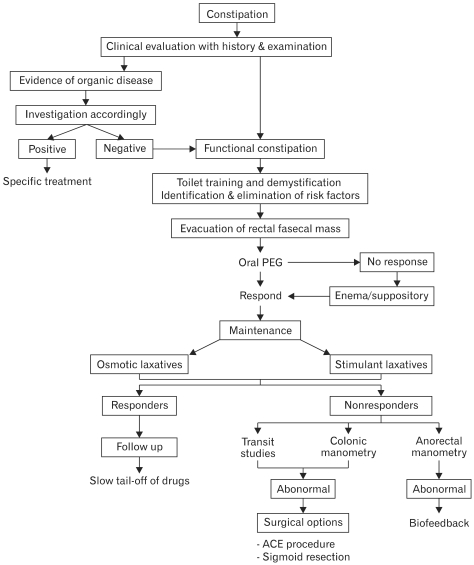
In the management of chronic constipation, a trustworthy relationship between the patient, parents and clinician is of paramount importance. The key steps in management include education and demystification, treatment of fecal impaction, maintenance therapy and close follow-up. Steps in management of childhood constipation are illustrated in the Figure.
Figure.
Algorithm of management of childhood constipation. PEG, polyethylene glycol; ACE, antegrade conlonic enema.
Education
The general public has various concepts on constipation which may interfere with proper clinical management. Therefore educating the parents and patients about pathophysiology and precipitating factors will help to alleviate anxiety, minimize accusations and increase their involvement in management. Approximately 15% of children with constipation improve with nonaccusatory education, demystification and toilet training.75 It is also worth mentioning to parents that the progress of treatment is often irregular and is marked by periods of improvement alternating with deterioration. Therefore, the duration of maintenance therapy ranges from 6 to 24 months.21,76
Behavioral Therapy
Behavioral therapies for constipation are designed to regularize toilet routines, discourage stool withholding and improve understanding of defecation dynamics. Several protocolized behavioral programs has been used as therapeutic interventions.77,78 The stepwise approach described by van Dijk et al78 included several steps. Psycho-education is used as the first step to change behavior of parents and child towards constipation. Reduction of anxiety towards defecation using education and models is helpful to promote successful defecation. In the next step, child is taught of straining techniques such as relaxation of legs and feet, to take a deep breath and hold it and how to push down while holding one's breath. Finally the behavior is reinforced by motivation and reward system and develops toilet routine without avoidance.78 A randomized control trial which compared conventional treatment alone and conventional treatment with added behavioral therapy failed to demonstrate significant difference in improvement of defecation frequency and fecal soiling.79 In contrast to this, a web based behavioral therapy plus laxatives with conventional treatment showed reduced fecal soiling, increased defecation in the toilet, and increased unprompted trips to the toilet in study group.80
Nevertheless, use of these non-invasive steps would enhance passing stools, improve strained family relationships and build a rapport between clinician and family. Therefore, behavioral therapy involving steps described above should be used in children presenting with chronic constipation.
Biofeedback Therapy
Biofeedback uses electrical or mechanical devices to increase the awareness of physiological functions of anal sphincter by providing the patient with visual, verbal and or auditory information and enhances self-control on body functions.81 During biofeedback, patients are provided with visual graphs of their rectal pressure and electromyography of external anal sphincter and also taught to relax external anal sphincter with the rise of rectal pressure. This therapy is likely to be beneficial for the subgroup of patients with pelvic floor dyssynergia and enable them to relax their pelvic floor and external anal sphincter in achieving normal defecation. In agreement with this, several previous studies have shown the efficacy of biofeedback therapy in correcting abnormal defecation dynamics.33,82,83 However, it failed to demonstrate an additional therapeutic value in clinical improvement of chronic constipation in children.65 Therefore, at present, biofeedback therapy seems to be beneficial for only a small subgroup of children with chronic constipation who have pelvic floor dyssynergia.
Dietary Measures
It is a wide spread practice to instruct patients with constipation to increase fluid and fiber intake. According to 2 studies, increased fluid intake only resulted in increased urine output and had no effect on stool output or consistency.84,85 In agreement with this, another study showed that increased water intake by 50% did not improve stool frequency or consistency.86
Low fiber intake has been recognized as a risk factor for constipation. Adequate intake of dietary fiber (age + 5 in grams) reduces risk of constipation, but further increase in fiber has no proven therapeutic value. Previous clinical trials failed to show significant improvement of bowel habits after fiber treatment compared to placebo and traditional treatments such as lactulose.87,88 In one study, a subgroup analysis showed that children with prolonged basal colonic transit times significantly increases number of bowel motions after administration of high fiber diet.89
Disimpaction
Evacuation of feces accumulated in the rectum is the key therapeutic step in successful management of constipation. Several studies have assessed the value of polyethylene glycol (PEG) in fecal disimpaction. One study proved that PEG 3350 without electrolytes has cleared fecal impaction in 75% of children with constipation and children using higher doses had more clearance than those using lower doses.41 In agreement with this, Pashanker et al90 showed that, after 8 weeks of treatment with PEG without electrolytes, children had less fecal soiling, painful defecation, fecal impaction and rectal dilatation.
