Abstract
Rationale
Tobacco withdrawal is characterized by a negative mood state and relatively mild somatic symptoms. Increased noradrenergic transmission has been reported to play an important role in opioid withdrawal, but little is known about the role of noradrenergic transmission in nicotine withdrawal.
Objectives
The aim of these experiments was to investigate the effects of prazosin, clonidine, and propranolol on the negative mood state and somatic signs associated with nicotine withdrawal in rats.
Methods
A discrete-trial intracranial self-stimulation procedure was used to assess the negative affective state of nicotine withdrawal. Elevations in brain reward thresholds are indicative of a deficit in brain reward function.
Results
In all the experiments, the nicotinic acetylcholine receptor antagonist mecamylamine (3 mg/kg) elevated the brain reward thresholds of the nicotine-treated rats and did not affect those of the control rats. The α1-adrenergic receptor antagonist prazosin (0.0625 and 0.125 mg/kg) dose-dependently attenuated the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. The α2-adrenergic receptor agonist clonidine (10–40 μg/kg) and the nonselective β-adrenergic receptor antagonist propranolol (2.5–10 mg/kg) did not attenuate the elevations in brain reward thresholds associated with nicotine withdrawal. Mecamylamine (2 mg/kg) induced more somatic signs in the nicotine-treated rats than in the control rats. Clonidine and propranolol, but not prazosin, decreased the total number of somatic signs associated with nicotine withdrawal.
Conclusion
Blockade of α1-adrenergic receptors attenuates the deficit in brain reward function associated with nicotine withdrawal. Antagonism of β-adrenergic receptors or stimulation of α2-adrenergic receptors attenuates the somatic symptoms of nicotine withdrawal.
Keywords: Prazosin, clonidine, propranolol, norepinephrine, adrenergic receptors, nicotine, withdrawal, brain reward function, rats
INTRODUCTION
Tobacco addiction is a chronic disorder that is characterized by loss of control over smoking, withdrawal symptoms, and relapse after periods of abstinence (American Psychiatric Association 2000). Abrupt cessation of smoking in humans is characterized by negative affective symptoms including depressed mood and anxiety as well as somatic symptoms such as bradycardia and gastrointestinal discomfort (Hughes et al. 1991). The negative emotional state associated with tobacco withdrawal provides a powerful motivation for the continuation of smoking (Bruijnzeel and Gold 2005; Koob and Le Moal 2005). Although the somatic signs of nicotine withdrawal may also contribute to smoking, it has been suggested that the affective withdrawal signs play a more important role in the continuation of smoking and relapse (Koob et al. 1997; Markou et al. 1998).
Animal experiments have shown that antagonism of nicotinic acetylcholine receptors (nAChRs) or discontinuation of nicotine administration induces elevations in brain reward thresholds in the intracranial self-stimulation (ICSS) procedure (Bruijnzeel et al. 2007; Epping-Jordan et al. 1998). Elevations in brain reward thresholds are mediated by a decreased sensitivity to the rewarding electrical stimuli and reflect a dysphoric or anhedonic state (Barr and Markou 2005). Dysphoria is a hallmark feature of drug withdrawal and elevations in brain reward thresholds have been observed during amphetamine, cocaine, morphine, fentanyl, and alcohol withdrawal (Bruijnzeel et al. 2006; Markou and Koob 1991; Schulteis et al. 1994; Schulteis et al. 1995; Wise and Munn 1995).
