Abstract
Background
Recommendations vary regarding immediate antimicrobial treatment versus watchful waiting for children younger than 2 years of age with acute otitis media.
Methods
We randomly assigned 291 children 6 to 23 months of age, with acute otitis media diagnosed with the use of stringent criteria, to receive amoxicillin–clavulanate or placebo for 10 days. We measured symptomatic response and rates of clinical failure.
Results
Among the children who received amoxicillin–clavulanate, 35% had initial resolution of symptoms by day 2, 61% by day 4, and 80% by day 7; among children who received placebo, 28% had initial resolution of symptoms by day 2, 54% by day 4, and 74% by day 7 (P = 0.14 for the overall comparison). For sustained resolution of symptoms, the corresponding values were 20%, 41%, and 67% with amoxicillin–clavulanate, as compared with 14%, 36%, and 53% with placebo (P = 0.04 for the overall comparison). Mean symptom scores over the first 7 days were lower for the children treated with amoxicillin–clavulanate than for those who received placebo (P = 0.02). The rate of clinical failure — defined as the persistence of signs of acute infection on otoscopic examination — was also lower among the children treated with amoxicillin–clavulanate than among those who received placebo: 4% versus 23% at or before the visit on day 4 or 5 (P<0.001) and 16% versus 51% at or before the visit on day 10 to 12 (P<0.001). Mastoiditis developed in one child who received placebo. Diarrhea and diaper-area dermatitis were more common among children who received amoxicillin–clavulanate. There were no significant changes in either group in the rates of nasopharyngeal colonization with nonsusceptible Streptococcus pneumoniae.
Conclusions
Among children 6 to 23 months of age with acute otitis media, treatment with amoxicillin–clavulanate for 10 days tended to reduce the time to resolution of symptoms and reduced the overall symptom burden and the rate of persistent signs of acute infection on otoscopic examination. (Funded by the National Institute of Allergy and Infectious Diseases; ClinicalTrials.gov number, NCT00377260.)
Acute otitis media is the most frequently diagnosed illness in children in the United States1 and the most commonly cited indication for antimicrobial therapy in children2; in the United States, most children with acute otitis media have routinely been treated with antimicrobial drugs. However, a watchful-waiting strategy, in which treatment is reserved for children whose condition does not improve without medication, has long been applied in several European countries in the interest of minimizing the use of antimicrobial drugs.3 In the Netherlands and Scotland, that strategy has been recommended officially for children as young as 6 months of age.4,5 In 2004, a clinical practice guideline issued by the American Academy of Pediatrics and the American Academy of Family Physicians endorsed watchful waiting as an option for children 6 to 23 months of age in whom illness is “nonsevere” (defined, in an adaptation of an earlier definition,6 by the presence of mild otalgia and a temperature of less than 39°C during the preceding 24 hours) and in whom the diagnosis of acute otitis media is uncertain.7 A similar recommendation, but without reference to diagnostic certainty, was issued recently by the Infectious Diseases and Immunization Committee of the Canadian Paediatric Society.8
The adoption of a strategy of watchful waiting has been based on the results of clinical trials3 that showed relatively high rates of spontaneous improvement in children with acute otitis media. However, in those trials, as in earlier trials involving children with otitis media, there were substantial limitations — most notably, the lack of stringent diagnostic criteria, the inclusion of few very young children, and the use of an antimicrobial drug that had limited efficacy or that was administered in suboptimal doses.9 Moreover, rates of spontaneous improvement similar to the rates seen in those studies among children receiving placebo have not been found uniformly.6 Therefore, for children with acute otitis media, the circumstances in which immediate antimicrobial treatment is the preferred strategy have remained unclear. We undertook this clinical trial to determine the extent to which antimicrobial treatment affects the course of both symptoms and signs of acute otitis media, irrespective of the apparent severity of the disease, among children 6 to 23 months of age in whom the diagnosis of acute otitis media is quite certain. Because amoxicillin–clavulanate has been shown to be the most effective treatment for acute otitis media, we chose it as the active treatment in our study.7,10
Methods
Eligibility and Enrollment
We conducted this trial between November 2006 and March 2009 at the Children's Hospital of Pittsburgh and Armstrong Pediatrics, an affiliated private practice in Kittanning, Pennsylvania. The study protocol was approved by the institutional review board at the University of Pittsburgh; written informed consent was obtained from a parent of each enrolled child. The protocol, including the statistical analysis plan, is available with the full text of this article at NEJM.org. The authors attest that the study was performed in accordance with the protocol and the statistical analysis plan.