Another study showed that PEG 3350 plus electrolytes is more effective in disimpaction compared to suppositories or rectal enemas.91 In addition, health costs and hospital admissions were reduced when using PEG compared to enemas and suppositories.91 Furthermore, PEG 3350 plus electrolytes was effective in clearing fecal retention in chronic treatment resistant constipation. In another study, 90% of children with treatment resistant constipation were successfully treated with PEG.92
Administration of enemas to relieve rectal fecal loading has long been practiced in management of childhood constipation. It is important that clinicians use the rectal route, only when oral drugs have failed. Insertion of rectal enema may be extremely disturbing to the child who might already have anal fissures. Therefore, it needs to be given under sedation to minimize pain and psychological effects. Otherwise it may disturb the good relationship and understanding between clinician and child, which is essential in the long term management.
Maintenance Therapy
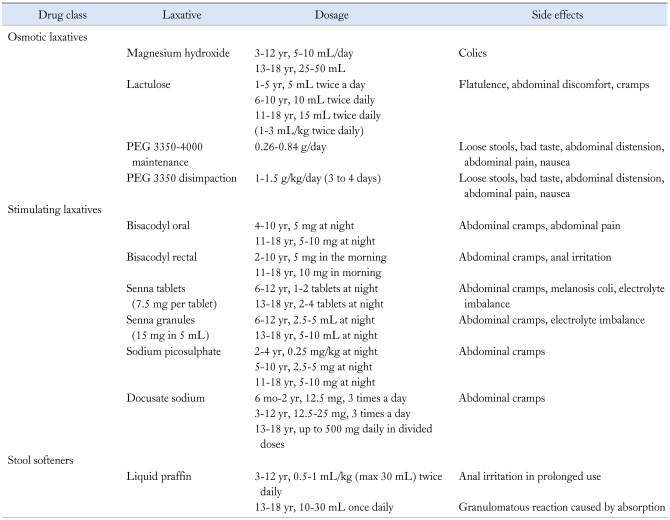
After achieving disimpaction it is vital to start daily oral laxatives to keep the stool soft thereby to prevent re-impaction. The duration of the maintenance phase needs to be individualized and may vary from months to years. Parents and children need to be counselled regarding the importance of this stage and should keep a regular bowel chart. Parents need to be advised on different alternatives to use if the child does not pass stools on a regular basis. A close follow-up is crucial during the initial period of maintenance to avoid recurrence. The main pharmacological agents used for maintenance are osmotic and stimulant laxatives.31,93 Table 3 gives recommended dosage of laxatives commonly used during this phase.
Table 3.
Drugs Commonly Used in the Management of Childhood Constipation
PEG, polyethylene glycol.
Osmotic laxatives
Lactulose. It is an unabsorbable, osmotically active carbohydrate which drags water into the gut, keeps the stools soft and facilitates passage of stools without pain. Two randomized controlled trials comparing lactitol and lactulose have found that both are equally effective in increasing stool frequency and normalizing stool consistency.31,94,95 When lactulose was compared with senna in a crossover trial, both drugs were found to be effective in improving defecation frequency.96
Polyethylene glycol. PEG is a non absorbable compound and is not digested by colonic bacteria. Its mechanism of action is increasing osmotic load in the large intestine which results in expansion of stool volume.97 In a prospective observational study, Pashankar et al98 failed to find any side effects following PEG therapy. PEG has not altered the serum electrolyte, osmolality and albumin levels of plasma and liver and renal functions.98 In a double blind randomized controlled trial, Candy et al92 noted that PEG with electrolytes was more effective compared to lactulose in increasing defecation frequency in children with intractable constipation. Furthermore, children on PEG plus electrolytes had less fecal impaction and did not need rescue medication.92 In a randomized, crossover study comparing PEG with lactulose, parents felt that PEG was more effective compared to lactulose, even though there was no difference in frequency of bowel motions, stool form or easy passage of stools between 2 groups.99 Two other double blind randomized controlled trials failed to demonstrate a significant difference in stool frequency between PEG and lactulose.100,101Two studies comparing PEG without electrolytes with milk of magnesia found no difference between 2 drugs with regard to outcome.102,103 Therefore current evidence shows no advantage of one osmotic laxative over the others during the maintenance phase of management.
Stimulant laxatives
Good quality clinical trials are lacking on effectiveness of stimulant laxative as maintenance therapy of childhood constipation and therefore it is difficult to draw evidence based conclusions. An open label randomized controlled trial comparing senna and lactulose showed no difference in bowel frequency. However, number of patients passing normal stools each day was significantly higher in patients receiving lactulose.94 Sondheimer and Gervaise104 noted that children using fecal softeners (mineral oil) had more daily motions and less fecal soiling than senna. Other stimulant laxatives such as bisacodyl, docusate sodium and sodium picosulphate have not been evaluated in randomized controlled trials.
New Therapeutic Options
Tegaserod
It is a serotonin receptor agonist which stimulates the peristaltic reflex, enhances intestinal secretions and decreases visceral sensitivity.105 It also act as a prokinetic agent in the upper and lower gastrointestinal tract.106 There are several trials of this drug in adults with constipation predominant irritable bowel syndrome and constipation. However the efficacy of this drug has not been evaluated in children.
Probiotics
A randomized control trial on the effectiveness of Lactobacillus GG as an adjunct to lactulose for children with constipation failed to show additional therapeutic benefit.107 Another study that compared the efficacy of probiotics containing Lactobacillus casei ramnosus with magnesium oxide to a placebo, failed to show a significant difference in final outcome between probiotics and MgO.108 More robust therapeutic trials are needed before recommending probiotics for routine management of constipation.