Extensive evidence indicates that noradrenergic transmission plays a critical role in regulating mood states, drug withdrawal, and drug intake in drug dependent animals. Recent studies point towards a critical role for the α1-adrenergic receptor in drug intake. For example, the α1-adrenergic receptor antagonist prazosin reduces heroin and cocaine self-administration in rats (6–12 hours/day access to drugs of abuse) (Greenwell et al. 2009; Wee et al. 2008). The activation of α1-adrenergic receptors may also play a role in the expression of drug withdrawal as prazosin diminishes weight loss in mice withdrawing from morphine (Ozdogan et al. 2003). Numerous studies suggest that stimulation of α2-adrenergic receptors also attenuates opioid withdrawal. The α2-adrenergic receptor agonist clonidine prevents the morphine withdrawal-induced decrease in operant responding for food and prevents naloxone-induced conditioned place aversion in morphine dependent animals (Kosten 1994; Sparber and Meyer 1978). It is important to note that clonidine also attenuates opioid withdrawal symptoms in humans (Gold et al. 1978). Clinical evidence suggests that clonidine improves smoking cessation rates in humans (Glassman et al. 1988; Gourlay et al. 2004). Gourlay and colleagues conducted a meta-analysis to investigate the effects of clonidine on smoking cessation (Gourlay et al. 2004). They used the data from six clinical studies and only one of these studies reported that clonidine improves smoking cessation rates. The analysis of the pooled data sets suggests that clonidine, slightly, but significantly improves smoking cessation rates (RR = 1.63). Wide scale usage of clonidine for smoking cessation might be somewhat hampered by its side effects which include sedation, dry mouth, and hypotension (Eisenach et al. 1996). It has been suggested that these side effects are mediated by the stimulation of α2-adrenergic receptors (Timmermans et al. 1981).
The β-adrenergic receptors have also been implicated in drug withdrawal. The nonselective β-adrenergic receptor antagonist propranolol decreases the number of somatic signs associated with morphine withdrawal and attenuates morphine-withdrawal induced conditioned place aversion (Harris and Aston-Jones 1993). The opioid receptor antagonist naloxone increases the release of norepinephrine in the central nucleus of the amygdala (CeA) and the bed nucleus of the stria terminalis (BNST) in morphine dependent animals (Fuentealba et al. 2000; Schulteis et al. 1994; Watanabe et al. 2003). In addition, blockade of β-adrenergic receptors in the CeA and BNST prevents morphine withdrawal-induced conditioned place aversion (Delfs et al. 2000; Watanabe et al. 2003). These studies point towards a critical role for increased noradrenergic transmission in the CeA and BNST in the negative mood state associated with opioid withdrawal.
Although the aforementioned studies suggest that the noradrenergic system plays a role in drug dependence, very little research has been done to investigate the role of noradrenergic transmission in the negative mood state and somatic signs associated with nicotine withdrawal. It is hypothesized here that inhibition of noradrenergic transmission will attenuate the negative mood state and somatic signs associated with nicotine withdrawal. In these experiments, the effects of the α1-adrenergic receptor antagonist prazosin, the α2-adrenergic receptor agonist clonidine, and the nonselective β-adrenergic receptor antagonist propranolol on the negative affective state and somatic signs associated with precipitated nicotine withdrawal was investigated. Prazosin, clonidine, and propranolol inhibit noradrenergic transmission in the brain, either by blocking α1 or β-adrenergic receptors or by stimulating presynaptic α2-adrenergic receptors. It should be noted that besides stimulating α2-adrenergic receptors, clonidine has also been shown to stimulate imidazoline-1 (I1) receptors (Buccafusco et al. 1995; Ernsberger et al. 1987). It has been suggested that clonidine may at least partly attenuate the affective signs of morphine withdrawal by stimulating I1 receptors (Georges et al. 2005). The negative affective state associated with nicotine withdrawal was investigated using a discrete-trial ICSS procedure. This procedure was used in all the experiments to assess the negative affective aspects of nicotine withdrawal as it provides a quantitative measure of the emotional aspects of drug withdrawal (Bruijnzeel et al. 2006; Schulteis et al. 1995; Wise and Munn 1995).
MATERIALS AND METHODS
Animals
Male Wistar rats (Charles River, Raleigh, NC) weighing 250–300 gram at the beginning of the experiments were used. Animals were group-housed (two per cage) in a temperature- and humidity-controlled vivarium and maintained on a 12 hour light–dark cycle (lights off at 8 AM). All testing occurred at the beginning of the dark cycle. Food and water was available ad libitum in the home cages. All subjects were treated in accordance with the National Institutes of Health guidelines regarding the principles of animal care. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) and approved by the University of Florida Institutional Animal Care and Use Committee.
Drugs
Nicotine hydrogen tartrate salt, mecamylamine hydrochloride, prazosin hydrochloride, clonidine hydrochloride, and DL-propranolol hydrochloride were purchased from Sigma (Sigma–Aldrich, St. Louis, MO, USA). Nicotine, mecamylamine, clonidine, and propranolol were dissolved in sterile saline (0.9 % sodium chloride) and prazosin was dissolved in distilled water. Propranolol and prazosin were dissolved by heating. All the injections (subcutaneous [sc]/intraperitoneally [ip]) were administered in a volume of 1 ml/kg body weight. Drug doses refer to the salt form.
