Abstract
Cell-cell communication in bacteria, called quorum sensing, relies on production, release, and detection of signaling molecules, termed autoinducers. Communication enables populations of cells to synchronize gene expression and therefore behave as a group in a manner akin to cells in multicellular organisms. Most quorum-sensing systems allow communication within an individual species of bacteria. However, one autoinducer, called AI-2, is produced and recognized by many different bacterial species, indicating that some bacteria communicate across species boundaries. Current studies are aimed at discovering the role that AI-2 plays in gene regulation. Differential gene expression in response to AI-2 may cause bacterial behavioral changes, such as biofilm formation or transition to a pathogenic state. Interestingly, multiple mechanisms to detect AI-2 exist. These differences likely reflect variations in the role that AI-2 plays for different bacteria. Additionally, structural analyses of the AI-2 receptor in V. harveyi have provided insight into bacterial trans-membrane signal transduction. A further understanding of bacterial quorum-sensing processes may facilitate development of new technologies aimed at interfering with bacterial communication and virulence.
Bacteria, long thought to exist as asocial entities, are increasingly acknowledged to use communication mechanisms to coordinate behaviors within a population. Cell-to-cell communication in bacteria, also known as quorum sensing, relies on the ability of bacteria to produce, secrete, and detect small molecules, termed autoinducers, in their surrounding environment. Scores of examples have been characterized in which a particular bacterial species produces a specific autoinducer molecule that is recognized only by that species. In addition, a newly discovered signaling molecule, called AI-2, is produced by many species of bacteria and is capable of eliciting responses in different species of bacteria.
Quorum sensing allows control of behaviors on a community-wide level. Bacterial cells detect the buildup of secreted autoinducers and respond with programmed changes in gene expression. Bacterial activities regulated by quorum sensing include biofilm development, virulence factor regulation, bioluminescence induction, antibiotic production, sporulation, and competence initiation. The understanding that bacteria produce and respond to extracellular signals makes it possible to develop technologies aimed at obstructing bacterial communication systems. By interfering with these systems, it may be possible to alter the course of a bacterium’s ability to harm humans, animals, and industrial processes. The discovery that a single type of signaling molecule, AI-2, contributes to the control of behaviors in diverse types of bacteria raises the possibility that a single drug or class of inhibitors may be able to target communication in many bacterial species.
Intra- and Interspecies Quorum Sensing
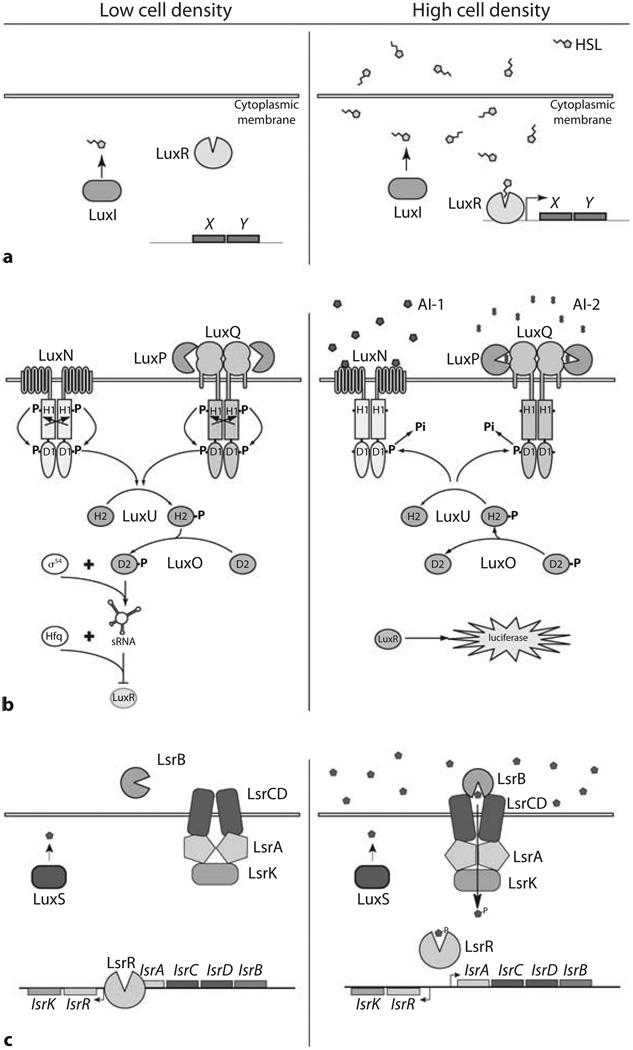
The ability of bacteria to produce and release signals to coordinate gene expression in a population was discovered in experimentally tractable bacteria whose quorum-sensing-dependent behaviors were easily quantifiable. The first bacterial cell-cell communication system studied at the molecular level was that of Vibrio fischeri that produces density-dependent bioluminescence [1]. The V. fischeri quorum-sensing system has become the paradigm for Gram-negative bacterial communication networks (fig. 1a). Typically, V. fischeri and other Gram-negative quorum-sensing bacteria have a LuxI enzyme that produces an acylated homoserine lactone (AHL) autoinducer by reacting S-adenosyl methionine (SAM) with an acylated acyl-carrier protein [2]. Each species uses a unique LuxI enzyme to generate an AHL autoinducer with a particular acyl side-chain making these signals species-specific. The AHL molecules diffuse across the membrane into the surrounding environment. Detection of AHL autoinducers is accomplished by LuxR-type proteins which have the dual ability of binding AHLs and DNA. At high cell densities, i.e., at high autoinducer concentrations, LuxR binds to a cognate AHL and undergoes a conformational change allowing the LuxR-AHL complex to bind DNA and control transcription of target genes. In V. fischeri, one of the targets for LuxR regulation is the set of genes encoding luciferase, and thus, at high autoinducer concentrations, LuxR promotes the expression of bioluminescence. Many other Gram-negative bacterial species use analogous LuxI/LuxR-type circuits, but their target genes are variable. For example, Pseudomonas aeruginosa controls biofilm development and secretion of enzymes contributing to its pathogenesis, Agrobacterium tumefaciens and Rhizobium leguminosarum regulate transfer of plasmid DNA between cells, and Serratia marcescens controls sliding motility. Each of these organisms uses a unique LuxI/R pathway or set of LuxI/R pathways, each utilizing different AHLs to regulate the above and other behaviors.