Surgery
Surgical options need to be considered only when medical therapy fails in long standing constipation. Children with loaded rectum who do not respond to enemas may need manual evacuation.109 Sigmoid resection and removal of dilated megasigmoid is a successful surgical intervention in some patients with severe constipation.110 Antegrade colonic enema via appendicocaecostomy is another surgical therapeutic option in severe functional constipation.111,112 Possible complications of this intervention include stenosis of the cutaneous opening, leakage around the cecostomy tube and displacement of the tube.113,114
Prognosis
Van Ginkel et al76 noted that 30% of children with constipation continued to have symptoms beyond puberty with several complications associated with it. A recent systematic review on prognosis of childhood constipation noted that the majority of them recover within 6-12 months of therapy and the recovery rate had no relationship with the age of onset, positive family history, defecation frequency and presence of fecal incontinence.115
Conclusion
Chronic constipation is a common pediatric problem affecting children worldwide. Exact etiology is unclear in the majority and is thought to be functional in origin. Constipation is a clinical diagnosis and investigations are rarely warranted, unless clues are found in the history or physical examination or poor response to therapy. Key steps in the management include education, rectal disimpaction, maintenance and follow-up. Approximately 30% of affected children will continue to have symptoms beyond puberty contrary to the common belief that children outgrow constipation.
Footnotes
Financial support: None.
Conflicts of interest: None.
References
- 1.Hyams J, Colletti R, Faure C, et al. Functional gastrointestinal disorders: working group report of the first world congress of paediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. 2002;35(suppl 2):S110–S117. doi: 10.1097/00005176-200208002-00008. [DOI] [PubMed] [Google Scholar]
- 2.Kokkonen J, Haapalahti M, Tikkanen S, Karttunen R, Savilahti E. Gastrointestinal complaints and diagnosis in children: a population based study. Acta Paediatr. 2004;93:880–886. [PubMed] [Google Scholar]
- 3.de Araújo Sant'Anna AM, Calçado AC. Constipation in school-aged children at public schools in Rio de Janeiro, Brazil. J Pediatr Gastroenterol Nutr. 1999;29:190–193. doi: 10.1097/00005176-199908000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Wald ER, Di Lorenzo C, Cipriani L, Colborn DK, Burgers R, Wald A. Bowel habits and toilet training in a diverse population of children. J Pediatr Gastroenterol Nutr. 2009;48:294–298. doi: 10.1097/mpg.0b013e31817efbf7. [DOI] [PubMed] [Google Scholar]
- 5.Devanarayana NM, Rajindrajith S. Bowel habits and behaviours related to defecation in 10 to 16 year olds: impact of socio-economic characteristics and emotional stress. J Pediatr Gastroenterol Nutr. 2011 doi: 10.1097/MPG.0b013e3181fd082b. (In press) [DOI] [PubMed] [Google Scholar]
- 6.Rasquin-Weber A, Hyman PE, Cucchiara S, et al. Childhood functional gastrointestinal disorders. Gut. 1999;45(suppl 2):II60–II68. doi: 10.1136/gut.45.2008.ii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loening-Baucke V. Functional fecal retention with encopresis in childhood. J Pediatr Gastroenterol Nutr. 2004;38:79–84. doi: 10.1097/00005176-200401000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Voskuijl WP, Heijmans J, Heijmans HS, Taminiau JA, Benninga MA. Use of Rome II criteria in childhood defecation disorders: applicability in clinical and research practice. J Pediatr. 2004;145:213–217. doi: 10.1016/j.jpeds.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 9.Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devanarayana NM, Adhikari C, Pannala W, Rajindrajith S. Prevalence of functional gastrointestinal diseases in a cohort of Sri Lankan adolescents: comparison between Rome II and Rome III criteria. J Trop Pediatr. doi: 10.1093/tropej/fmq039. Published Online First: 4 June 2010. doi: 10.1093/tropej/fmq039. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. 2006;101:2401–2409. doi: 10.1111/j.1572-0241.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 12.Iacono G, Merolla R, D'Amico D, et al. Gastrointestinal symptoms in infancy: a population-based prospective study. Dig Liver Dis. 2005;37:432–438. doi: 10.1016/j.dld.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Ip KS, Lee WT, Chan JS, Young BW. A community-based study of the prevalence of constipation in young children and the role of dietary fiber. Hong Kong Med J. 2005;11:431–436. [PubMed] [Google Scholar]
- 14.Rajindrajith S, Devanarayana N, Mettananda S, et al. Constipation and functional faecal retention in a group of school children in a district in Sri Lanka. Sri J Child Health. 2009;38:60–64. [Google Scholar]