Surgical Procedures
Electrode implantations
At the beginning of all the intracranial surgeries, the rats were anesthetized with an isoflurane/oxygen vapor mixture (1–3% isoflurane) and placed in a Kopf stereotaxic frame (David Kopf Instruments, Tujunga, CA). The incisor bar was set 5 mm above the interaural line. The rats were prepared with stainless steel bipolar electrodes (model MS303/2 Plastics One, Roanoke, VA) 11 mm in length in the medial forebrain bundle at the level of the posterior lateral hypothalamus (AP −0.5 mm; ML ±1.7 mm; DV −8.3 mm from dura). The electrodes were permanently secured to the skull using dental cement anchored with four skull screws.
Osmotic minipump implantations
The rats were prepared with osmotic minipumps (model 2ML4, 28 day pumps, Durect Corporation, Cupertino, CA) filled with either saline or nicotine. The pumps were implanted subcutaneously under isoflurane/oxygen (1–3% isoflurane) anesthesia. The nicotine concentration was adjusted to compensate for differences in body weight and to deliver 9 mg/kg/day of nicotine salt (3.16 mg/kg/day nicotine base). This dose of nicotine does not have a long-term effect on brain reward thresholds or response latencies of rats in the ICSS procedure (Harrison et al. 2001).
Apparatus
Intracranial self-stimulation chambers
The experimental apparatus consisted of twelve Plexiglas chambers (30.5 × 30 × 17 cm; Med Associates, Georgia, VT), each housed in a sound-attenuating melamine chamber (Med Associates, Georgia, VT). The operant conditioning chambers consisted of a metal grid floor and a metal wheel (5 cm wide) centered on a sidewall. A photobeam detector recorded every 90 degrees of rotation. Brain stimulation was delivered by constant current stimulators (Model 1200C, Stimtek, Acton, MA). Subjects were connected to the stimulation circuit through bipolar leads (Plastics One, Roanoke, VA). A computer controlled the stimulation parameters and data collection.
Experimental procedures
Intracranial self-stimulation procedure
Rats were trained on a modified discrete-trial ICSS procedure (Kornetsky and Esposito 1979), as described previously (Bruijnzeel et al. 2007; Markou and Koob 1992). The rats were trained to turn the wheel on a fixed ratio 1 (FR1) schedule of reinforcement. Each quarter turn resulted in the delivery of a 0.5 second train of 0.1 millisecond cathodal square-wave pulses at a frequency of 100 Hz. After the successful acquisition of responding, the rats were trained on a discrete-trial current-threshold procedure. The rats were subsequently tested on a current-threshold procedure in which stimulation intensities varied according to the classical psychophysical method of limits. The test session provided two variables: brain reward thresholds and response latencies. The brain reward threshold was defined as the midpoint between stimulation intensities that supported responding and current intensities that failed to support responding. It was considered a failed response when the rat responded to fewer than two of the three trials for two consecutive blocks of trials. The response latency was defined as the time interval between the beginning of the non-contingent stimulus and a positive response.
Somatic withdrawal signs
Rats were observed for 10 minutes in a Plexiglas observation chamber (25 × 25 × 46 cm; L × W × H). The rats were habituated to the observation chamber for 5 minutes per day on 3 consecutive days prior to testing. The following somatic signs were recorded: body shakes, cheek tremors, escape attempts, eye blinks, gasps, genital licks, head shakes, ptosis, teeth chattering, writhes, and yawns (Malin et al. 1992; Rylkova et al. 2008). Ptosis was counted once per minute if present continuously. The total number of somatic signs was defined as the sum of the individual occurrences. For the final statistical analyses the signs were divided into the following categories: abdominal constrictions which included gasps and writhes; shakes included head shakes and body shakes; facial fasciculations included cheek tremors and teeth chattering; eye blinks; ptosis; yawns; other signs occurred occasionally and included escape attempts and genital licks.
