Abstract
Background/Aims
High-resolution manometry (HRM) with pressure topography is used to subtype achalasia cardia, which has therapeutic implications. The aim of this study was to compare the clinical characteristics, manometric variables and treatment outcomes among the achalasia subtypes based on the HRM findings.
Methods
The patients who underwent HRM at the Asian Institute of Gastroenterology, Hyderabad between January 2008 and January 2009 were enrolled. The patients with achalasia were categorized into 3 subtypes: type I - achalasia with minimum esophageal pressurization, type II - achalasia with esophageal compression and type III - achalasia with spasm. The clinical and manometric variables and treatment outcomes were compared.
Results
Eighty-nine out of the 900 patients who underwent HRM were diagnosed as achalasia cardia. Fifty-one patients with a minimum follow-up period of 6 months were included. Types I and II achalasia were diagnosed in 24 patients each and 3 patients were diagnosed as type III achalasia. Dysphagia and regurgitation were the main presenting symptoms in patients with types I and II achalasia. Patients with type III achalasia had high basal lower esophageal sphincter pressure and maximal esophageal pressurization when compared to types I and II. Most patients underwent pneumatic dilatation (type I, 22/24; type II, 20/24; type III, 3/3). Patients with type II had the best response to pneumatic dilatation (18/20, 90.0%) compared to types I (14/22, 63.3%) and III (1/3, 33.3%).
Conclusions
The type II achalasia cardia showed the best response to pneumatic dilatation.
Keywords: Balloon dilatation, Esophageal achalasia, Esophageal motility disorder
Introduction
Achalasia cardia is a motility disorder of the esophagus with an estimated prevalence of 0.5-1.0/100,000 population per year. It is characterized by dysphagia for solids and liquids. The other features include regurgitation of undigested food, chest pain, weight loss and respiratory symptoms.1,2 Incomplete relaxation of lower esophageal sphincter (LES) on swallowing, absence of esophageal peristalsis and elevated basal LES pressures1,2 are the findings on esophageal manometry of achalasia cardia. High-resolution manometry (HRM) is replacing conventional pull through manometry as it is more sensitive and easier to perform and analyze.3-5 HRM provides detailed pressure topography of the esophagus and allows a better identification of compartmentalized distal esophageal pressurization than conventional manometry.6-9 HRM with pressure topography is being used to classify achalasia into different subtypes.10,11
The treatment modalities for achalasia cardia include drug therapy, endoscopic pneumatic dilatation, endoscopic botulinum injection and surgery. The predictors of therapeutic failure for endoscopic pneumatic dilatation are younger age (40-45 years), male, dilated oesophagus, use of a small sized balloon (30 mm), repeated dilatations and a post dilatation LES pressure > 10 mmHg.12-14 The subtypes of achalasia cardia show variable response to endoscopic pneumatic dilatation. This variation in therapeutic outcome with pneumatic dilatation would allow us to categorize patients into different subgroups. The aim of this study was to compare the clinical characteristics, manometric variables and treatment outcomes within the subtypes of achalasia cardia based on the HRM findings. This is the first study in the Indian population.
Materials and Methods
The study was conducted at the Asian Institute of Gastroenterology, Hyderabad between January 2008 and January 2009. The patients underwent HRM and found to have impaired LES relaxation were further analyzed. They underwent an upper gastrointestinal endoscopy to rule out secondary achalasia. The study protocol was approved by the Asian Institute of Gastroenterology Institutional Review Board. The inclusion and exclusion criteria were as follows: patients of any age presenting to the outpatient department or referred for esophageal manometry and diagnosed as achalasia cardia were included. Patients were excluded with the followings: (1) previous treatment for achalasia cardia, (2) patients with esophageal lesions such as webs, rings, strictures or malignancy of esophagus or stomach, (3) previous history of esophageal or gastric surgeries and (4) history of nasal surgery during the last 6 months.
Manometry was performed with a 16 channel water perfused catheter which has 8 channels 1 cm apart at the lower end and the remaining 8 channels 3 cm apart (Dent sleeve International Limited, manufactured by Mui scientific, Ontario, Canada). The data were analyzed using Trace 1.2 V software (Geoff Hebbard, Royal Melbourne Hospital, Victoria, Australia). The manometry catheter was introduced by the transnasal route. The protocol included a basal LES pressure recording for 3 minutes, followed by ten 5 mL wet swallows with an interval of 30 seconds between each of the swallows.
Each of the 10 wet swallow frames included: upper esophageal sphincter relaxation, esophageal body contraction and LES relaxation. The esophageal body contraction was clearly visualized by the isobaric colour contour plot, which provided a continuous depiction of pressure along the entire recording segment. This allowed for a complete spatial and temporal analysis of esophageal motor events.