Fig. 1.
Quorum-sensing signaling networks. Low cell density (left sides of panels), High cell density (right sides of panels). a A LuxI/LuxR quorum-sensing circuit. The LuxI enzyme produces an AHL molecule that diffuses into the surroundings. At high cell densities, the LuxR protein binds the AHL and subsequently binds to DNA promoting the transcription of genes X and Y. b V. harveyi AI-1- and AI-2-dependent circuits. At low cell density, LuxN and LuxQ exist in kinase mode autophosphorylating at histidine (H1) and aspartic acid (D1) residues. Phosphate is passed to LuxU at the histidine (H2) site, and then to LuxO at an aspartic acid (D2). LuxO-P, with sigma factor σ54, activates transcription of the sRNA genes. The sRNAs, with the RNA chaperone Hfq repress luxR expression. At high cell densities, AI-1 binds LuxN and AI-2 binds LuxP-LuxQ. Binding of autoinducers causes LuxN and LuxQ to switch to phosphatase mode. Phosphate is drained from LuxU and LuxO, terminating expression of the sRNA genes. LuxR is derepressed, and luciferase is expressed. c The E. coli and S. typhimurium Lsr system. At low cell densities, LsrR binds to DNA and represses expression of the lsr operon. At high cell densities, AI-2 is imported via the Lsr transporter and is phosphorylated by LsrK. AI-2-phosphate binds to LsrR causing it to release DNA, thus derepressing lsr transcription [adapted from 8, 28].
In Vibrio harveyi, a bioluminescent bacterial species related to V. fischeri, the quorum-sensing circuitry is more complex (fig. 1b). V. harveyi relies on multiple autoinducers to regulate light production. The predominant autoinducer contributing to luciferase production is an AHL-denoted HAI-1 (V. harveyi autoinducer-1). HAI-1 is N-(3- hydroxybutanoyl)homoserine lactone and it is synthesized by LuxM. The HAI-1 receptor, LuxN, is a membrane-spanning histidine sensor kinase protein belonging to the well-conserved family of bacterial two-component signal transduction proteins.
Two-component histidine sensor kinases autophosphorylate by transferring phosphate from ATP to a conserved histidine residue located on the kinase. A phosphorylation cascade ensues, where phosphate passes from histidine (H1) to aspartic acid (D1), and phosphate can continue to a second histidine (H2) and second aspartic acid (D2), located on proteins connected to, or separate from, the kinase. Phosphorylation of the terminal aspartic acid generally causes activation of a DNA-binding response regulator protein. In the absence of HAI-1, LuxN autophosphorylates on the conserved H1 residue, which is then passed on to D1 located within the C-terminal domain of LuxN [3]. Phosphate is sequentially passed to H2 on LuxU, a phosphotransfer protein, and then on to D2 located on LuxO, the DNA-binding response regulator [4]. Phosphorylation of LuxO promotes the expression of genes encoding five small regulatory RNAs (sRNAs) [5]. These five sRNAs, together with the sRNA chaperone Hfq, bind to and block translation of the luxR mRNA. (LuxR of V. harveyi is not a member of the canonical V. fischeri LuxI/LuxR family of proteins; it does not bind HSLs.) The V. harveyi’s LuxR protein directly activates transcription of luciferase and regulates nearly 100 other genes [6]. When V. harveyi reaches high cell density, HAI-1 concentrations are also high. When bound to autoinducer, the enzymatic activity of LuxN switches from kinase to phosphatase, and LuxN drains phosphate from LuxU and LuxO. Dephosphorylated LuxO is unable to activate transcription of sRNA genes, and consequently, LuxR expression is derepressed, and luciferase is expressed, and light is produced.
Interestingly, in the absence of the HAI-1 system, V. harveyi continues to control luciferase production as a function of cell population density, albeit at 1,000 times lower light production per cell. V. harveyi mutants were screened for those unable to regulate light production in a background lacking the HAI-1 system, and a new autoinducer detection system was discovered [7]. Two genes identified in the screen encode components of the autoinducer receptor. The first gene, luxP, encodes a periplasmic binding protein LuxP, similar to the Escherichia coli ribose-binding protein. The second gene, named luxQ, encodes a two-component histidine kinase similar to LuxN. A third gene, luxS, encodes the autoinducer synthase, LuxS, which produces a signal which was named, for lack of a specific chemical nature, AI-2.