- 15.Kajiwara M, Inoue K, Usui A, Kurihara M, Usui T. The micturition habits and prevalence of day time urinary incontinence in Japanese primary school children. J Urol. 2004;171:403–407. doi: 10.1097/01.ju.0000101907.87169.06. [DOI] [PubMed] [Google Scholar]
- 16.Uğuralp S, Karaoğlu L, Karaman A, Demircan M, Yakinci C. Frequency of enuresis, constipation and enuresis associated with constipation in a group of school children aged 5-9 years in Malatya, Turkey. Turk J Med Sci. 2003;33:315–320. [Google Scholar]
- 17.Bakwin H, Davidson M. Constipation in twins. Am J Dis Child. 1971;121:179–181. doi: 10.1001/archpedi.1971.02100130133018. [DOI] [PubMed] [Google Scholar]
- 18.Rajindrajith S, Devanarayana NM, Adhikari C, Pannala W, Benninga MA. Constipation in children: an epidemiological study in Sri Lanka using Rome III criteria. Arch Dis Child. doi: 10.1136/adc.2009.173716. Published Online First: 23 Jun 2010. doi: 10.1136/adc.2009.173716. [DOI] [PubMed] [Google Scholar]
- 19.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology. 2009;136:741–754. doi: 10.1053/j.gastro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Lee WT, Ip KS, Chan JS, Lui NW, Young BW. Increased prevalence of constipation in pre-school children is attributable to under-consumption of plant foods: a community-based study. J Paediatr Child Health. 2008;44:170–175. doi: 10.1111/j.1440-1754.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- 21.Roma E, Adamidis D, Nikolara R, Constantopoulos A, Messaritakis J. Diet and chronic constipation in children: the role of fiber. J Pediatr Gastroenterol Nutr. 1999;28:169–174. doi: 10.1097/00005176-199902000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Morais MB, Vitolo MR, Aguirre AN, Fagundes-Neto U. Measurement of low dietary fiber intake as a risk factor for chronic constipation in children. J Pediatr Gastroenterol Nutr. 1999;29:132–135. doi: 10.1097/00005176-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Lee WTK, Leung SSF, Leung DMK. The current dietary practice of Hong Kong adolescents. Asia Pacific J Clin Nutr. 1994;8:83–87. [PubMed] [Google Scholar]
- 24.Golder JM, Erhardt JG, Scherbaum V, Saeed M, Biesalski HK, Fürst P. Dietary intake and nutritional intake of women and pre-school children in the Republic of the Maldives. Public Health Nutr. 2001;4:773–780. doi: 10.1079/phn2000101. [DOI] [PubMed] [Google Scholar]
- 25.Inan M, Aydiner CY, Tokuc B, et al. Factors associated with childhood constipation. J Paediatr Child Health. 2007;43:700–706. doi: 10.1111/j.1440-1754.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 26.Devanarayana NM, Rajindrajith S. Association between constipation and stressful life events in a cohort of Sri Lankan children and adolescents. J Trop Pediatr. 2010;56:144–148. doi: 10.1093/tropej/fmp077. [DOI] [PubMed] [Google Scholar]
- 27.Iacono G, Cavataio F, Montalto G, et al. Intolerance of cow's milk and chronic constipation in children. N Engl J Med. 1998;339:1100–1104. doi: 10.1056/NEJM199810153391602. [DOI] [PubMed] [Google Scholar]
- 28.Daher S, Tahan S, Solé D, et al. Cow's milk protein intolerance and chronic constipation in children. Pediatr Allergy Immunol. 2001;12:339–342. doi: 10.1034/j.1399-3038.2001.0o057.x. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham C, Taylor HG, Minich NM, Hack M. Constipation in very-low-birth-weight children at 10 to 14 years of age. J Pediatr Gastroenterol Nutr. 2001;33:23–27. doi: 10.1097/00005176-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Ludvigsson JF Abis Study Group. Epidemiological study of constipation and other gastrointestinal symptoms in 8000 children. Acta Paediatr. 2006;95:573–580. doi: 10.1080/08035250500452621. [DOI] [PubMed] [Google Scholar]
- 31.Benninga MA, Voskuijl WP, Taminiau JA. Childhood constipation: is there new light in the tunnel? J Pediatr Gastroenterol Nutr. 2004;39:448–464. doi: 10.1097/00005176-200411000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Borowitz SM, Cox DJ, Tam A, Ritterband LM, Sutphan JL, Penberthy JK. Precipitant of constipation during early childhood. J Am Board Fam Pract. 2003;16:213–218. doi: 10.3122/jabfm.16.3.213. [DOI] [PubMed] [Google Scholar]