To be eligible for enrollment in the study, children were required to have received at least two doses of pneumococcal conjugate vaccine and to have acute otitis media that was diagnosed on the basis of three criteria: the onset, within the preceding 48 hours, of symptoms that parents rated with a score of at least 3 on the Acute Otitis Media Severity of Symptoms (AOM-SOS) scale11,12 (on which scores range from 0 to 14, with higher scores indicating greater severity of symptoms), the presence of middle-ear effusion, and moderate or marked bulging of the tympanic membrane or slight bulging accompanied by either otalgia or marked erythema of the membrane. The AOM-SOS scale consists of seven discrete items: tugging of ears, crying, irritability, difficulty sleeping, diminished activity, diminished appetite, and fever. Parents are asked to rate these symptoms, in comparison with the child's usual state, as “none,” “a little,” or “a lot,” with corresponding scores of 0, 1, and 2 (see Fig. 1 in the Supplementary Appendix, available at NEJM.org). Children were excluded if they had another acute illness (e.g., pneumonia) or a chronic illness (e.g., cystic fibrosis), were allergic to amoxicillin, had received more than one dose of an antimicrobial drug within the previous 96 hours, had had otalgia for longer than 48 hours, or had perforation of the tympanic membrane.
Randomization
We stratified children according to whether they had a history of recurrent acute otitis media (defined as three or more episodes in the preceding 6 months or four or more episodes over the course of 12 months) and according to their exposure or nonexposure to three or more children for at least 10 hours per week. At each study site, within each stratum, we randomly assigned children in blocks of four, in a 1:1 ratio, to receive a 10-day course of either amoxicillin–clavulanate (Augmentin ES, GlaxoSmithKline), at a daily dose of 90 mg of amoxicillin per kilogram of body weight combined with 6.4 mg of clavulanate per kilogram, or placebo; each study drug was administered in two doses per day. The placebo was prepared by the research pharmacy at the Children's Hospital of Pittsburgh according to the formula for placebo specified in the application to the Food and Drug Administration for the labeling information for Augmentin ES and was similar to that product in appearance and taste. The parents, the research personnel, and the health care providers who were not associated with the study remained unaware of the children's group assignments throughout the study. Parents were advised to administer acetaminophen as needed for the relief of symptoms.
Assessment of Symptoms
We assessed symptoms with the use of a structured interview of one of the child's parents; assessments were performed by telephone every day until the first follow-up visit and in person at each visit. We also asked the parents about loss of time at work or the need for alternative day-care arrangements because of the child's illness. Parents were asked to record their child's AOM-SOS scores and other pertinent clinical information in a diary twice a day for 3 days and once a day thereafter.
Otoscopic Examination, Overall Assessment, and Management
All the study clinicians were otoscopists who had successfully completed an otoscopic validation program,13 and their findings on otoscopic examination determined the diagnoses for the study; whenever possible, however, we also obtained otoendoscopic photographs of the children's tympanic membranes (see Fig. 2, 3, and 4 in the Supplementary Appendix). The children received the study drug on day 1; thereafter, we assessed them during the course of therapy, usually on day 4 or 5; at the end of therapy, usually on day 10 to 12; and at a follow-up visit, usually on day 21 to 25. Hereinafter, we use those days to designate the three types of visit, irrespective of individual variations in the actual days of the visits. At each visit, we categorized the children as having met the criteria for either clinical success or clinical failure. We defined clinical failure at or before the day 4–5 visit as either a lack of substantial improvement in symptoms, a worsening of signs on otoscopic examination, or both, and clinical failure at the day 10–12 visit as the failure to achieve complete or nearly complete resolution of symptoms and of otoscopic signs, without regard to the persistence or resolution of middle-ear effusion. We treated children who met the criteria for clinical failure with a standardized 10-day regimen of orally administered amoxicillin, at a dose of 90 mg per kilogram per day, and cefixime, at a dose of 8 mg per kilogram per day. Once a child had met the criteria for clinical failure, he or she remained in that category for the analyses. At the day 21–25 visit, the reappearance of acute otitis media in a child who had previously been categorized as having met the criteria for clinical success was considered to be a relapse. When feasible, we obtained nasopharyngeal specimens from the children for culturing, at study entry and at the day 10–12 and day 21–25 visits.
Outcomes
All outcome measures were prespecified. The primary outcome measures were the time to resolution of symptoms and the symptom burden over time. The time to resolution of symptoms was measured in two ways: the time to the first recording of an AOM-SOS score of 0 or 1 and the time to the second of two successive recordings of that score. The symptom burden over time was measured by calculating the mean AOM-SOS score in the two groups each day over the first 7 days of follow-up and the groups' weighted mean scores for that period. The secondary outcomes were overall clinical efficacy, the use of acetaminophen, the occurrence of adverse events, nasopharyngeal colonization rates, and the use of health care resources.
Statistical Analysis
We estimated that with a sample of 120 children who could be evaluated in each study group, the study would have 80% power to detect a 66% lower rate of resolution of symptoms in the placebo group as compared with the amoxicillin–clavulanate group. All the analyses were based on the intention-to-treat principle, were performed with the use of two-sided tests, and included adjustment for the study stratification variables. We compared the time to the resolution of symptoms between the study groups using life-table plots, and we conducted tests of equal hazard functions using a proportional-hazards model. We compared the mean AOM-SOS scores in the two groups at individual assessments each day over the first 7 days of follow-up using generalized estimating equations, and the groups' weighted mean scores for that period (taking into account that observations were made twice daily during the first 3 days of follow-up) using regression analysis. For analyses of clinical success or failure, we used logistic regression. To determine whether variables were prognostic or effect modifiers, we used the proportional-hazards model or logistic-regression models, as appropriate. We used McNemar's test for analyses of nasopharyngeal colonization rates.