V. harveyi integrates AI-2 information directly into the quorum-sensing pathway described above. LuxP and LuxQ work together as the AI-2 receptor complex (see structural details below). At low cell densities, when AI-2 concentrations are low, LuxQ acts as a kinase in a manner analogous to LuxN. Phosphate follows the typical H1 to D1 transfer on LuxQ itself, and is then passed on to H2 of LuxU and D2 of LuxO. When both HAI-1 and AI-2 concentrations are low, the kinase activities of both LuxN and LuxQ maximize LuxO-phosphate levels in the cell, generating high levels of the sRNAs. Consequently, little LuxR protein is made, and the cells do not produce light. The phosphorylation pathway reverses under high autoinducer concentrations. AI-2 binds to LuxP, switching LuxQ from kinase to phosphatase, lowering levels of LuxU-P and LuxO-P. Ultimately, LuxR is derepressed and light is produced.
The luxS gene exists in over half of all sequenced bacterial genomes. Importantly, species containing luxS genes generate and secrete AI-2 activity into their surroundings. One question that has emerged is whether AI-2 is an important communication signal in other bacteria, and if so, what do these bacteria control with AI-2 information? For E. coli and Salmonella typhimurium, AI-2 plays a regulatory role. In these bacteria, a set of genes called lsr (luxs regulated) are induced that encode components of the machinery used for the import and processing of AI-2 [8, 9]. This transporter is so effective that levels of AI-2 are diminished to near background levels outside the cells.
The AI-2 receptor in the Lsr system, LsrB, is a periplasmic binding protein similar to V. harveyi’s AI-2 receptor LuxP. While the overall fold of this family of proteins is highly conserved (the prototype being the ribose binding protein, RbsB), primary sequence similarity between LsrB, LuxP and RbsB is low. RbsB’s similarity to LuxP and LsrB is 47 and 41%, respectively. However, LuxP is only 11% similar to LsrB. Low sequence similarity reflects the fact that each protein interacts with different types of downstream components. Instead of interacting with a histidine sensor kinase protein, as is the case for LuxP, the LsrB protein delivers AI-2 to an ABC transporter that imports AI-2 into the cytoplasm (fig. 1c). Transport of AI-2 is coupled to its phosphorylation and sequestration, which is dependent on LsrK kinase activity [9]. Phosphorylated AI-2 subsequently binds and deactivates the transcriptional repressor LsrR. Transcription increases of the genes encoding the Lsr transporter and modification enzymes used to import, modify, and degrade AI-2. This positive feedback is required for rapid induction of the Lsr system and removal of AI-2 from the environment.
To date, several studies have identified additional genes differentially regulated in response to AI-2 or LsrR/LsrK in both E. coli and S. typhimurium strains, including pathogenic species. However, the ability of the Lsr transporter to rapidly reduce AI-2 levels in the surrounding environment has led to the hypothesis that E. coli and S. typhimurium not only rely on AI-2 to identify their ‘quorum’, but also to diminish AI-2 levels to ‘confuse’ other species occupying the same environment. As proof of this principle, E. coli and V. harveyi were grown together and the effects of this co-culture on V. harveyi luciferase production was monitored. In the mixed species set up, as V. harveyi population numbers increased, luciferase production was induced. However, as AI-2 concentrations climbed, E. coli, in turn, induced its Lsr transporter, and AI-2 levels in the co-culture dropped dramatically. The rate of light production from V. harveyi declined, and the cells’ gene expression pattern was maintained at the level appropriate for cell densities 10- to 100-fold below their actual population number [9]. Few bacterial species exist in pure culture in nature, and it is likely that communication networks are subject to quorum-sensing eavesdropping and interference, as the Lsr studies imply.