- 33.Benninga MA, Buller HA, Taminiau JA. Biofeedback training in chronic constipation. Arch Dis Child. 1993;68:126–129. doi: 10.1136/adc.68.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Plas RN, Benninga MA, Staalman CR, et al. Megarectum in constipation. Arch Dis Child. 2000;83:52–58. doi: 10.1136/adc.83.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benninga MA, Büller HA, Tytgat GN, Akkermans LM, Bossuyt PM, Taminiau JA. Colonic transit time in constipated children: does pediatric slow-transit constipation exist? J Pediatr Gastroenterol Nutr. 1996;23:241–251. doi: 10.1097/00005176-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Gutiérrez C, Marco A, Nogales A, Tebar R. Total and segmental colonic transit time and anorectal manometry in children with chronic idiopathic constipation. J Pediatr Gastroenterol Nutr. 2002;35:31–38. doi: 10.1097/00005176-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Zaslavsky C, da Silveria TR, Maguilnik I. Total and segmental colonic transit time with radio-opaque markers in adolescents with functional constipation. J Pediatr Gastroenterol Nutr. 1998;27:138–142. doi: 10.1097/00005176-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Hutson JM, Chow CW, Borg J. Intractable constipation with a decrease in substance P-immunoreactive fibres: is it a variant of intestinal neuronal dysplasia? J Pediatr Surg. 1996;31:580–583. doi: 10.1016/s0022-3468(96)90501-1. [DOI] [PubMed] [Google Scholar]
- 39.Wheatley JM, Hutson JM, Chow CW, Oliver M, Hurley MR. Slow transit constipation in childhood. J Pediatr Surg. 1999;34:829–832. doi: 10.1016/s0022-3468(99)90381-0. [DOI] [PubMed] [Google Scholar]
- 40.Stanton MP, Hutson JM, Simpson D, et al. Colonic manometry via appendicostomy shows reduced frequency, amplitude and length of propagating sequences in children with slow-transit constipation. J Pediatr Surg. 2005;40:1138–1145. doi: 10.1016/j.jpedsurg.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 41.Loening-Baucke V, Swidsinski A. Constipation as cause of acute abdominal pain in children. J Pediatr. 2007;151:666–669. doi: 10.1016/j.jpeds.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Plunkett A, Phillips CP, Beattie RM. Management of chronic functional constipation in childhood. Paediatr Drugs. 2007;9:33–46. doi: 10.2165/00148581-200709010-00004. [DOI] [PubMed] [Google Scholar]
- 43.Loening-Baucke V. Prevalence rates for constipation and faecal and urinary incontinence. Arch Dis Child. 2007;92:486–489. doi: 10.1136/adc.2006.098335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gold DM, Levine J, Weinstein TA, Kessler BH, Pettei MJ. Frequency of digital rectal examination in children with chronic constipation. Arch Pediatr Adolesc Med. 1999;153:377–379. doi: 10.1001/archpedi.153.4.377. [DOI] [PubMed] [Google Scholar]
- 45.Barr RG, Levine MD, Wilkinson RH, Mulvihill D. Chronic and occult stool retention: a clinical tool for its evaluation in school-aged children. Clin Pediatr (Phila) 1979;18:674, 676, 677–679. doi: 10.1177/000992287901801103. [DOI] [PubMed] [Google Scholar]
- 46.Blethyn AJ, Jenkins HR, Roberts R, Verrier Jones K. Radiological evidence of constipation in urinary tract infection. Arch Dis Child. 1995;73:534–535. doi: 10.1136/adc.73.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leech SC, McHugh K, Sullivan PB. Evaluation of a method of assessing faecal loading on plain abdominal radiographs in children. Pediatr Radiol. 1999;29:255–258. doi: 10.1007/s002470050583. [DOI] [PubMed] [Google Scholar]
- 48.Reuchlin-Vroklage LM, Bierma-Zeinstra S, Benninga MA, Berger MY. Diagnostic value of abdominal radiography in constipated children: a systematic review. Arch Pediatr Adolesc Med. 2005;159:671–678. doi: 10.1001/archpedi.159.7.671. [DOI] [PubMed] [Google Scholar]
- 49.de Lorijn F, van Rijn RR, Heijmans J, et al. The Leech method for diagnosing constipation: intra- and interobserver variability and accuracy. Pediatr Radiol. 2006;36:43–49. doi: 10.1007/s00247-005-0031-z. [DOI] [PubMed] [Google Scholar]
- 50.Jackson CR, Lee RE, Wylie AB, Adams C, Jaffray B. Diagnostic accuracy of Barr and Blethyn radiological scoring systems for childhood constipation assessed using colonic transit time as the gold standard. Pediatr Radiol. 2009;39:664–667. doi: 10.1007/s00247-009-1205-x. [DOI] [PubMed] [Google Scholar]
- 51.Arhan P, Devroede G, Jehannin B, et al. Segmental colonic transit time. Dis colon Rectum. 1981;24:625–629. doi: 10.1007/BF02605761. [DOI] [PubMed] [Google Scholar]
- 52.Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 53.Bouchoucha M, Devroede G, Arhan P, et al. What is the meaning of colorectal transit time measurement? Dis Colon Rectum. 1992;35:773–782. doi: 10.1007/BF02050328. [DOI] [PubMed] [Google Scholar]
- 54.Cook BJ, Lim E, Cook D, et al. Radionuclear transit to assess sites of delay in large bowel transit in children with chronic idiopathic constipation. J Pediatr Surg. 2005;40:478–483. doi: 10.1016/j.jpedsurg.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 55.Bautista Casasnovas A, Varela Cives R, Villanueva Jeremias A, Castro-Gago M, Cadranel S, Tojo Sierra R. Measurement of colonic transit time in children. J Pediatr Gastroenterol Nutr. 1991;13:42–45. doi: 10.1097/00005176-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 56.Wagener S, Shankar KR, Turnock RR, Lamont GL, Baillie CT. Colonic transit time - what is normal? J Pediatr Surg. 2004;39:166–169. doi: 10.1016/j.jpedsurg.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 57.de Lorijn F, van Wijk MP, Reitsma JB, van Ginkel R, Taminiau JA, Benninga MA. Prognosis of constipation: clinical factors and colonic transit time. Arch Dis Child. 2004;89:723–727. doi: 10.1136/adc.2003.040220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papadopoulou A, Clayden GS, Booth IW. The clinical value of solid marker transit studies in childhood constipation and soiling. Eur J Pediatr. 1994;153:560–564. doi: 10.1007/BF02190658. [DOI] [PubMed] [Google Scholar]