Experimental design
Experiment 1: Effects of prazosin on nicotine withdrawal
First, the effects of prazosin alone (0.0625–2 mg/kg, ip) on brain reward thresholds and response latencies were investigated. Rats (n = 9) were prepared with electrodes and trained on the ICSS procedure. When stable baseline brain reward thresholds were achieved, defined as less than 10% variation within a 5-day period, the injections with prazosin started. Prazosin (0.0625–2 mg/kg, ip) was administered according to a Latin-square and administered 30 minutes before the rats were placed in the ICSS test chambers. There were at least two drug-free days between successive test days. ICSS testing continued on these drug-free days.
Second, in another group of animals the effects of the high doses of prazosin (0.25–1 mg/kg, ip) on the elevations in brain reward thresholds and somatic signs associated with precipitated nicotine withdrawal was investigated. When stable baseline brain reward thresholds were achieved, the drug naive rats were prepared with 28-day osmotic minipumps containing either saline (n = 8) or nicotine (9 mg/kg/day of nicotine salt, n = 9). The non-competitive and nonspecific nAChR antagonist mecamylamine was used to precipitate withdrawal. Mecamylamine (3 mg/kg, sc) injections started at least 6 days after the implantation of the minipumps to allow the development of nicotine dependence. The dose of mecamylamine was based on previous studies that reported that 3 mg/kg of mecamylamine induces a 40% elevation in brain reward thresholds in nicotine dependent rats and does not affect the brain reward thresholds of control rats (Bruijnzeel et al. 2007; Bruijnzeel et al. 2009). Prazosin (0.25–1 mg/kg, ip) was administered according to a Latin-square design 25 minutes prior to treatment with mecamylamine. The pretreatment interval was based on previous studies that investigated the effects of prazosin on morphine withdrawal and heroin self-administration in rats (Greenwell et al. 2009; Ozdogan et al. 2003). The rats were placed in the ICSS test chambers 5 minutes after the administration of mecamylamine. It was ensured that the minimum time-interval between the mecamylamine injections was at least 3 days to maintain nicotine dependence and allow the brain reward thresholds to return to baseline levels. The serum elimination half-life of mecamylamine is approximately 1 hour (Debruyne et al., 2003). In a separate group of animals, the effect of prazosin (0.25–1 mg/kg, ip) on the somatic signs associated with precipitated nicotine withdrawal was investigated. Rats were prepared with 28-day osmotic minipumps containing either saline (n = 8) or nicotine (9 mg/kg/day of nicotine salt, n = 10). The nAChR antagonist mecamylamine (2 mg/kg, sc) was used to precipitate nicotine withdrawal. A slightly lower dose of mecamylamine was used in the somatic withdrawal experiment than in the ICSS experiment as relatively low doses of mecamylamine induce a large increase in the number of somatic signs in nicotine-treated rats. Furthermore, high doses of mecamylamine increase the number of somatic signs in control animals (Malin et al. 1994). Mecamylamine injections started at least 6 days after the implantation of the minipumps. Prazosin (0.25–1 mg/kg, ip) was administered according to a Latin-square design 25 minutes prior to the administration of mecamylamine. Five minutes after the administration of mecamylamine, the rats were placed in Plexiglas observation cages and somatic signs were recorded for 10 minutes by an experienced observer who was blind to the treatment conditions. The time-interval between the mecamylamine injections was at least 72 hours.
Third, the effects of the low doses (0.0625 and 0.125 mg/kg, ip) of prazosin on the elevations in brain reward thresholds (saline, n = 9; nicotine, n = 9) and somatic signs (nicotine, n = 10) associated with precipitated nicotine withdrawal was investigated. This experiment was conducted in a similar manner as the experiment described above, with the exception that the effects of prazosin on somatic withdrawal signs were investigated in the ICSS animals starting 3 days after the ICSS studies were completed. The nicotine-ICSS group consisted of 9 animals and the nicotine-somatic signs group consisted of 10 animals. This discrepancy in group sizes was due to the fact that one of the ICSS rats was unstable and therefore this rat was not included in the ICSS part of this study.