Achalasia was diagnosed as an impaired LES relaxation on deglutition (mean integrated relaxation pressure ≥ 15 mmHg) and aperistalsis of the esophageal body. The integrated relaxation pressure was defined as the LES relaxation pressure for 4 seconds within the relaxation window. Integrated relaxation pressure can be contiguous or non-contiguous and is usually less than 15 mmHg.11
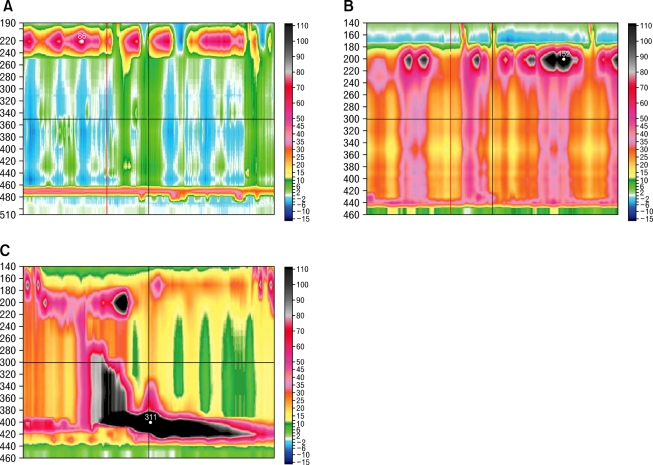
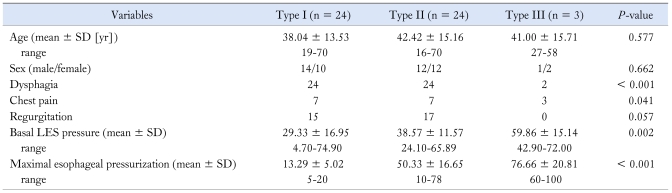
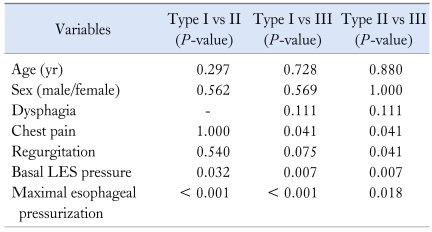
The patients with impaired LES relaxation were divided into 3 subtypes based on the dominant feature of the distal esophageal pressure after swallowing.10,11 Clinical, manometric variables and treatment outcomes were compared among the 3 subtypes. For type I achalasia (classic achalasia), the distal esophageal pressure was less than 30 mmHg in more than 8 out of the 10 wet swallows (Figure A). For type II achalasia (achalasia with esophageal compression), at least 2 out of the 10 wet swallows were associated with a pan esophageal pressurization greater than 30 mmHg (Figure B). For type III achalasia (spastic achalasia), there were 2 or more spastic contractions (contractile front velocity [CFV] > 8 cm/s)11 with or without periods of compartmentalized pressurization (Figure C). The CFV was calculated from the slope of the line connecting the 30 mmHg isobaric contour at the proximal margin of S2 and the distal margin of the S3. Endoscopic pneumatic dilatation was performed using a Rigiflex balloon with a 35 mm diameter (Microvasive). The balloon was placed over an endoscopically introduced guide wire and positioned across the LES. After confirmation of the position by fluoroscopy, the balloon was left inflated for 1 minute. The need for further dilatation was determined by the persistence of symptoms 4 weeks after treatment. The patients were followed for 6 months after endoscopic pneumatic dilatation. A successful treatment response was defined as symptomatic relief requiring no further intervention up to 6 months after a single intervention.13
Figure.
Achalasia subtypes. (A) Type I (classic achalasia), there is no significant pressurization within the body of the esophagus. (B) Type II (achalasia with compression), there is rapid pan-esophageal pressurization. (c) Type III (spastic achalasia), this swallow shows spastic contraction.
Statistical Methods
The clinical and manometric parameters were summarized using mean ± SD. The data was analyzed by Statistical Package for Social Sciences (SPSS) 13.0 version (SPSS Inc, Chicago, IL, USA). The Chi-square test was used to compare the categorical variables among the subtypes. The comparison among the 3 subtypes for normal continuous data was performed using a one way analysis of variance. The comparison among the 3 subtypes for abnormal continuous data was performed using the Kruskal-Wallis test. The comparison between 2 subtypes was performed using the t test for normal continuous data and the Wilcox Rank Sum test for abnormal continuous data. The predictors of response to pneumatic dilatation among the different subtypes were assessed using the multivariate logistic regression analysis. A P-value of less than 0.05 was considered statistically significant. For calculation of odds ratio, achalasia subtype I was considered as the control because it represented the classic definition of achalasia cardia.