AI-2 Identification
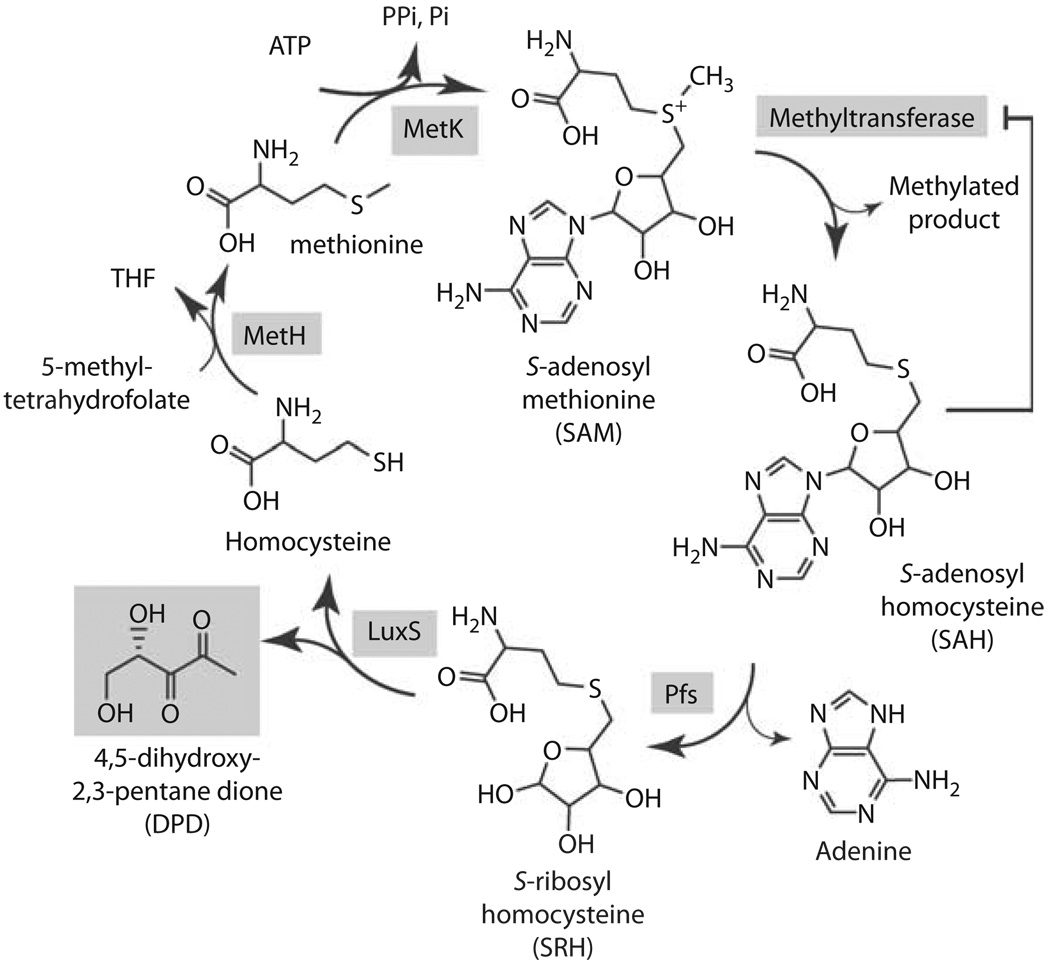
The LuxS protein does not to share sequence homology to LuxI-type autoinducer syntheses, and common extraction techniques used to purify AHLs from bacterial cultures proved unsuccessful for isolating AI-2 activity, suggesting that it was a novel chemical. Clues to LuxS’s function and to AI-2’s chemical nature came from the notion that LuxS may be involved in a pathway with other genes lying within close proximity on the chromosome. luxS was often found near genes involved in methionine metabolism and the reactive methyl cycle. In Borellia burgdorferi, for example, luxS is preceded in an operon by the genes metK and pfs. MetK catalyzes the production of SAM by combining adenosine with methionine (fig. 2). SAM is an essential coenzyme whose function is to provide methyl groups to numerous substrates, for example, in the biosynthesis of DNA, RNA, fatty acids, and proteins. SAM-dependent transmethylation reactions result in the release of S-adenosyl homocysteine (SAH) as a byproduct. SAH inhibits transmethylation reactions and is therefore toxic. The second gene found near luxS in B. burgdorferi was pfs. The Pfs enzyme detoxifies SAH by removing adenine and generating S-ribosyl homocysteine (SRH). Cells break down SRH into homocysteine and 4,5-dihydroxy-2,3-pentanedione (DPD); however, no enzyme had ever been identified to catalyze this reaction. Indeed, as predicted, LuxS catalyzes this final reaction. Thus, bacteria that use Pfs and LuxS to recycle SAM pools generate DPD as an additional byproduct, and DPD is responsible for AI-2 activity [10].
Fig. 2.
Reactive methyl cycle and production of DPD. SAM-dependent methyltransferases convert SAM to SAH, accumulation of which confers product-feedback inhibition on methyltransferase reactions. SAH is detoxified to SRH by Pfs. SRH is converted to homocysteine and DPD by LuxS. Homocysteine can be recycled to SAM via MetH, which generates methionine, and MetK.
DPD and AI-2 Activity
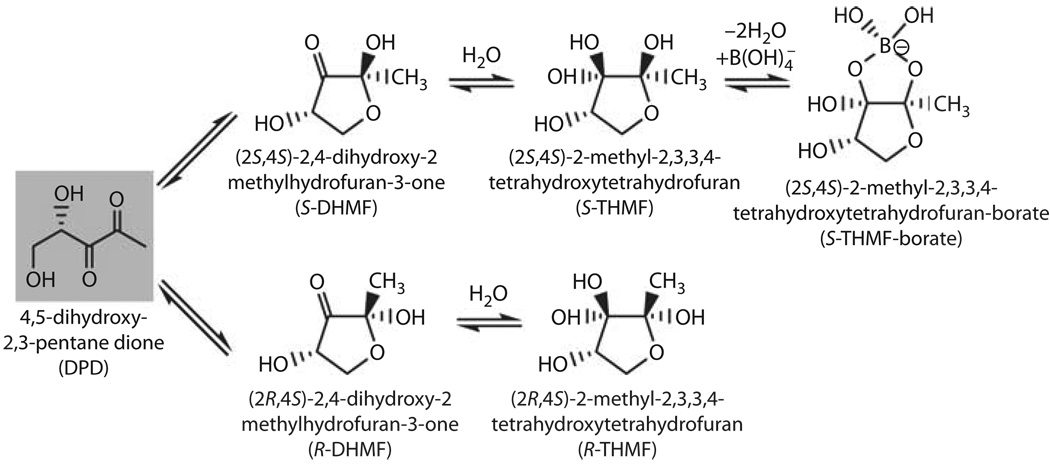
Use of DPD as an intercellular signaling molecule is interesting from the standpoint that this molecule is inherently unstable. DPD spontaneously cyclizes to form two enantiomers, 2S,4S-DHMF and 2R,4S-DHMF (fig. 3) [11]. Hydration of the ketone in aqueous solutions leads to formation of S- and R-THMF. The multiple derivatives of DPD raised the question: which of these isomers is recognized as AI-2 by bacteria? Because conversion between these molecules is spontaneous and fast, isolation or synthesis of each compound for biological activity testing was impossible. Thus, determining which forms of DPD are active came from biochemical and structural studies of various DPD receptors. Crystals of V. harveyi’s LuxP protein containing the AI-2 ligand within the binding pocket showed that a borated adduct of the S-THMF molecule is the V. harveyi signal [12]. Consistent with this, Vibrio species are found in marine environments where borate is plentiful. In contrast, levels of borate are low in terrestrial environments, yet bacteria such as E. coli and S. typhimurium also produce and respond to AI-2. These findings support the idea that borated DPD may not be the only active form of the molecule. Indeed, crystal structures of LsrB, the AI-2 binding protein from S. typhimurium contained the non-borated R-THMF ligand [11].