Experiment 2: Effects of clonidine on nicotine withdrawal
The aim of the first part of this experiment was to investigate the effect of clonidine (10–40 μg/kg, sc) on the elevations in brain reward thresholds associated with mecamylamine (3 mg/kg, sc) precipitated nicotine withdrawal (saline, n = 10; nicotine, n = 11). The second part of this experiment investigated the effect of clonidine (10–40 μg/kg, sc) on the somatic signs associated with mecamylamine (2 mg/kg, sc) precipitated nicotine withdrawal (saline, n = 8; nicotine, n = 10). The ICSS experiment and the somatic withdrawal experiment were conducted in separate groups of animals. For both the ICSS experiment and the somatic withdrawal experiment, clonidine was administered according to a Latin-square design 25 minutes prior to treatment with mecamylamine. The pretreatment interval and clonidine doses were based on previous studies that investigated the effects of clonidine on morphine withdrawal-induced disruption of operant responding for food pellets and stress-induced reinstatement of nicotine seeking in rats (Sparber and Meyer 1978; Zislis et al. 2007). The rats were placed in the ICSS test chambers or in the behavioral observation chambers 5 minutes after the administration of mecamylamine. These experiments were conducted as described under experiment 1 with the exception that the rats received clonidine instead of prazosin.
Experiment 3: Effects of propranolol on nicotine withdrawal
The aim of the first part of this experiment was to investigate the effect of propranolol (2.5–10 mg/kg, ip) on the elevations in brain reward thresholds associated with mecamylamine (3 mg/kg, sc) precipitated nicotine withdrawal (saline, n = 11; nicotine, n = 12). The second part of this experiment investigated the effects of propranolol (2.5–10 mg/kg, ip) on the somatic signs associated with mecamylamine (2 mg/kg, sc) precipitated nicotine withdrawal (saline, n = 8; nicotine, n = 10). The ICSS experiment and the somatic withdrawal experiment were conducted in separate groups of animals. For both the ICSS experiment and the somatic withdrawal experiment, propranolol was administered according to a Latin-square design 25 minutes prior to treatment with mecamylamine. The pretreatment interval and doses were based on previous studies that investigated the effects of propranolol on somatic morphine withdrawal signs and anxiety-like behavior in rats (Harris and Aston-Jones 1993; Yang et al. 1990). This experiment was the same as experiment 2 with the exception that propranolol was administered prior to treatment with mecamylamine.
Statistical analyses
ICSS parameters (brain reward thresholds and response latencies) were expressed as a percentage of the pre-test day values. Total overall somatic signs and percent changes in ICSS parameters were analyzed using a mixed design analyses of variance (ANOVA) with the dose of prazosin, clonidine, or propranolol as the within-subjects factor and pump content (saline or nicotine) as the between-subjects factor. Newman-Keuls post hoc tests were conducted when the ANOVA revealed statistically significant effects. In experiment 1, an additional ANOVA, with prazosin as within-subjects factor, was conducted to investigate if prazosin (0.25–1 mg/kg) affected the brain reward thresholds of the saline-treated control rats. The effects of prazosin (0.0625–2 mg/kg) on ICSS parameters in drug free control rats (no saline or nicotine pumps and no mecamylamine pretreatment) was investigated with an ANOVA and the dose of prazosin was within-subjects factor. One way ANOVA’s were used to verify that the ICSS parameters (absolute brain reward thresholds and response latencies) between the saline rats and nicotine rats did not differ prior to the implantation of the minipumps or prior to the onset of the injections with mecamylamine. The individual somatic signs (abdominal constrictions, shakes, etc.) were analyzed with nonparametric tests because of the small number of specific individual signs recorded in the saline groups. The Kruskal Wallis test (independent samples) was used to compare somatic signs between the nicotine and saline groups. The Wilcoxon signed-rank test (related samples) was used to make comparisons between drug doses within a specific treatment group (nicotine or saline). For all the experiments, the criterion for significance was set at 0.05. The statistical analyses were performed using PASW Statistics 18 for Windows software.
RESULTS
Experiment 1: Effects of prazosin on nicotine withdrawal
First, the effects of prazosin on brain reward thresholds and response latencies of rats in the ICSS procedure were investigated. The administration of prazosin did not affect the brain reward thresholds or the response latencies (Table 1).
Table 1.