Results
Eighty-nine of the 900 patients who underwent HRM were diagnosed as achalasia cardia. Fifty-one patients with a mean follow-up of 6 months were included in the study.
Achalasia Subtypes
Types I and II achalasia were observed in 24 patients each and type III achalasia in 3 patients. The mean age of patients between types II and III were similar, compared to type I with younger age (P = 0.577). The male to female ratio in all the groups were similar (P = 0.662). Dysphagia and regurgitation were more frequently presenting symptoms in types I and II (P < 0.001 and P = 0.057, respectively) than type III. The patients with type III had a higher incidence of chest pain when compared to the others (P = 0.041) (Table 1).
Table 1.
Clinical and Manometric Characteristics of the Achalasia Subtypes
LES, lower esophageal sphincter.
An intergroup analysis was performed to investigate the differences in terms of clinical variables and manometric parameters among the 3 subtypes (Table 2). The clinical variables were not significantly different among the 3 subgroups except chest pain in type III achalasia. However, the manometric parameters were different among the 3 subgroups. The patients with type III had spastic contraction in most of the swallows, and also had higher basal LES pressure and maximal esophageal pressurization when compared to types I and II (P < 0.001) (Table 1). For patients with type II achalasia, the swallows were associated with compression while the patients with type I had failed swallows.
Table 2.
Comparison of Clinical and Manometric Characteristics among the Subtypes of Achalasia
LES, lower esophageal sphincter.
Clinical Outcomes
Endoscopic pneumatic dilatation was performed in 45 out of the 51 patients, endoscopic injection of botulinum neurotoxin in 4, pharmacological treatment in 1 and Heller's myotomy in 1 respectively. Since the number of patients who underwent botulinum toxin injection and surgery were small, they were not included in the clinical outcome analysis.
Data from 45 patients who had undergone endoscopic pneumatic dilatation was used to assess the treatment outcomes. A subgroup analysis was performed to investigate the differences in terms of clinical variables, manometric parameters and response to endoscopic pneumatic dilatation among the 3 subtypes. The response to pneumatic dilatation was best in type II (18/20, 90.0%) compared to type I (14/22, 63.0%) and type III (1/3, 33.3%). The other variables which have an influence on the response to endoscopic pneumatic dilatation were age, sex, and the duration of symptoms. Multivariate logistic regression analysis of response to endoscopic pneumatic dilatation with age, sex, the duration of symptoms and types of achalasia was performed (Table 3). Among these variables, the types of achalasia was found to have a significant correlation with response to endoscopic pneumatic dilatation (P = 0.042) (Table 3). The other variables were not statistically significant. According to data, patients with type II achalasia had the best response to endoscopic pneumatic dilatation: type II vs I (P = 0.045), type II vs III (P = 0.016) and type I vs III (P = 0.315). The analysis of the odds ratio for patients with type II achalasia showed that they responded better to pneumatic dilatation (OR, 5.14; 95% CI, 0.94-28.14) when compared to type I achalasia.
Table 3.
Predictors of Response to Pneumatic Dilatation
Discussion
This is the first manometric study of the Indian population to classify the subtypes of achalasia. The frequency of subtypes I and II was the same in the present study, different from the findings reported by Pandolfino et al10 where type II was more common.
Type I achalasia was more common in the younger age group with dysphagia and regurgitation as the main features. Type II achalasia had similar features to type I except age (Table 1).
The cause of chest pain, a common symptom associated with achalasia, remains unknown. Eckardt et al15 studied chest pain in patients with achalasia and reported an association with a younger age and short duration of symptoms. However, there was no correlation with the manometric or radiographic findings. In our study, patients with type III achalasia had chest pain. The pathogenesis of chest pain associated with type III achalasia may be related to the high esophageal pressurization.
The rate of therapeutic failure is high in younger patients.16 Chuah et al17 showed that older patients (> 45 years) had an overall favorable rate of clinical remission in a prospective 7-year follow-up study of endoscopic pneumatic dilatation. Ghoshal et al18 in their study of therapeutic failure after pneumatic dilatation showed on multivariate analysis that male gender was associated with poor outcome. Age, grade of dysphagia, esophageal dilatation and pre-dilatation LES pressure did not affect the outcome.18 In our study, response to pneumatic dilatation had no correlation with age (P = 0.366) and sex (P = 0.344).
The major finding in our study was that HRM could predict the response to endoscopic pneumatic dilatation in achalasia. Type II achalasia was a strong positive predictor of response to endoscopic pneumatic dilatation and type III achalasia was a strong negative predictor. Patients with type II achalasia tended to have a less severe dilatation of esophagus compared to type I and might represent an early stage of the disease. Hence, they responded better to treatment. Patients with type I tended to have severe esophageal dilatation due to prolonged functional obstruction at the LES with minimal contractility of esophageal body and might represent a late stage in the natural history of achalasia. Patients with type II had preserved longitudinal muscle contraction and sufficient excitation of the circular muscle to generate substantial intrabolus pressure in the esophageal body. Thus, reduction of the LES pressure with therapy would give good response to these patients.
