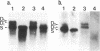Abstract
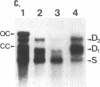
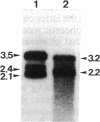
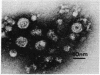
A human hepatocellular carcinoma cell line (Huh6-c15) was transfected with a recombinant DNA molecule that consists of tandemly arranged hepatitis B virus (HBV) genome and a neomycin-resistant gene. One clone resistant to G-418 produces and releases surface antigen and e antigen into medium at a high level and accumulates core particles intracellularly. This clone has a chromosomally integrated set of the original recombinant DNA and produces a 3.5-kilobase transcript corresponding to the pregenome RNA as well as HBV DNAs in an extrachromosomal form. Most of these DNAs were in single-stranded or partially double-stranded form and were packaged in the intracellular core particles. In the medium, particles were detected that contained HBV DNA and were morphologically indistinguishable from Dane particles. These results demonstrate that the HBV genome in an integrated state acted as a template for viral gene expression and replication. The cells were maintained for more than 6 months without losing the ability to produce the extrachromosomal HBV DNA and Dane-like particles. Thus, the cells can be used as a model system for analyses of gene expression and DNA replication of HBV in human hepatocytes.
Full text
PDF
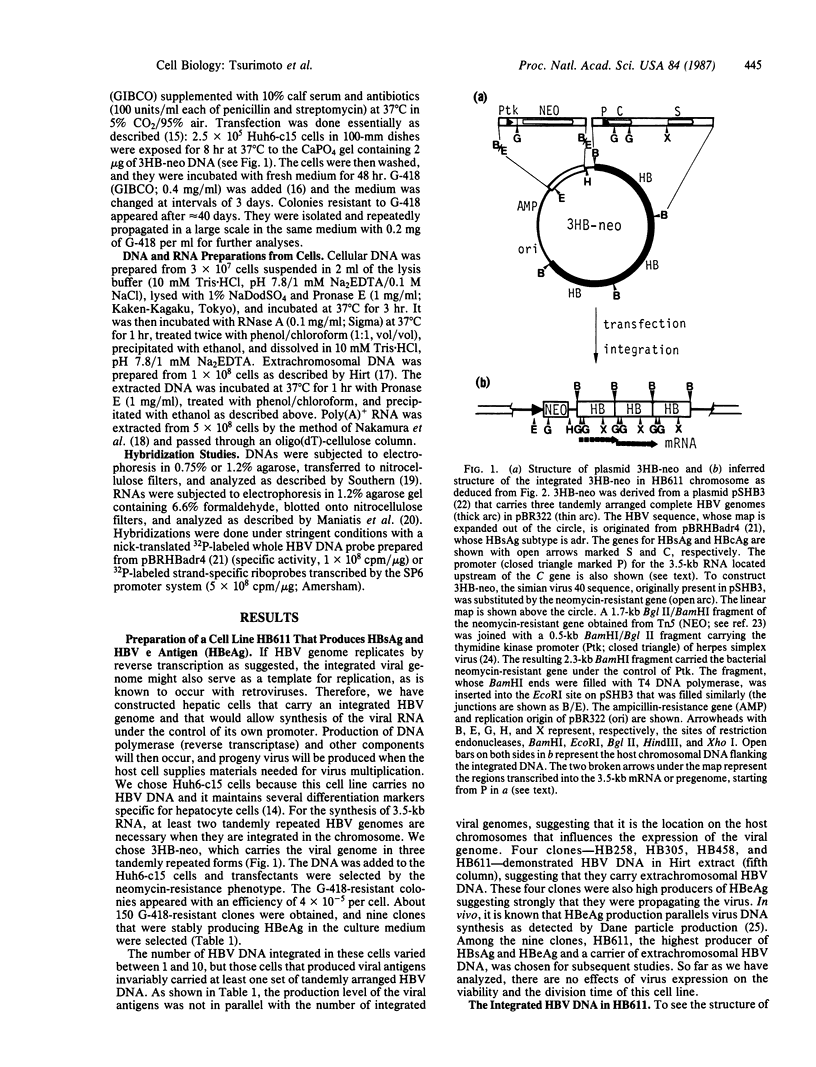



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cattaneo R., Will H., Schaller H. Hepatitis B virus transcription in the infected liver. EMBO J. 1984 Sep;3(9):2191–2196. doi: 10.1002/j.1460-2075.1984.tb02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dane D. S., Cameron C. H., Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970 Apr 4;1(7649):695–698. doi: 10.1016/s0140-6736(70)90926-8. [DOI] [PubMed] [Google Scholar]
- Elfassi E., Romet-Lemonne J. L., Essex M., Frances-McLane M., Haseltine W. A. Evidence of extrachromosomal forms of hepatitis B viral DNA in a bone marrow culture obtained from a patient recently infected with hepatitis B virus. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3526–3528. doi: 10.1073/pnas.81.11.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G. H., Ganem D., Varmus H. Mapping the major transcripts of ground squirrel hepatitis virus: the presumptive template for reverse transcriptase is terminally redundant. Cell. 1985 Aug;42(1):297–308. doi: 10.1016/s0092-8674(85)80125-2. [DOI] [PubMed] [Google Scholar]
- Fujiyama A., Miyanohara A., Nozaki C., Yoneyama T., Ohtomo N., Matsubara K. Cloning and structural analyses of hepatitis B virus DNAs, subtype adr. Nucleic Acids Res. 1983 Jul 11;11(13):4601–4610. doi: 10.1093/nar/11.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich W. H., Robinson W. S. Hepatitis B virus contains protein attached to the 5' terminus of its complete DNA strand. Cell. 1980 Oct;21(3):801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- Hirschman S. Z., Gerber M., Garfinkel E. Purification of naked intranuclear particles from human liver infected by hepatitis B virus. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3345–3349. doi: 10.1073/pnas.71.9.