Effects of prazosin on brain reward thresholds and response latencies.
| Prazosin dose (mg/kg) | Thresholds | Latencies | ||
|---|---|---|---|---|
| Abs. (μA) | Percentage | Abs. (s) | Percentage | |
| 0 | 93.3 ± 9.4 | 98.5 ± 2.6 | 3.2 ± 0.1 | 99.3 ± 3.6 |
| 0.0625 | 95.3 ± 10.1 | 97.0 ± 2.5 | 3.4 ± 0.1 | 104.2 ± 2.2 |
| 0.125 | 94.2 ± 9.9 | 99.2 ± 3.0 | 3.3 ± 0.1 | 100.4 ± 3.5 |
| 0.25 | 95.0 ± 10.9 | 95.9 ± 1.9 | 3.5 ± 0.1 | 105.4 ± 2.8 |
| 0.5 | 91.7 ± 10.2 | 95.1 ± 1.7 | 3.6 ± 0.1 | 104.7 ± 3.8 |
| 1 | 96.8 ± 9.9 | 100.3 ± 2.8 | 3.5 ± 0.1 | 106.4 ± 4.2 |
| 2 | 90.5 ± 9.7 | 98.2 ± 1.9 | 3.5 ± 0.1 | 105.7 ± 3.6 |
Percentage indicates that the brain reward thresholds and response latencies are expressed as a percentage of the pre-test day values. The number of rats per group was 9. Abbreviations: Abs., absolute.
In the high-doses study (0.25–1 mg/kg), there were no differences in absolute brain reward thresholds and response latencies between the saline group and the nicotine group on the test-day prior to the minipump implantations or on the test-day prior to the first mecamylamine/prazosin injections (Table 2). The administration of 3 mg/kg of mecamylamine elevated the brain reward thresholds of the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated rats (Figure 1A; Treatment: F1,15=10.28, P<0.006). Pretreatment with prazosin affected the brain reward thresholds of the nicotine-treated rats and the saline-treated rats differently (Dose × Treatment interaction: F3,45=3.95, P<0.01). Newman–Keuls posthoc comparisons indicated that 0.25 and 0.5 mg/kg of prazosin attenuated the elevations in brain reward thresholds associated with mecamylamine-precipitated nicotine withdrawal. Posthoc comparisons demonstrated that prazosin (0.25, 0.5, or 1 mg/kg) did not significantly elevate the brain reward thresholds of the rats chronically treated with saline. Furthermore, an additional ANOVA analysis did not reveal an effect of prazosin-dose on brain reward thresholds in the rats chronically treated with saline. Mecamylamine increased the response latencies of the nicotine-treated rats compared to those of the saline-treated rats (Figure 1B; Treatment: F1,15=7.26, P<0.02). This ANOVA analysis compared the collapsed response latencies of all the nicotine groups with the collapsed response latencies of all the saline groups. Posthoc comparisons did not reveal a significant difference in response latencies between the nicotine and saline groups. The administration of prazosin did not alter the response latencies.
Table 2.
Absolute baseline brain reward thresholds and response latencies.
| Thresholds (μA) | Latencies (s) | |||
|---|---|---|---|---|
| Saline | Nicotine | Saline | Nicotine | |
| Expt. 1: Prazosin (0.25–1 mg/kg) | ||||
| Prior pump implantation | 96.6 ± 10.6 | 96.7 ± 8.0 | 3.4 ± 0.2 | 3.0 ± 0.1 |
| Prior first injection | 103.9 ± 13.2 | 94.3 ± 3.1 | 3.5 ± 0.2 | 3.0 ± 0.1 |
| Expt. 1: Prazosin (0.0625, 0.125 mg/kg) | ||||
| Prior pump implantation | 136.9 ± 11.0 | 129.2 ± 10.2 | 3.7 ± 0.1 | 3.5 ± 0.2 |
| Prior first injection | 127.8 ± 12.5 | 125.6 ± 7.6 | 3.6 ± 0.2 | 3.3 ± 0.1 |
| Exp. 2: Clonidine (10–40 μg/kg) | ||||
| Prior pump implantation | 120.0 ± 9.2 | 110.0 ± 8.0 | 3.2 ± 0.1 | 3.2 ± 0.1 |
| Prior first injection | 115.0 ± 6.7 | 112.5 ± 3.1 | 3.0 ± 0.1 | 3.1 ± 0.1 |
| Exp. 3: Propranolol (2.5–10 mg/kg) | ||||
| Prior pump implantation | 118.0 ± 6.1 | 114.8 ± 9.3 | 3.4 ± 0.1 | 3.3 ± 0.1 |
| Prior first injection | 113.4 ± 9.3 | 112.7 ± 6.4 | 3.2 ± 0.1 | 3.3 ± 0.1 |
Prior refers to the test-day immediately before the implantation of the osmotic minipumps or the start of the mecamylamine injections.