Fig. 3.
Interconverting forms of DPD. Cyclization of DPD generates two major stereoisomers S-DHMF and R-DHMF. Hydration in aqueous solution forms S-THMF and R-THMF. S-THMF is able to complex with borate, forming S-THMF-borate [adapted from 11].
Knowing the two AI-2 structures provided a mechanism by which interspecies communication occurs. The LuxS enzyme, regardless of bacterial species origin, is responsible for the production of the linear DPD molecule, which through spontaneous rearrangements, produces a pool of interconverting compounds. A molecule recognized by one species can rearrange to become the signal recognized by another species. It remains to be seen whether forms of DPD other than the two now known are recognized as AI-2 signals, or whether other types of adducts can be made from DPD to generate new, unanticipated AI-2 molecules.
Environmental conditions affect the equilibrium of AI-2 molecules, and may contribute to a bacterium’s ability to monitor its surrounding environment. For instance, V. cholerae, a bacterium that contains luxS and responds to AI-2, has a life cycle that alternates between marine environments and the human intestine. As discussed above, the predominant form of AI-2 in the ocean is S-THMF-borate. However, in the human gastrointestinal tract, where only trace amounts of borate are available, AI-2 likely exists only in non-borated forms. It is possible that V. cholerae distinguishes these niches by measuring the availability of borated AI-2. Therefore, in addition to population density, information encoded within AI-2 can also include the status of the surrounding environment. It is also possible that bacteria detect multiple forms of AI-2 using different protein receptors. If so, this would allow direct monitoring of different AI-2 forms and the proportions of each.
Discriminating between AI-2 Signaling Effects and LuxS Metabolic Roles
A large effort has been underway to test the roles that LuxS and AI-2 play in controlling gene regulation. luxS null mutations have been generated in different bacterial species, and subsequent studies have ranged in scope from measuring changes in expression of a specific gene of interest in some species, to whole genome transcription profiles in others. For instance, specific virulence factors have been monitored in luxS mutants of Clostridium perfringens, Shigella flexneri, Actinobacillus actinomycetemcomitans and Streptococcus pyogenes [13–16]. Likewise, whole genome expression profiles have been compared between wild-type strains and luxS mutant for many species, including E. coli W113 and E. coli O157:H7, Neisseria meningitidis,Streptococcus pneumoniae, and Streptococcus mutans [17–21]. Typically, large numbers of genes are differentially regulated in luxS mutants (ranging from <1 to 10% of the genome) with many of these genes being unresponsive to exogenously added AI-2. The inability to complement luxS mutants with artificially added autoinducer raises the question of whether luxS mutations affect gene regulation in an AI-2-dependent manner, or whether the ramification of luxS mutations stem from alterations in SAM, SAH, or SRH levels. Several possibilities in addition to disruption of the reactive methyl cycle may also explain the lack of ability to complement luxS phenotypes, including the appropriate timing of signal presentation, the percent of the population responding to the signal, the concentration of the signal, if the signal requires chemical modification, or if feedback regulation affects the ability of cells to respond to AI-2.
In some quorum-sensing systems, a temporary window of time exists during which cells are able to respond to extracellular signals. Studies of competence development, a quorum-sensing-controlled process in S. pneumoniae, have shown that only 15–30 min exist when cells develop and maintain maximum competence [22]. After this interval, the response to the competence-stimulating autoinducer rapidly decreases. Shut-down of competence is now partially understood to be controlled by degradation of transcription factors responsible for induction of the competent state [23]. This stands as a clear example that programs triggered by autoinducers can be heavily regulated processes, and precise temporal conditions must be met to generate full responses. In the case of AI-2, cells experiencing only the correct concentrations of autoinducer may also be critical for proper responses. Recent studies of biofilm formation within mixed bacterial cultures of Streptococcus oralis and Actinomyces naeslundii found that optimal AI-2 concentrations exist for biofilm formation, and above and below this concentration, biofilm development is decreased [24].
Effects of added autoinducers on a bacterial culture also may be difficult to observe when only a subpopulation of cells is responsive to the extracellular signal. In Bacillus subtilis, competence develops in only 10% of the population in response to the competence-inducing autoinducer ComX [25]. Without an easily tractable phenotype, like uptake of DNA, effects on gene expression via microarray or proteomic analysis, or other methods that measure the mean change of expression of target genes in the population, may be nearly impossible to observe.
When using AI-2 to artificially stimulate cells, another difficulty to be considered is that some species of bacteria in which luxS has been deleted may be locked in a state that is unresponsive to exogenously added autoinducer. In E. coli and S. typhimurium, as mentioned, AI-2 is imported into the cell by the Lsr transporter. Transport occurs quickly due to upregulation of the Lsr apparatus, and AI-2 is completely removed from the surrounding environment in <2 h. In luxS mutants, exogenously supplied AI-2 requires approximately 60 min longer than in the wild type for proper induction of the Lsr system [8]. The response is possible only because a secondary, promiscuous transporter is capable of importing some AI-2, which can then kick-start the system. For species with other types of positive feedback regulation, it may be the case that deletion of luxS abolishes the basal level of AI-2 required to ensure that detection systems are primed for induction. Without this, cells are unable to respond to exogenously added AI-2.