Results
Study Population
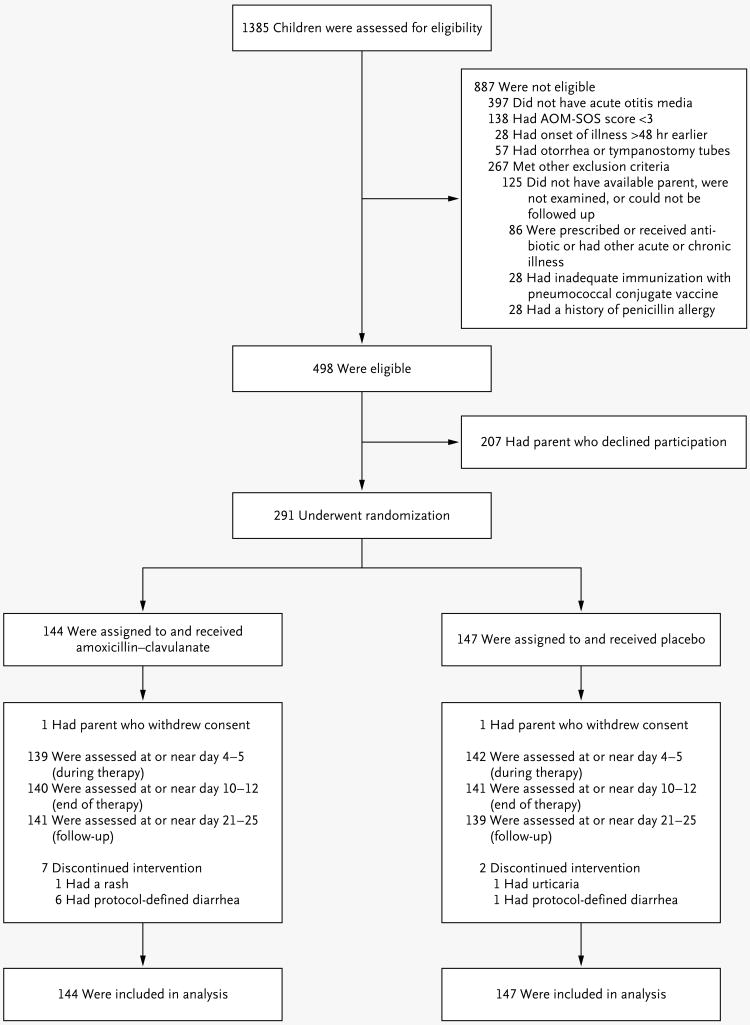
A total of 1385 children were screened; 498 of the children were eligible for the clinical trial and 291 were enrolled (Fig. 1). Selected demographic and clinical characteristics of the children are shown in Table 1. There were no significant differences between enrolled children and children whose parents withheld consent or between the children in the two study groups. We found no significant correlation between children's AOM-SOS scores at entry and the degree of bulging of their affected tympanic membranes. A total of 96% of the children completed all scheduled study visits, and 85% reportedly received all scheduled medication doses during days 1 through 3 and at least 80% of doses overall.
Figure 1. Enrollment, Randomization, and Follow-up of Children in the Study.
Mean scores on the Acute Otitis Media Severity of Symptoms (AOM-SOS) scale range from 0 to 14, with higher scores indicating greater severity of symptoms.
Table 1. Selected Demographic and Clinical Characteristics, According to Study Group*.