- 59.Martelli H, Faverdin C, Devroede G, et al. Can functional constipation begin at birth? Gastroenterol Int. 1998;11:1–11. [Google Scholar]
- 60.Nurko S. Gastrointestinal manometry: methodology and indications. In: Walker WA, Goulet O, Kleinman RE, Sherman PM, Schneider BL, Sanderson IR, editors. Pediatric gastrointestinal disease. 4th ed. Ontario: BC Decker; 2004. pp. 1786–1808. [Google Scholar]
- 61.Lee JH, Choe YH, Lee SK, Seo JM, Kim JH, Suh YL. Allergic proctitis and abdominal distension mimicking Hirschsprung's disease in infants. Acta Paediatr. 2007;96:1784–1789. doi: 10.1111/j.1651-2227.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 62.Meunier P, Marechal JM, Mollard P. Accuracy of the manometric diagnosis of Hirschsprung's disease. J Pediatr Surg. 1978;13:411–415. doi: 10.1016/s0022-3468(78)80466-7. [DOI] [PubMed] [Google Scholar]
- 63.Low PS, Quak SH, Prabhakaran K, Joseph VT, Chiang GS, Aiyathurai EJ. Accuracy of anorectal manometry in the diagnosis of Hirschsprung's disease. J Pediatr Gastroenterol Nutr. 1989;9:342–346. doi: 10.1097/00005176-198910000-00013. [DOI] [PubMed] [Google Scholar]
- 64.Kong MS, Lin JN, Chuang JH, Wang KL. Screening Hirschsprung's disease by anorectal manometry. Chin J Gastroenterol. 1993;10:29–32. [Google Scholar]
- 65.van der Plas RN, Benninga MA, Büller HA, et al. Biofeedback training in treatment of childhood constipation: a randomized controlled study. Lancet. 1996;348:776–778. doi: 10.1016/s0140-6736(96)03206-0. [DOI] [PubMed] [Google Scholar]
- 66.van Ginkel R, Büller HA, Boeckxstaens GE, van der Plas RN, Taminiau JA, Benninga MA. The effect of anorectal manometry on the outcome of treatment in severe childhood constipation: a randomized, controlled trial. Pediatrics. 2001;108:E9. doi: 10.1542/peds.108.1.e9. [DOI] [PubMed] [Google Scholar]
- 67.Loening-Baucke V. Biofeedback treatment for chronic constipation and encopresis in childhood: long term outcome. Pediatrics. 1995;96(1 Pt 1):105–110. [PubMed] [Google Scholar]
- 68.Di Lorenzo C, Hillemeier C, Hyman P, et al. Manometry studies in children: minimum standards for procedures. Neurogastroenterol Motil. 2002;14:411–420. doi: 10.1046/j.1365-2982.2002.00347.x. [DOI] [PubMed] [Google Scholar]
- 69.Villarreal J, Sood M, Zangen T, et al. Colonic diversion for intractable constipation in children: colonic manometry helps guide clinical decisions. J Pediatr Gastroenterol Nutr. 2001;33:588–591. doi: 10.1097/00005176-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 70.Gertken JT, Cocjin J, Pehlivanov N, Danda C, Hyman PE. Comorbidities associated with constipation in children referred for colon manometry may mask functional diagnosis. J Pediatr Gastroenterol Nutr. 2005;41:328–331. doi: 10.1097/01.mpg.0000173605.62141.27. [DOI] [PubMed] [Google Scholar]
- 71.Kayaba H, Hebiguchi T, Yoshino H, et al. Fecoflowmetric evaluation of anorectal function and ability to defecate in children with idiopathic chronic constipation. Pediatr Surg Int. 2003;19:251–255. doi: 10.1007/s00383-002-0844-x. [DOI] [PubMed] [Google Scholar]
- 72.Bijoś A, Czerwionka-Szaflarska M, Mazur A, Romañczuk W. The usefulness of ultrasound examination of the bowel as a method of assessment of functional chronic constipation in children. Pediatr Radiol. 2007;37:1247–1252. doi: 10.1007/s00247-007-0659-y. [DOI] [PubMed] [Google Scholar]
- 73.Klijn AJ, Asselman M, Vijverberg MA, Dik P, de Jong TP. The diameter of the rectum on ultrasonography as a diagnostic tool for constipation in children with dysfunctional voiding. J Urol. 2004;172(5 Pt 1):1986–1988. doi: 10.1097/01.ju.0000142686.09532.46. [DOI] [PubMed] [Google Scholar]
- 74.Keshtgar AS, Ward HC, Clayden GS, Sanei A. Thickening of the internal anal sphincter in idiopathic constipation in children. Pediatr Surg Int. 2004;20:817–823. doi: 10.1007/s00383-004-1233-4. [DOI] [PubMed] [Google Scholar]
- 75.van der Plas RN, Benninga MA, Taminiau JA, Büller HA. Treatment of defaecation problems in children: the role of education, demystification and toilet training. Eur J Pediatr. 1997;156:689–692. doi: 10.1007/s004310050691. [DOI] [PubMed] [Google Scholar]
- 76.van Ginkel R, Reitsma JB, Büller HA, van Wijk MP, Taminiau JA, Benninga MA. Childhood constipation: longitudinal follow-up beyond puberty. Gastroenterology. 2003;125:357–363. doi: 10.1016/s0016-5085(03)00888-6. [DOI] [PubMed] [Google Scholar]