In conclusion, this study presented the clinical and manometric predictors for treatment response of achalasia and the clinical usefulness of HRM. The results showed that the patients with type II achalasia responded better to endoscopic pneumatic dilatation compared to the others. The subtyping of achalasia by HRM may allow the clinician to direct therapy and predict outcomes.
Acknowledgements
The authors would like to thank the manometry technician Mr. Sunil and statistician Mr. Yanadi Reddy for their help during the study.
Footnotes
Financial support: None.
Conflicts of interest: None.
References
- 1.Richter JE. Oesophageal motility disorders. Lancet. 2001;358:823–828. doi: 10.1016/S0140-6736(01)05973-6. [DOI] [PubMed] [Google Scholar]
- 2.Hirano I, Tatum RP, Shi G, Sang Q, Joehl RJ, Kahrilas PJ. Manometric heterogeneity in patients with idiopathic achalasia. Gastroenterology. 2001;120:789–798. doi: 10.1053/gast.2001.22539. [DOI] [PubMed] [Google Scholar]
- 3.Bredenoord AJ, Smout AJ. High-resolution manometry. Dig Liver Dis. 2008;40:174–181. doi: 10.1016/j.dld.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Salvador R, Dubecz A, Polomsky M, et al. A new era in esophageal diagnostics: the image-based paradigm of high-resolution manometry. J Am Coll Surg. 2009;208:1035–1044. doi: 10.1016/j.jamcollsurg.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Fisichella PM, Patti MG. The evolution of oesophageal function testing and its clinical applications in the management of patients with oesophageal disorders. Dig Liver Dis. 2009;41:626–629. doi: 10.1016/j.dld.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Kahrilas PJ, Ghosh SK, Pandolfino JE. Esophageal motility disorders in terms of pressure topography: the Chicago Classification. J Clin Gastroenterol. 2008;42:627–635. doi: 10.1097/MCG.0b013e31815ea291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox M, Hebbard G, Janiak P, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16:533–542. doi: 10.1111/j.1365-2982.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 8.Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103:27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 9.Kahrilas PJ, Sifrim D. High-resolution manometry and impedance-pH/ manometry: valuable tools in clinical and investigational esophagology. Gastroenterology. 2008;135:756–769. doi: 10.1053/j.gastro.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandolfino JE, Fox MR, Bredenoord AJ, Kahrilas PJ. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil. 2009;21:796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vantrappen G, Hellemans J, Deloof W, Valembois P, Vandenbroucke J. Treatment of achalasia with pneumatic dilatations. Gut. 1971;12:268–275. doi: 10.1136/gut.12.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boeckxstaens GE. Achalasia. Best Pract Res Clin Gastroenterol. 2007;21:595–608. doi: 10.1016/j.bpg.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Ferri LE, Cools-Lartigue J, Cao J, et al. Clinical predictors of achalasia. Dis Esophagus. 2010;23:76–81. doi: 10.1111/j.1442-2050.2009.01006.x. [DOI] [PubMed] [Google Scholar]
- 15.Eckardt VF, Stauf B, Bernhard G. Chest pain in achalasia: patient characteristics and clinical course. Gastroenterology. 1999;116:1300–1304. doi: 10.1016/s0016-5085(99)70493-2. [DOI] [PubMed] [Google Scholar]
- 16.Tuset JA, Luján M, Huguet JM, Canelles P, Medina E. Endoscopic pneumatic balloon dilation in primary achalasia: predictive factors, complications, and long-term follow-up. Dis Esophagus. 2009;22:74–79. doi: 10.1111/j.1442-2050.2008.00874.x. [DOI] [PubMed] [Google Scholar]
- 17.Chuah SK, Hu TH, Wu KL, et al. Clinical remission in endoscope-guided pneumatic dilation for the treatment of esophageal achalasia: 7-year follow-up results of a prospective investigation. J Gastrointest Surg. 2009;13:862–867. doi: 10.1007/s11605-009-0804-z. [DOI] [PubMed] [Google Scholar]
- 18.Ghoshal UC, Kumar S, Saraswat VA, Aggarwal R, Misra A, Choudhuri G. Long-term follow-up after pneumatic dilation for achalasia cardia: factors associated with treatment failure and recurrence. Am J Gastroenterol. 2004;99:2304–2310. doi: 10.1111/j.1572-0241.2004.40099.x. [DOI] [PubMed] [Google Scholar]