| Characteristic | Amoxicillin–Clavulanate Group (N = 144) | Placebo Group (N = 147) | All Children (N = 291) |
|---|---|---|---|
| Age at entry — no. (%) | |||
| 6–11 mo | 83 (58) | 78 (53) | 161 (55) |
| 12–17 mo | 35 (24) | 42 (29) | 77 (26) |
| 18–23 mo | 26 (18) | 27 (18) | 53 (18) |
| Sex — no. (%) | |||
| Male | 75 (52) | 80 (54) | 155 (53) |
| Female | 69 (48) | 67 (46) | 136 (47) |
| Race — no. (%)† | |||
| White | 66 (46) | 65 (44) | 131 (45) |
| Black | 62 (43) | 58 (39) | 120 (41) |
| Other | 16 (11) | 24 (16) | 40 (14) |
| Maternal level of education — no./total no. (%) | |||
| Less than high school | 24/144 (17) | 16/146 (11) | 40/290 (14) |
| High-school graduate | 90/144 (62) | 95/146 (65) | 185/290 (64) |
| College graduate | 30/144 (21) | 35/146 (24) | 65/290 (22) |
| Type of health insurance — no./total no. (%) | |||
| Private | 43/141 (30) | 42/145 (29) | 85/286 (30) |
| Medicaid | 98/141 (70) | 103/145 (71) | 201/286 (70) |
| Exposure to other children — no. (%)‡ | |||
| Yes | 69 (48) | 72 (49) | 141 (48) |
| No | 75 (52) | 75 (51) | 150 (52) |
| AOM-SOS score§ | |||
| Baseline score — no. (%) | |||
| 3–5 | 37 (26) | 33 (22) | 70 (24) |
| 6–8 | 46 (32) | 51 (35) | 97 (33) |
| 9–11 | 46 (32) | 44 (30) | 90 (31) |
| 12–14 | 15 (10) | 19 (13) | 34 (12) |
| Mean baseline score | 7.69±2.85 | 7.90±2.87 | 7.80±2.86 |
| Laterality of acute otitis media — no. (%) | |||
| Bilateral | 75 (52) | 77 (52) | 152 (52) |
| Unilateral | 69 (48) | 70 (48) | 139 (48) |
| Degree of tympanic membrane bulging in worse ear — no. (%) | |||
| Slight | 42 (29) | 39 (27) | 81 (28) |
| Moderate | 63 (44) | 70 (48) | 133 (46) |
| Marked | 39 (27) | 38 (26) | 77 (26) |
There were no significant differences in characteristics between the two study groups.
Race was reported by the parents.
Exposure to other children was defined as exposure to at least three children for at least 10 hours per week.
The Acute Otitis Media Severity of Symptoms (AOM-SOS) scale measures seven discrete items: tugging of ears, crying, irritability, difficulty sleeping, diminished activity, diminished appetite, and fever. Parents are asked to rate these symptoms, in comparison with the child's usual state, as “none,” “a little,” or “a lot,” with corresponding scores of 0, 1, and 2. Thus, total scores range from 0 to 14, with higher scores indicating greater severity of symptoms.
Efficacy of Treatment
Symptomatic Response
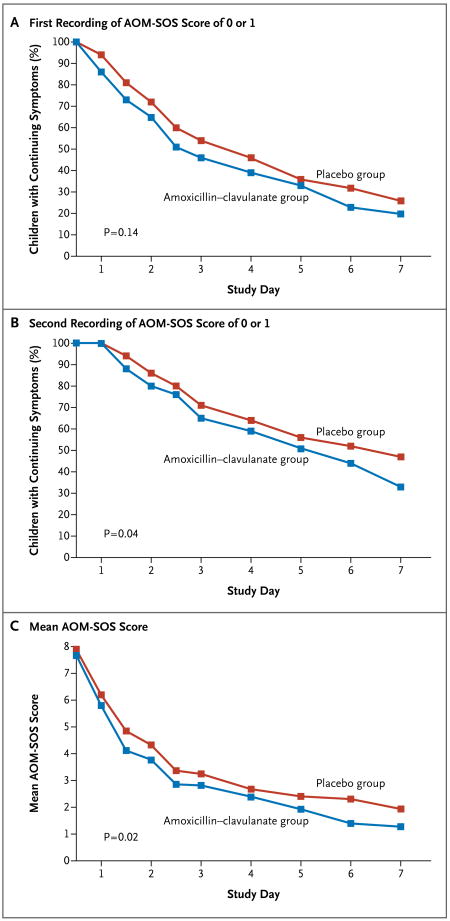
The children's symptomatic response during the first 7 days of follow-up is summarized in Figure 2. The time to resolution of symptoms is shown according to two criteria: the time to the first recording of an AOM-SOS score of 0 or 1 (Fig. 2A) and the time to the second of two successive recordings of that score (Fig. 2B). (In 54% of the instances in which scores fell to 0 or 1, a subsequent score was higher than 1.) Among the children who were treated with amoxicillin–clavulanate, the initial resolution of symptoms occurred by day 2 in 35% of the children, by day 4 in 61%, and by day 7 in 80%; the corresponding values among children who received placebo were 28%, 54%, and 74% (P = 0.14 for the overall comparison). The resolution of symptoms as defined by two successive recordings of an AOM-SOS score of 0 or 1 occurred by day 2 in 20% of the children who were treated with amoxicillin–clavulanate, by day 4 in 41%, and by day 7 in 67%; the corresponding values for children who received placebo were 14%, 36%, and 53% (P = 0.04 for the overall comparison).
Figure 2. Resolution of Children's Symptoms during the First 7 Days of Follow-up.
During the first 3 days, the Acute Otitis Media Severity of Symptoms (AOM-SOS) scale11,12 was administered twice daily; thereafter, it was administered once daily. Panels A and B show the proportion of children in whom symptoms had not resolved. Resolution of symptoms is defined in Panel A as the first recording of an AOM-SOS score of 0 or 1 and in Panel B as the second of two successive recordings of a score of 0 or 1. Panel C shows the mean scores on the AOM-SOS scale over the course of the first 7 days of the study treatment. Since multiple assessments were made on the first 3 study days, numbers on the x axis in all three panels indicate the end of a study day.