Finally, phenotypes stemming from luxS mutations may be due to a combination of effects on the active methyl cycle as well as a loss of the signaling molecule AI-2. Genomic, proteomic, and metabalomic studies may provide initial clues for discriminating between gene and protein level changes in response to AI-2 concentrations versus metabolic disturbances. A recent study in S. mutans that measured changes in gene expression using microarray analysis compared a wild-type strain to a luxS mutant and to a luxS mutant supplemented with chemically synthesized DPD. Over 500 genes (30% of genome) showed changes in gene expression in the luxS mutant, but only 59 of these genes were complemented by exogenously supplied DPD [21] .These results indicate that the large majority of genes whose expression changed as a result of the luxS mutation were not responsive to DPD under conditions tested. But the finding that a substantial number of genes are controlled by AI-2 provides footing for future studies to optimize conditions that may give insight to how S. mutans detects AI-2.
Further complications due to signal redundancy also complicate these analyses. In fact, the species in which AI-2 was discovered, V. harveyi, does not have an obvious luxS phenotype. A V. harveyi luxS mutant decreases light production about 100-fold when grown to high cell density as compared to wild type [26]. However, a 100-fold loss in light output accounts for less than 1% of the overall change in light production observed when AI-1 and AI-2 autoinducers are removed simultaneously.
A Structural Study of the AI-2 Receptor of Vibrio harveyi
One long-term goal of quorum-sensing studies is to identify compounds that can interfere with bacterial communication. To fully understand how small molecule antagonists interfere with cell-cell communication systems, the endogenous signaling molecules and the mechanisms by which information is relayed into the cell must be defined. The identification of the AI-2 signal, along with the identification of the proteins used to convert AI-2 information into target gene expression, makes such studies feasible. Of particular interest is to understand how AI-2 ligand binding in the periplasm leads to changes in enzymatic activity of the LuxQ sensor kinase in the cytoplasm.
Genetic studies had indicated that LuxP controls LuxQ’s ability to switch from the kinase state (at low AI-2 concentrations) to the phosphatase state (at high AI-2 concentrations), because deletions of luxP cause LuxQ to remain locked in the kinase state [27]. To determine how conformational changes in LuxP control LuxQ enzymatic activity, combined structural and genetic studies were performed. V. harveyi LuxP and the periplasmic domain of LuxQ (LuxQp) were expressed in E. coli in the presence and absence of AI-2, and the protein complexes were purified and crystallized. LuxP and LuxQp have two sites of interaction, and each is required for proper switching between kinase and phosphatase activities.
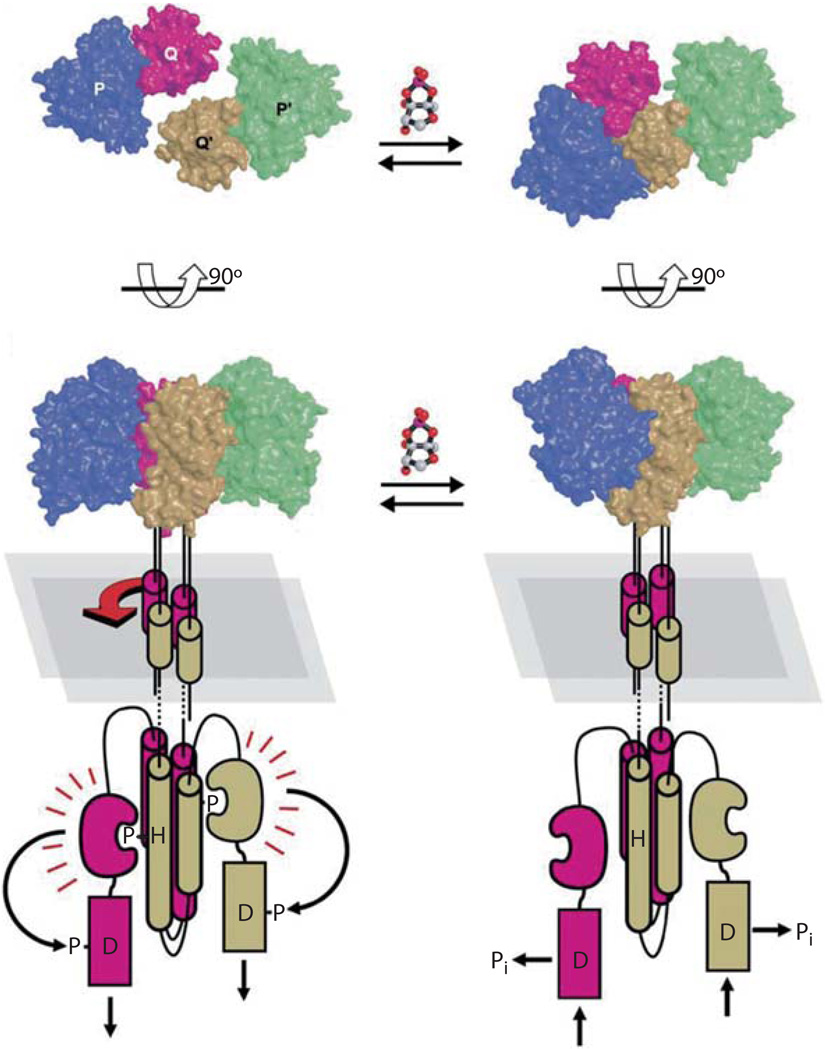
In protein complexes lacking AI-2, LuxP is in an open conformation and LuxP interacts with LuxQp in a 1:1 stoichiometry forming a complex (the LuxPQp monomeric subunit) [27]. Contacts between LuxP and LuxQp occur at a location distal to the membrane, and this interaction is necessary for LuxQ kinase activity because deleting this region causes LuxQ to favor the phosphatase state in an AI-2-independent manner. The structure of the holo-LuxP-LuxQp protein complex displayed two major differences from the apo-complex structure [28]. First, a second site of interaction between LuxP and LuxQ was evident. While the first site of interaction is identical to that observed in the apo-complex, the second site of interaction occurs because AI-2 binding induces a major conformational change in LuxP, and allows new contacts to form between LuxP and a second LuxQpsubunit (LuxQp′). The second major structural difference in the holo-complex is the generation of an asymmetric dimer, consisting of two LuxPQp monomers (designated LuxPQp-LuxP′Qp′). When LuxP simultaneously contacts LuxQp and LuxQp′, the complex is held together in an orientation that is asymmetric along the axis that is normal to the cytoplasmic membrane.