- 77.Felt B, Wise CG, Olson A, Kochhar P, Marcus S, Coran A. Guideline for the management of pediatric idiopathic constipation and soiling. Arch Pediatr Adolesc Med. 1999;153:380–385. doi: 10.1001/archpedi.153.4.380. [DOI] [PubMed] [Google Scholar]
- 78.van Dijk M, Benninga MA, Grootenhuis MA, Nieuwenhuizen AM, Last BF. Chronic childhood constipation: a review of the literature and the introduction of a protocolized behavioral intervention program. Patient Educ Couns. 2007;67:63–77. doi: 10.1016/j.pec.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 79.van Dijk M, Bongers ME, de Vries GJ, Grootenhuis MA, Last BF, Benninga MA. Behavioral therapy for childhood constipation: a randomized, controlled trial. Pediatrics. 2008;121:e1334–e1341. doi: 10.1542/peds.2007-2402. [DOI] [PubMed] [Google Scholar]
- 80.Ritterband LM, Cox DJ, Walker LS, et al. An internet intervention as an adjunctive therapy for pediatric encopresis. J Consult Clin Psychol. 2003;71:910–917. doi: 10.1037/0022-006X.71.5.910. [DOI] [PubMed] [Google Scholar]
- 81.Loening-Baucke V. Biofeedback training in children with functional constipation. A critical review. Dig Dis Sci. 1996;41:65–71. doi: 10.1007/BF02208585. [DOI] [PubMed] [Google Scholar]
- 82.Wald A, Chandra R, Gabel S, Chiponis D. Evaluation of biofeedback in childhood encopresis. J Pediatr Gastroenterol Nutr. 1987;6:554–558. doi: 10.1097/00005176-198707000-00011. [DOI] [PubMed] [Google Scholar]
- 83.Loening-Baucke V. Modulation of abnormal defecation dynamics by biofeedback treatment in chronically constipated children with encopresis. J Pediatr. 1990;116:214–222. doi: 10.1016/s0022-3476(05)82877-x. [DOI] [PubMed] [Google Scholar]
- 84.Ziegenhagen DJ, Tewinkel G, Kruis W, Herrmann F. Adding more fluid to wheat bran has no significant effects on intestinal function of healthy subjects. J Clin Gastroenterol. 1991;13:525–530. doi: 10.1097/00004836-199110000-00010. [DOI] [PubMed] [Google Scholar]
- 85.Chung BD, Parekh U, Sellin JH. Effect of increased fluid intake on stool output in normal healthy volunteers. J Clin Gastroenterol. 1999;28:29–32. doi: 10.1097/00004836-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Young RJ, Beerman LE, Vanderhoof JA. Increasing oral fluid in chronic constipation in children. Gastroenterol Nurs. 1998;21:156–161. doi: 10.1097/00001610-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 87.Kokke FT, Scholtens PA, Alles MS, et al. A dietary fiber mixture versus lactulose in the treatment of childhood constipation: a double-blind randomized controlled trial. J Pediatr Gastroenterol Nutr. 2008;47:592–597. doi: 10.1097/mpg.0b013e318162c43c. [DOI] [PubMed] [Google Scholar]
- 88.Loening-Baucke V, Miele E, Staiano A. Fiber (glucomannan) is beneficial in the treatment of childhood constipation. Pediatrics. 2004;113(3 Pt 1):e259–e264. doi: 10.1542/peds.113.3.e259. [DOI] [PubMed] [Google Scholar]
- 89.Castillejo G, Bulló M, Anguera A, Escribano J, Salas-Salvadó J. A controlled, randomized double-blind trial to evaluate the effect of a supplement of cocoa husk that is rich in dietary fiber on colonic transit in constipated pediatric patients. Pediatrics. 2006;118:e641–e648. doi: 10.1542/peds.2006-0090. [DOI] [PubMed] [Google Scholar]
- 90.Pashankar DS, Bishop WP. Efficacy and optimal dose of daily polyethylene glycol 3350 for treatment of constipation and encopresis in children. J Pediatr. 2001;139:428–432. doi: 10.1067/mpd.2001.117002. [DOI] [PubMed] [Google Scholar]
- 91.Guest JF, Candy DC, Clegg JP, et al. Clinical and economic impact of using macroglo 3350 plus electrolytes in an outpatient setting compared to enemas and suppositories and manual evacuation to treat paediatric faecal impaction based on actual clinical practice in England and Wales. Curr Med Res Opin. 2007;23:2213–2225. doi: 10.1185/030079907X210462. [DOI] [PubMed] [Google Scholar]
- 92.Candy DC, Edwards D, Geraint M. Treatment of faecal impaction with polyethylene glycol plus electrolytes (PEG + E) followed by a double-blind comparison of PEG + E versus lactulase as maintenance therapy. J Pediatr Gastroenterol Nutr. 2006;43:65–70. doi: 10.1097/01.mpg.0000228097.58960.e6. [DOI] [PubMed] [Google Scholar]
- 93.British national formulary for children. London: BMJ publishing group; 2005. Gastrointestinal system; pp. 72–79. [Google Scholar]
- 94.Martino AM, Pesce F, Rosati U. The effects of lactitol in the treatment of intestinal stasis in childhood. Minerva Pediatr. 1992;44:319–323. [PubMed] [Google Scholar]
- 95.Pitzalis G, Deganello F, Mariani P, et al. Lactitol in chronic idiopathic constipation in children. Pediatr Med Chir. 1995;17:223–226. [PubMed] [Google Scholar]