The mean AOM-SOS scores in the two treatment groups over the first 7 days (Fig. 2C) were lower in the amoxicillin–clavulanate group than in the placebo group at each time point (P = 0.02) and at the 10–12 day visit (1.59±0.21 vs. 2.46±0.20; difference, 0.87; 95% confidence interval [CI], 0.29 to 1.45; P = 0.003). The 7-day weighted mean (±SE) score was also significantly lower in the amoxicillin–clavulanate group than in the placebo group (2.79±0.16 vs. 3.42±0.18; difference, 0.63; 95% CI, 0.15 to 1.10; P = 0.01). The degree of difference between the two treatment groups varied according to the severity of the children's symptoms at trial entry; among children whose initial AOM-SOS score was 8 or less, the scores were 2.21±0.16 vs. 2.58±0.19 (difference, 0.37; 95% CI, −0.13 to 0.87; P = 0.14), and among children whose initial score was higher than 8, the values were 3.59±0.27 vs. 4.50±0.28 (difference, 0.91; 95% CI, 0.13 to 1.68; P = 0.02).
Clinical Failure
Children who received amoxicillin–clavulanate were less likely than children receiving placebo to have evidence of clinical failure at or before the day 4–5 visit (4% vs. 23%; difference, 19%; 95% CI, 12 to 27; P<0.001) and at or before the day 10–12 visit (16% vs. 51%; difference, 35%; 95% CI, 25 to 45; P<0.001). In the two groups combined, AOM-SOS scores at the time of clinical failure were 2 or higher in 33 of 39 children (85%) who met the criteria for clinical failure at or before the day 4–5 visit and in 60 of 95 children (63%) who met the criteria for clinical failure at or before the day 10–12 visit; the proportions did not differ significantly between the two study groups. The mean (±SD) scores at the day 4–5 visit were 5.0±3.2 among children who met the criteria for clinical failure at that visit and 2.2±2.3 among children who did not meet the criteria for clinical failure at that visit; the corresponding values at the day 10–12 visit were 3.7±3.3 and 1.2±1.8. No child was categorized as having met the criteria for clinical failure on the basis of symptoms alone.
Prognostic Factors and Effect Modifiers
At day 10 to 12, the clinical results were less favorable among children who were exposed to three or more children for at least 10 hours per week than among children who were not (P = 0.007), among children with higher AOM-SOS scores at entry than among those with lower scores (P = 0.004), among children with bilateral acute otitis media than among children with unilateral acute otitis media (P = 0.002), and among children with more bulging of the tympanic membrane than among children with less bulging (P<0.001) (Table 2; and see Table 2 in the Supplementary Appendix for the multiple logistic-regression analysis). Among children in the trial whose illness would have been considered severe (as indicated by moderate or severe otalgia or a body temperature of more than 39°C during the preceding 24 hours), the rates of clinical failure at or before the day 10–12 visit were 19% (12 of 63 children) among those receiving amoxicillin–clavulanate and 61% (40 of 66) among those receiving placebo. Corresponding rates among children whose illness would have been considered nonsevere were 14% (11 of 79) and 43% (33 of 77). Among the 17 interactions that we tested between a demographic or clinical variable and the assigned study treatment, one was significant: children 18 to 23 months of age who received amoxicillin–clavulanate had a higher rate of clinical failure than did children 12 to 17 months of age and children 6 to 11 months of age (38% vs. 12% and 11%, respectively) (see Table 1 in the Supplementary Appendix for additional details).
Table 2. Clinical Failure Rates at or before the Day 10–12 Visit, According to Study Group and Demographic and Clinical Characteristics at Entry*.
| Characteristic at Entry | Amoxicillin–Clavulanate Group (N = 144) | Placebo Group (N = 147) | All Children (N = 291) | P Value† |
|---|---|---|---|---|
| no. of children with clinical failure/total no. (%)‡ | ||||
| Exposure to other children§ | 0.007 | |||
| No | 10/73 (14) | 29/73 (40) | 39/146 (27) | |
| Yes | 13/69 (19) | 44/70 (63) | 57/139 (41) | |
| AOM-SOS score | 0.004 | |||
| ≤8 | 9/82 (11) | 35/81 (43) | 44/163 (27) | |
| >8 | 14/60 (23) | 38/62 (61) | 52/122 (43) | |
| Laterality of acute otitis media | 0.002 | |||
| Unilateral | 6/68 (9) | 29/70 (41) | 35/138 (25) | |
| Bilateral | 17/74 (23) | 44/73 (60) | 61/147 (41) | |
| Degree of tympanic membrane bulging in worse ear | <0.001 | |||
| Slight or moderate | 12/103 (12) | 46/106 (43) | 58/209 (28) | |
| Marked | 11/39 (28) | 27/37 (73) | 38/76 (50) | |
| No. of unfavorable characteristics | <0.001 | |||
| 0 | 1/19 (5) | 4/15 (27) | 5/34 (15) | |
| 1 | 4/42 (10) | 14/46 (30) | 18/88 (20) | |
| 2 | 6/44 (14) | 27/46 (59) | 33/90 (37) | |
| 3 | 8/26 (31) | 20/28 (71) | 28/54 (52) | |
| 4 | 4/11 (36) | 8/8 (100) | 12/19 (63) | |
For each characteristic, the favorable category is listed first and the unfavorable second. All the tests for interactions between study group and the individual characteristics were nonsignificant. The odds ratios for the individual unfavorable characteristics, adjusted for study group and for the remaining three characteristics, ranged from 2.0 to 2.3 (see Table 2 in the Supplementary Appendix for additional details). AOM-SOS denotes the Acute Otitis Media Severity of Symptoms scale.