Interestingly, although a large conformational change occurs in each LuxP subunit upon binding AI-2, virtually no conformational change is observed in either LuxQp subunit. It appears that signal propagation does not occur through intramolecular conformational changes that are passed across the membrane into the cytoplasmic domains of the protein. Rather, in the case of LuxQ, the relative orientation in which the periplasmic LuxQ subunits are arranged around the axis of symmetry determines the enzymatic state of the receptor.
The current model suggests that when AI-2 concentrations are low, LuxP is in an open conformation and only one contact is made with its paired LuxQ subunit. The orientation of LuxQ-LuxQ′ dimers is determined by membrane-spanning and cytoplasmic domains of the protein and the dimer is situated in a symmetric orientation (fig. 4, left). Upon binding AI-2, LuxP undergoes large movements to close around the ligand. This movement reorients LuxP, making it possible to contact the LuxQp′ subunit. This contact drives rotation between the two LuxQ subunits into an asymmetric position (fig. 4, right). This asymmetric orientation must signal to the cytoplasmic domains to switch kinase activity off. The phosphatase activity of LuxQ is located in the C-terminal aspartic acid (D1)-containing domain, and is not dependent on autoinducer concentration. Therefore, the activity of LuxQ’s kinase domain determines whether phosphate flows toward LuxU and LuxO or away from them. When the kinase is active, it overrides the phosphatase activity; when the kinase is inactivated, the phosphatase activity prevails, and phosphate is drained away from LuxU and LuxO.
Fig. 4.
Model for AI-2 dependent LuxPQ receptor activity. The top panels display the LuxPQp receptor complex from a ‘top-down’ view, and the lower panels are a 90° rotation, showing the complex from the side. In the absence of AI-2 (left) LuxQ-LuxQ9 dimers exist in a symmetric orientation both in the periplasm and the cytoplasm, and therefore are in kinase mode. AI-2 binding to LuxP (right) causes a conformational change in LuxP, and induces interactions with LuxQ9. Simultaneous contacts of LuxP with LuxQ and LuxQ9 rotates the complex into an asymmetric orientation. Rotation of the periplasmic domains is conferred to the cytoplasmic domains, switching LuxQ from kinase to phosphatase mode [figure 4 reproduced with permission, 28].
The AI-2-LuxP-LuxQp receptor complex is one of only a few bacterial signal transduction receptor complexes with structures that have been determined with and without bound ligand. Another such receptor system in which structural data are available is the chemotaxis receptor. Chemotaxis systems are used by bacteria to detect and subsequently move towards attractant and away from repellant molecules. Chemotaxis receptors measure changes in concentration of the attractants or repellants and relay this information to a signaling network which controls the bacterium’s swimming direction. These receptors cluster to form arrays of protein complexes in the membrane. Clustering of receptors is proposed to provide a mechanism to detect small fluctuations in attractant/repellant concentrations and then respond with large changes in swimming behavior. Unlike the chemotaxis receptors, the LuxP-LuxQ quorum-sensing receptor does not appear to form higher-order clusters [28]. If clustering of receptors provides added sensitivity to small changes in chemotaxis attractants, perhaps arranging quorum-sensing receptors as independent detectors protects the cell from responding to minor fluctuations in autoinducers. This arrangement likely allows cells to measure the slow build-up of autoinducers and at a critical concentration, commit to new behaviors that require a change in global transcription.