- 96.Perkin JM. Constipation in childhood: a controlled comparison between lactulose and standardized senna. Curr Med Res Opin. 1977;4:540–543. doi: 10.1185/03007997709115268. [DOI] [PubMed] [Google Scholar]
- 97.Schiller LR, Emmett M, Santa Ana CA, Fordtran JS. Osmotic effects of polyethylene glycol. Gastroenterology. 1988;94:933–941. doi: 10.1016/0016-5085(88)90550-1. [DOI] [PubMed] [Google Scholar]
- 98.Pashankar DS, Loening-Baucke V, Bishop WP. Safety of polyethylene glycol 3350 for the treatment of chronic constipation in children. Arch Pediatr Adolesc Med. 2003;157:661–664. doi: 10.1001/archpedi.157.7.661. [DOI] [PubMed] [Google Scholar]
- 99.Gremse DA, Hixon J, Crutchfield A. Comparison of polyethylene glycol 3350 and lactulose for treatment of chronic constipation in children. Clin Pediatr (Phila) 2002;41:225–229. doi: 10.1177/000992280204100405. [DOI] [PubMed] [Google Scholar]
- 100.Dupont C, Leluyer B, Maamri N, et al. Double-blind randomized evaluation of clinical and biological tolerance of polyethylene glycol 4000 versus lactulose in constipated children. J Pediatr Gastroenterol Nutr. 2005;41:625–633. doi: 10.1097/01.mpg.0000181188.01887.78. [DOI] [PubMed] [Google Scholar]
- 101.Voskuijl W, de Lorijn F, Verwijs W, et al. PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial. Gut. 2004;53:1590–1594. doi: 10.1136/gut.2004.043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Loening-Baucke V. Polyethylene glycol without electrolytes for children with constipation and encopresis. J Pediatr Gastroenterol Nutr. 2002;34:372–377. doi: 10.1097/00005176-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 103.Loening-Baucke V, Pashanker DS. A randomized, prospective comparison study of polyethylene glycol 3350 without electrolytes and milk of magnesia for children with constipation and fecal incontinence. Pediatrics. 2006;118:528–535. doi: 10.1542/peds.2006-0220. [DOI] [PubMed] [Google Scholar]
- 104.Sondheimer JM, Gervaise EP. Lubricant versus laxative in the treatment of chronic functional constipation of children: a comparative study. J Pediatr Gastroenterol Nutr. 1982;1:223–226. doi: 10.1097/00005176-198201020-00012. [DOI] [PubMed] [Google Scholar]
- 105.Degen L, Petrig C, Studer D, Schroller S, Beglinger C. Effects of tegaserod on gut transit in male and female subjects. Neurogastroenterol Motil. 2005;17:821–826. doi: 10.1111/j.1365-2982.2005.00715.x. [DOI] [PubMed] [Google Scholar]
- 106.Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G. Tegaserod accelerates orocaecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000;118:463–468. doi: 10.1016/s0016-5085(00)70251-4. [DOI] [PubMed] [Google Scholar]
- 107.Banaszkiewicz A, Szajewska H. Ineffectiveness of Lactobacillus GG as an adjunct to lactulose for the treatment of constipation in children: a double-blind, placebo-controlled randomized trial. J Pediatr. 2005;146:364–349. doi: 10.1016/j.jpeds.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 108.Bu LN, Chang MH, Ni YH, Chen HL, Cheng CC. Lactobacillus casei rhamnosus Lcr35 in children with chronic constipation. Pediatr Int. 2007;49:485–490. doi: 10.1111/j.1442-200X.2007.02397.x. [DOI] [PubMed] [Google Scholar]
- 109.Clayden GS, Adeyinka T, Kufeji D, Keshtgar AS. Surgical management of severe chronic constipation. Arch Dis Child. 2010;95:859–860. doi: 10.1136/adc.2009.180802. [DOI] [PubMed] [Google Scholar]
- 110.Levitt MA, Carney DE, Powers CJ, Tantoco JG, Caty MG. Laparoscopically assisted colon resection for severe idiopathic constipation with megarectosigmoid. Pediatr Endosurg Innov Tech. 2003;7:285–289. [Google Scholar]
- 111.Marshall J, Hutson JM, Anticich N, Stanton MP. Antegrade continence enemas in the treatment of slow-transit constipation. J Pediatr surg. 2001;36:1227–1230. doi: 10.1053/jpsu.2001.25768. [DOI] [PubMed] [Google Scholar]
- 112.Youssef NN, Barksdale E, Jr, Griffiths JM, Flores AF, Di Lorenzo C. Management of intractable constipation with antegrade enemas in neurologically intact children. J Pediatr Gastroenterol Nutr. 2002;34:402–405. doi: 10.1097/00005176-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 113.Mousa HM, van den Berg MM, Caniano DA, Hogan M, Di Lorenzo C, Hayes J. Cecostomy in children with defecation disorders. Dig Dis Sci. 2006;51:154–160. doi: 10.1007/s10620-006-3101-7. [DOI] [PubMed] [Google Scholar]
- 114.Cascio S, Flett ME, De La Hunt M, Barrett AM, Jaffray B. MACE or caecostomy button for idiopathic constipation in children: a comparison of complications and outcomes. Pediatr Surg Int. 2004;20:484–487. doi: 10.1007/s00383-004-1220-9. [DOI] [PubMed] [Google Scholar]
- 115.Pijpers MA, Bongers ME, Benninga MA, Berger MY. Functional constipation in children: a systematic review on prognosis and predictive factors. J Pediatr Gastroenterol Nutr. 2010;50:256–268. doi: 10.1097/MPG.0b013e3181afcdc3. [DOI] [PubMed] [Google Scholar]