For the individual characteristics, P values are for the comparison, in the study groups combined, between children with the favorable characteristic and children with the unfavorable characteristic. For the number of unfavorable characteristics, the P value is for the test for linear trend from none to four.
Data were not available for some children because of missed visits.
Exposure to other children was defined as exposure to at least three children for at least 10 hours per week.
Relapse and Residual Middle-Ear Effusion
Among children who met the criteria for clinical success at the day 10–12 visit, the rate of relapse noted at or before the day 21–25 visit was 16% (19 of 119 children) in the amoxicillin–clavulanate group and 19% (13 of 70) in the placebo group (P = 0.56). At the day 21–25 visit, 71 of the 141 children in the amoxicillin–clavulanate group (50%) had middle-ear effusion (29 unilateral and 42 bilateral) and 87 of 139 in the placebo group (63%) had middle-ear effusion (32 unilateral and 55 bilateral) (P = 0.05).
Nasopharyngeal Colonization and Other Outcomes
From day 1 to the day 21–25 visit, no significant changes occurred in either group in the rate of colonization with nonsusceptible strains of Streptococcus pneumoniae (minimum inhibitory concentration, ≥0.1 μg per milliliter) (see Table 3 in the Supplementary Appendix for details). The rates of clinical failure had no apparent relationship to the children's initial colonization status in either group. We found no significant differences between the amoxicillin–clavulanate group and the placebo group in either the mean daily number of doses of acetaminophen administered (0.37 and 0.43, respectively; P = 0.35) or the use of health care resources.
Complications and Adverse Events
Mastoiditis developed in one child in the placebo group on day 5. Dermatitis in the diaper area and protocol-defined diarrhea occurred commonly, and often together, among children receiving antimicrobial agents. Details concerning complications and adverse events are summarized in Table 3.
Table 3. Complications and Adverse Events According to Study Group and Treatment Received at the Time of Occurrence*.
| Adverse Event | Amoxicillin–Clavulanate Group (N = 144) | Placebo Group (N = 147) | ||||
|---|---|---|---|---|---|---|
| During Receipt of Amoxicillin–Clavulanate |
During Receipt of Rescue Therapy |
Total | During Receipt of Placebo |
During Receipt of Rescue Therapy |
Total | |
|
number of children (percent) |
||||||
| Mastoiditis† | 0 | 0 | 0 | 1 (1) | 0 | 1 (1) |
| Perforation of tympanic membrane | 1 (1) | 0 | 1 (1) | 6 (4) | 1 (1) | 7 (5) |
| Protocol-defined diarrhea‡ | 34 (24) | 2 (1) | 36 (25) | 11 (7) | 11 (7) | 22 (15)§ |
| Diaper-area dermatitis | 67 (47) | 6 (4) | 73 (51) | 24 (16) | 27 (18) | 51 (35)¶ |
| Oral thrush | 7 (5) | 0 | 7 (5) | 0 | 1 (1) | 1 (1) |
| Vomiting‖ | 12 (8) | 0 | 12 (8) | 11 (7) | 1 (1) | 12 (8) |
| Rash** | 1 (1) | 0 | 1 (1) | 1 (1) | 1 (1) | 2 (1) |
In each study group, one child was not followed beyond enrollment. Comparisons were adjusted for study site, history or no history of recurrent acute otitis media, and exposure or no exposure to other children.
Mastoiditis developed on day 5 in an 11-month-old child with unilateral acute otitis media and a score on the Acute Otitis Media Severity of Symptoms (AOM-SOS) scale of 14 at entry and of 7 on day 5. A culture of middle-ear aspirate yielded serotype 19A Streptococcus pneumoniae with a penicillin minimum inhibitory concentration of 2 μg per milliliter. The child was hospitalized for 41 hours and treated initially with parenteral antibiotics and subsequently with oral antibiotics; he recovered uneventfully.
Diarrhea was defined in the protocol as three or more watery stools in 1 day or two watery stools daily for at least 2 days. Treatment was discontinued because of diarrhea in six children in the amoxicillin–clavulanate group and one child in the placebo group, in whom Clostridium difficile enteritis developed.
P = 0.05 for the difference between the amoxicillin–clavulanate group and the placebo group.
P = 0.008 for the difference between the amoxicillin–clavulanate group and the placebo group.
In the amoxicillin–clavulanate group, treatment was discontinued in one child because of vomiting.