Future Questions and Goals
As technologies continue to improve our ability to monitor gene, protein, and metabolite levels in living cells, and as we find new ways to identify small molecules produced by bacteria, discovery of new quorum-sensing systems will likely accelerate. To fulfill the promise that an understanding of quorum sensing will allow us to manipulate bacterial behaviors by interfering with communication systems, autoinducer analogs and signaling inhibitors will need to be synthesized, or natural ones discovered and tested in vivo. Large chemical libraries are becoming available, and experimental screening for quorum-sensing antagonists is a promising route. Likewise, rational design of compounds based on known autoinducers, and screens for compounds occurring naturally in the environment, could be alternative resources of inhibitors. If quorum-sensing systems are susceptible to chemical inhibition, development of novel antimicrobial agents could ensue. With respect to AI-2-based quorum-sensing systems, LuxS appears to be a promising target for directed inhibitors because the LuxS crystal structure has been solved, all LuxS proteins synthesize the same moiety, DPD, and its enzymatic mechanism is defined. By interfering with production of the AI-2 signaling molecule, communication could be terminated in a diverse array of species
References
- 1.Nealson KH, Platt T, Hastings JW. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970;104:313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 3.Freeman JA, Lilley BN, Bassler BL. A genetic analysis of the functions of LuxN: a two-component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol Microbiol. 2000;35:139–149. doi: 10.1046/j.1365-2958.2000.01684.x. [DOI] [PubMed] [Google Scholar]
- 4.Freeman JA, Bassler BL. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol. 1999;31:665–677. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- 5.Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Waters CM, Bassler BL. The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers. Genes Dev. 2006;20:2754–2767. doi: 10.1101/gad.1466506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassler BL, Wright M, Silverman MR. Multiple signaling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13:273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 8.Taga ME, Semmelhack JL, Bassler BL. The LuxS-dependent autoinducer AI-2 controls the expression of an ABC transporter that functions in AI-2 uptake in Salmonella typhimurium. Mol Microbiol. 2001;42:777–793. doi: 10.1046/j.1365-2958.2001.02669.x. [DOI] [PubMed] [Google Scholar]
- 9.Xavier KB, Bassler BL. Interference with AI-2-mediated bacterial cell-cell communication. Nature. 2005;437:750–753. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schauder S, Shokat K, Surette MG, Bassler BL. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 11.Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol Cell. 2004;15:677–687. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 13.Day WA, Jr, Maurelli AT. Shigella flexneri LuxS quorum-sensing system modulates virB expression but is not essential for virulence. Infect Immun. 2001;69:15–23. doi: 10.1128/IAI.69.1.15-23.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong KP, Chung WO, Lamont RJ, Demuth DR. Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS. Infect Immun. 2001;69:7625–7634. doi: 10.1128/IAI.69.12.7625-7634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyon WR, Madden JC, Levin JC, Stein JL, Caparon MG. Mutation of luxS affects growth and virulence factor expression in Streptococcus pyogenes. Mol Microbiol. 2001;42:145–157. doi: 10.1046/j.1365-2958.2001.02616.x. [DOI] [PubMed] [Google Scholar]
- 16.Ohtani K, Hayashi H, Shimizu T. The luxS gene is involved in cell-cell signalling for toxin production in Clostridium perfringens. Mol Microbiol. 2002;44:171–179. doi: 10.1046/j.1365-2958.2002.02863.x. [DOI] [PubMed] [Google Scholar]
- 17.DeLisa MP, Wu CF, Wang L, Valdes JJ, Bentley WE. DNA microarray-based identification of genes controlled by autoinducer 2-stimulated quorum sensing in Escherichia coli. J Bacteriol. 2001;183:5239–5247. doi: 10.1128/JB.183.18.5239-5247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dove JE, Yasukawa K, Tinsley CR, Nassif X. Production of the signaling molecule, autoinducer-2, by Neisseria meningitidis: lack of evidence for a concerted transcriptional response. Microbiology. 2003;149:1859–1869. doi: 10.1099/mic.0.26185-0. [DOI] [PubMed] [Google Scholar]
- 19.Joyce EA, Kawale A, Censini S, Kim CC, Covacci A, Falkow S. LuxS is required for persistent pneumococcal carriage and expression of virulence and biosynthesis genes. Infect Immun. 2004;72:2964–2975. doi: 10.1128/IAI.72.5.2964-2975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperandio V, Torres AG, Giron JA, Kaper JB. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J Bacteriol. 2001;183:5187–5197. doi: 10.1128/JB.183.17.5187-5197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sztajer H, Lemme A, Vilchez R, Schulz S, Geffers R, Yip CY, Levesque CM, Cvitkovitch DG, Wagner-Dobler I. Autoinducer-2-regulated genes in Streptococcus mutans UA159 and global metabolic effect of the luxS mutation. J Bacteriol. 2008;190:401–415. doi: 10.1128/JB.01086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotchkiss RD. Cyclical behavior in pneumococcal growth and transformability occasioned by environmental changes. Proc Natl Acad Sci USA. 1954;40:49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo P, Morrison DA. Transient association of an alternative sigma factor, ComX, with RNA polymerase during the period of competence for genetic transformation in Streptococcus pneumoniae. J Bacteriol. 2003;185:349–358. doi: 10.1128/JB.185.1.349-358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rickard AH, Palmer RJ, Jr, Blehert DS, Campagna SR, Semmelhack MF, Egland PG, Bassler BL, Kolenbrander PE. Autoinducer-2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Mol Microbiol. 2006;60:1446–1456. doi: 10.1111/j.1365-2958.2006.05202.x. [DOI] [PubMed] [Google Scholar]
- 25.Magnuson R, Solomon J, Grossman AD. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell. 1994;77:207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 26.Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signaling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 27.Neiditch MB, Federle MJ, Miller ST, Bassler BL, Hughson FM. Regulation of LuxPQ receptor activity by the quorum-sensing signal autoinducer-2. Mol Cell. 2005;18:507–518. doi: 10.1016/j.molcel.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Neiditch MB, Federle MJ, Pompeani AJ, Kelly RC, Swem DL, Jeffrey PD, Bassler BL, Hughson FM. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126:1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]