Figure 1.
Effect of the α1-adrenergic receptor antagonist prazosin (0.25–1 mg/kg) on the elevations in brain reward thresholds associated with mecamylamine-precipitated nicotine withdrawal (A; saline, n = 8; nicotine, n = 9) and response latencies (B). Asterisks (** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Plus signs (+ P<0.05) indicate lower brain reward thresholds compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle. Data are expressed as means ± SEM.
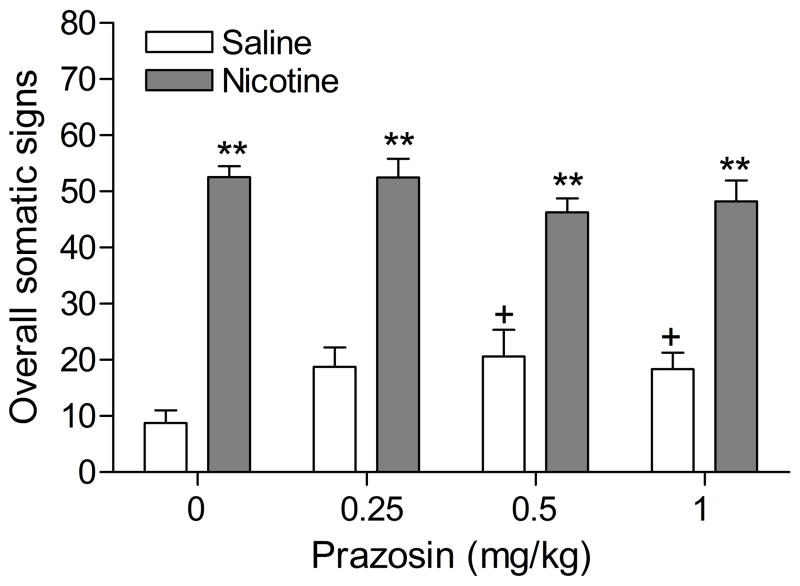
Precipitated nicotine withdrawal increased the total number of somatic signs (Figure 2; Treatment: F1,16=168.23, P<0.0001), facial fasciculations, yawns, abdominal constrictions, and eye blinks (see table 3 for the level of significance of individual signs). Prazosin (0.25–1 mg/kg) increased the total number of somatic signs in the control animals (Dose × Treatment: F3,48=3.26, P<0.03) and increased the number of occurrences of ptosis in the control animals (Table 3). Furthermore, prazosin increased the number of occurrences of ptosis, yawns, and shakes in the nicotine withdrawing rats and decreased the number of eye blinks in the nicotine withdrawing rats (Table 3).
Figure 2.
Effect of the α1-adrenergic receptor antagonist prazosin (0.25–1 mg/kg) on overall somatic signs associated with mecamylamine-precipitated nicotine withdrawal (A; saline, n = 8; nicotine, n = 10). Asterisks (** P<0.01) indicate a greater number of somatic signs in the nicotine-treated rats than in the saline-treated control group. Plus signs (+ P<0.05) indicate a greater number of somatic signs compared to those in rats chronically treated with saline and acutely treated with mecamylamine and vehicle. Data are expressed as means ± SEM.
Table 3.
Effects of prazosin on somatic nicotine withdrawal signs.