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschman S. Z., Price P., Garfinkel E., Christman J., Acs G. Expression of cloned hepatitis B virus DNA in human cell cultures. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5507–5511. doi: 10.1073/pnas.77.9.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kaplan P. M., Ford E. C., Purcell R. H., Gerin J. L. Demonstration of subpopulations of Dane particles. J Virol. 1976 Mar;17(3):885–893. doi: 10.1128/jvi.17.3.885-893.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers T. A., Greenberg H. B., Robinson W. S. Structure of hepatitis B Dane particle DNA and nature of the endogenous DNA polymerase reaction. J Virol. 1977 Aug;23(2):368–376. doi: 10.1128/jvi.23.2.368-376.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNab G. M., Alexander J. J., Lecatsas G., Bey E. M., Urbanowicz J. M. Hepatitis B surface antigen produced by a human hepatoma cell line. Br J Cancer. 1976 Nov;34(5):509–515. doi: 10.1038/bjc.1976.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt O., von Loringhoven A. F., Frösner G. G. Expression of hepatitis B virus core antigen gene is induced in human hepatoma cells by their growth in nude mice. J Gen Virol. 1984 Aug;65(Pt 8):1443–1448. doi: 10.1099/0022-1317-65-8-1443. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Robinson W. S. Hepatitis B virus DNA forms in nuclear and cytoplasmic fractions of infected human liver. Virology. 1984 Sep;137(2):390–399. doi: 10.1016/0042-6822(84)90231-9. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Nishide T., Emi M., Nakamura Y., Matsubara K. Corrected sequences of cDNAs for human salivary and pancreatic alpha-amylases [corrected]. Gene. 1984 May;28(2):263–270. doi: 10.1016/0378-1119(84)90265-8. [DOI] [PubMed] [Google Scholar]
- Nozaki C., Miyanohara A., Fujiyama A., Hamada F., Ohtomo N., Matsubara K. Two mammalian cell systems for propagation of the hepatitis B virus genome in extrachromosomal and chromosomally integrated states: production of the surface and e antigens. Gene. 1985;38(1-3):39–44. doi: 10.1016/0378-1119(85)90201-x. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Yin J. C., Yong-di Z., Johnson R. C., Reznikoff W. S. Genetic organization of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):99–105. doi: 10.1101/sqb.1981.045.01.018. [DOI] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science. 1986 Apr 25;232(4749):477–484. doi: 10.1126/science.3961490. [DOI] [PubMed] [Google Scholar]
- Shaul Y., Rutter W. J., Laub O. A human hepatitis B viral enhancer element. EMBO J. 1985 Feb;4(2):427–430. doi: 10.1002/j.1460-2075.1985.tb03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata T., Karasawa T., Abe K., Uzawa T., Suzuki H., Oda T., Imai M., Mayumi M., Moritsugu Y. Hepatitis B e antigen and infectivity of hepatitis B virus. J Infect Dis. 1977 Oct;136(4):571–576. doi: 10.1093/infdis/136.4.571. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum G. H., Korba B. E., Lechner J. F., Tokiwa T., Gazdar A. F., Seeley T., Siegel M., Leeman L., Autrup H., Harris C. C. High-frequency transfection and cytopathology of the hepatitis B virus core antigen gene in human cells. Science. 1983 Oct 28;222(4622):385–389. doi: 10.1126/science.6194563. [DOI] [PubMed] [Google Scholar]