In each group, treatment was discontinued in one child because of rash.
Discussion
In this clinical trial involving children 6 to 23 months of age with acute otitis media, children who were treated with amoxicillin–clavulanate, as compared with those who received placebo, had consistently more favorable short-term outcomes, including a sustained symptomatic response, an absence of otoscopic evidence of persistent middle-ear infection, and a reduced rate of residual middle-ear effusion. There were no suggestive or significant between-group differences in the use of analgesic drugs or health care resources. The interaction that we found between age and treatment is probably attributable to chance, since it involves small numbers of children and runs counter to other findings.14 In both study groups, the rates of clinical failure were greatest among children who were most severely affected initially. Nonetheless, the relative reduction in the risk of clinical failure at day 10 to 12 with amoxicillin–clavulanate treatment, as compared with placebo, was as large among the children who were least severely affected — those whose illnesses would have been considered non-severe as previously defined — as it was among children whose illnesses would have been considered severe. The principal side effects of treatment both with amoxicillin–clavulanate and with rescue antimicrobial agents were diarrhea and dermatitis in the diaper area, but the side effects were usually not severe enough to result in discontinuation of the offending drug. Treatment with amoxicillin–clavulanate was not associated with a detectable increase in nasopharyngeal colonization with nonsusceptible strains of S. pneumoniae, although the power to detect such emergent resistance was limited.
In keeping with recent recommendations,15 we chose the resolution of symptoms as the primary outcome of interest. Because we had previously noted that symptoms often recurred after having seemingly resolved, we defined the time of resolution in two ways: the time at which an AOM-SOS score of 0 or 1 was first recorded and the time of the second of two successive recordings of an AOM-SOS score of 0 or 1. We also measured the symptom burden over time, and we used a combination of symptomatic response and middle-ear findings to categorize overall outcomes as either clinical success or clinical failure. The differences in symptom scores between the two study groups were modest but consistent through the first 10 days of follow-up; the differences were observed mainly among the children with the most severe symptoms initially. In contrast, between-group differences in the overall clinical response, which included the symptomatic response and findings on otoscopic examination, were substantial and were observed not only among the children who had the most severe symptoms initially but also among the children who had the least severe symptoms. To our knowledge, a disparity of this nature has not been reported previously. These observations, together with the fact that among infants and young children, acute otitis media may be entirely asymptomatic16,17 and the fact that symptoms may not differentiate acute otitis media from other respiratory illnesses,18 suggest that overall clinical response constitutes the more telling measure of outcome. Regardless of the initial severity of symptoms, however, it is uncertain whether children who have become asymptomatic but have otoscopic findings that suggest persistent infection are thereby at increased risk for illness later. Also uncertain is the clinical significance of persistent middle-ear effusion in the apparent absence of infection. Because in young children otitis media with effusion is often a forerunner of acute otitis media,19 it is possible that the higher prevalence of persistent effusion among the children in the placebo group than among children in the amoxicillin–clavulanate group might have placed the children in the placebo group at greater risk for recurrent infection.
The differences in outcome in this trial between the children who were treated with amoxicillin–clavulanate and the children who received placebo were greater than the differences seen in most previous trials of antimicrobial agents — not because of better outcomes among the children treated with antimicrobial agents but because of higher rates of clinical failure among the children who received placebo. This finding, in turn, seems to be attributable to the stringent diagnostic criteria that we used to ensure that we would study only children in whom the diagnosis of acute otitis media was quite certain.20
In conclusion, among children 6 to 23 months of age with acute otitis media, treatment with amoxicillin–clavulanate for 10 days affords a measurable short-term benefit, irrespective of the apparent severity of the illness. The benefit must be weighed against concern not only about the side effects of the medication but also about the contribution of antimicrobial treatment to the emergence of bacterial resistance. These considerations underscore the need to restrict treatment to children whose illness is diagnosed with the use of stringent criteria.
Supplementary Material
Acknowledgments
Supported by a grant (3U01AI066007-02S1) from the National Institute of Allergy and Infectious Diseases.
Dr. Hoberman reports receiving honoraria and travel-expense reimbursement from GlaxoSmithKline, and Dr. Wald, grant support from Merck and GlaxoSmithKline.
We thank all the families who participated in this study; the many house officers at the Children's Hospital of Pittsburgh; the faculty and nurses at General Academic Pediatrics; Dr. Thomas G. Lynch, who facilitated onsite study-related activities at Armstrong Pediatrics in Kittanning, PA; the many Pittsburgh-area practitioners who referred children to the study; and, in particular, Dr. Michael D. Green, director of the Infectious Diseases Research Laboratory; Dr. Judith M. Martin, independent safety monitor; members of the data and safety monitoring board (Drs. Eugene D. Shapiro, Stephen I. Pelton, Margaret A. Kenna, Howard S. Faden, and George H. McCracken, Jr.); and Drs. Farukh M. Khambaty and Linda C. Lambert at the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases for administrative support.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Freid VM, Makuc DM, Rooks RN. Ambulatory health care visits by children: principal diagnosis and place of visit. Vital Health Stat. 1998;13:1–23. [PubMed] [Google Scholar]
- 2.Finkelstein JA, Metlay JP, Davis RL, Rifas-Shiman SL, Dowell SF, Platt R. Antimicrobial use in defined populations of infants and young children. Arch Pediatr Adolesc Med. 2000;154:395–400. doi: 10.1001/archpedi.154.4.395. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld RM, Vertrees JE, Carr J, et al. Clinical efficacy of antimicrobial drugs for acute otitis media: metaanalysis of 5400 children from thirty-three randomized trials. J Pediatr. 1994;124:355–67. doi: 10.1016/s0022-3476(94)70356-6. [DOI] [PubMed] [Google Scholar]
- 4.Appelman CL, van Balen FA, van de Lisdonk EH, van Weert HC, Eizenga WH. Otitis media acuta: NHG-standaard (eerste herziening) Huisarts Wet. 1999;42:362–6. In Dutch. [Google Scholar]
- 5.Scottish Intercollegiate Guidelines Network . Guideline no 66. Edinburgh: Royal College of Physicians in Edinburgh; 2003. Diagnosis and management of childhood otitis media in primary care. http://www.sign.ac.uk/guidelines/fulltext/66/index.html. [Google Scholar]
- 6.Kaleida PH, Casselbrant ML, Rockette HE, et al. Amoxicillin or myringotomy or both for acute otitis media: results of a randomized clinical trial. Pediatrics. 1991;87:466–74. [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451–65. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 8.Forgie S, Zhanel G, Robinson J. Management of acute otitis media. Paediatr Child Health (Oxford) 2009;14:457–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Paradise JL. Short-course antimicrobial treatment for acute otitis media: not best for infants and young children. JAMA. 1997;278:1640–2. [PubMed] [Google Scholar]
- 10.Hoberman A, Dagan R, Leibovitz E, et al. Large dosage amoxicillin/clavulanate, compared with azithromycin, for the treatment of bacterial acute otitis media in children. Pediatr Infect Dis J. 2005;24:525–32. doi: 10.1097/01.inf.0000164794.50281.1a. [DOI] [PubMed] [Google Scholar]
- 11.Shaikh N, Hoberman A, Paradise JL, et al. Development and preliminary evaluation of a parent-reported outcome instrument for clinical trials in acute otitis media. Pediatr Infect Dis J. 2009;28:5–8. doi: 10.1097/INF.0b013e318185a387. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh N, Hoberman A, Paradise JL, et al. Responsiveness and construct validity of a symptom scale for acute otitis media. Pediatr Infect Dis J. 2009;28:9–12. doi: 10.1097/INF.0b013e318185a3a0. [DOI] [PubMed] [Google Scholar]
- 13.Kaleida PH, Stool SE. Assessment of otoscopists' accuracy regarding middle-ear effusion: otoscopic validation. Am J Dis Child. 1992;146:433–5. doi: 10.1001/archpedi.1992.02160160053013. [DOI] [PubMed] [Google Scholar]
- 14.Hoberman A, Paradise JL, Burch DJ, et al. Equivalent efficacy and reduced occurrence of diarrhea from a new formulation of amoxicillin/clavulanate potassium (Augmentin) for treatment of acute otitis media in children. Pediatr Infect Dis J. 1997;16:463–70. doi: 10.1097/00006454-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Rockville, MD: Food and Drug Administration, Center for Drug Evaluation and Research; 2008. Guidance for industry — acute bacterial otitis media: developing drugs for treatment. Revision 1. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070947.pdf. [Google Scholar]
- 16.Hayden GF, Schwartz RH. Characteristics of earache among children with acute otitis media. Am J Dis Child. 1985;139:721–3. doi: 10.1001/archpedi.1985.02140090083037. [DOI] [PubMed] [Google Scholar]
- 17.Niemela M, Uhari M, Jounio-Ervasti K, Luotonen J, Alho OP, Vierimaa E. Lack of specific symptomatology in children with acute otitis media. Pediatr Infect Dis J. 1994;13:765–8. doi: 10.1097/00006454-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Laine MK, Tähtinen PA, Ruuskanen O, Huovinen P, Ruohola A. Symptoms or symptom-based scores cannot predict acute otitis media at otitis-prone age. Pediatrics. 2010;125(5):e1154–e1161. doi: 10.1542/peds.2009-2689. [DOI] [PubMed] [Google Scholar]
- 19.Paradise JL, Bernard BS, Colborn DK, Smith CG, Rockette HE. Otitis media with effusion (OME): highly prevalent and often the forerunner of acute otitis media (AOM) during the first year of life. Pediatr Res. 1993;33:121A. abstract. [Google Scholar]
- 20.Paradise JL. On classifying otitis media as suppurative or nonsuppurative, with a suggested clinical schema. J Pediatr. 1987;111:948–51. doi: 10.1016/s0022-3476(87)80226-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.