| Prazosin (mg/kg) | vehicle | 0.25 | 0.5 | 1 | |
|---|---|---|---|---|---|
| Abd. const. | Saline | 0.0 ± 0.0+ | 0.0 ± 0.0++ | 0.0 ± 0.0+ | 0.0 ± 0.0++ |
| Nicotine | 7.9 ± 1.8 | 8.1 ± 1.2 | 7.6 ± 1.7 | 8.1 ± 2.1 | |
| Eye blinks | Saline | 4.3 ± 0.6+ | 5.8 ± 0.9+ | 8.0 ± 2.2 | 3.9 ± 0.9 |
| Nicotine | 25.1 ± 2.9 | 14.4 ± 2.8** | 11.6 ± 2.9** | 7.6 ± 1.9** | |
| Ptosis | Saline | 0.5 ± 0.5 | 5.8 ± 1.3* | 4.1 ± 1.7 | 6.8 ± 1.1* |
| Nicotine | 4.8 ± 1.5 | 9.3 ± 1.3* | 9.2 ± 1.8* | 9.8 ± 1.4 | |
| Facial fasc. | Saline | 0.1 ± 0.0++ | 0.0 ± 0.0++ | 0.0 ± 0.0+ | 0.0 ± 0.0++ |
| Nicotine | 3.4 ± 0.9 | 3.2 ± 1.2 | 1.9 ± 0.6 | 2.4 ± 0.6 | |
| Yawns | Saline | 0.0 ± 0.0+ | 0.4 ± 0.4++ | 0.4 ± 0.2++ | 0.0 ± 0.0++ |
| Nicotine | 1.9 ± 0.8 | 5.7 ± 1.0* | 5.2 ± 0.8* | 7.2 ± 0.7** | |
| Shakes | Saline | 3.5 ± 2.0 | 6.5 ± 2.9 | 7.4 ± 4.0 | 7.5 ± 2.6 |
| Nicotine | 7.6 ± 2.2 | 11.8 ± 3.3 | 10.5 ± 3.3 | 13.1 ± 3.0* | |
| Other signs | Saline | 0.4 ± 0.2 | 0.4 ± 0.3 | 0.8 ± 0.3 | 0.3 ± 0.2 |
| Nicotine | 1.9 ± 1.4 | 0.0 ± 0.0 | 0.3 ± 0.3 | 0.0 ± 0.0 | |
Somatic signs in nicotine-treated (n = 10) and saline-treated rats (n = 8) as recorded during 10-minute observation periods. Abdominal constrictions include gasps and writhes; facial fasciculations include cheek tremors and teeth chattering; shakes include head shakes and body shakes; other signs include escape attempts and genital licks.
Plus signs (+ P<0.05, ++ P<0.01) indicate fewer somatic signs in the saline group than in the nicotine group.
Asterisks (* P<0.05, ** P<0.01) indicate a decrease or increase in the number of somatic signs compared to rats in the same treatment group (nicotine or saline) and acutely treated with vehicle. Data are expressed as means ± SEM.
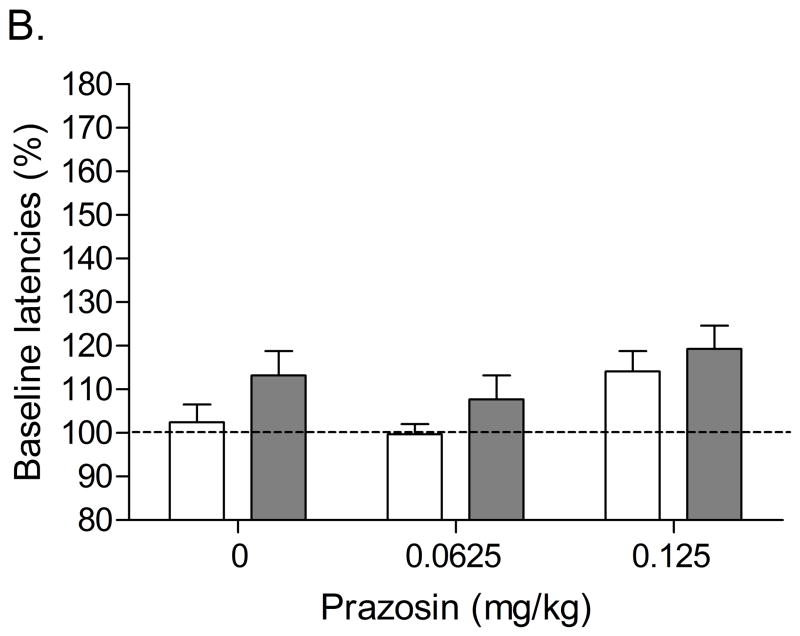
In the low-doses study (0.0625 and 0.125 mg/kg), there were no differences in absolute brain reward thresholds and response latencies between the saline group and the nicotine group on the test-day prior to the minipump implantations or on the test-day prior to the first mecamylamine/prazosin injections (Table 2). The administration of 3 mg/kg of mecamylamine also elevated the brain reward thresholds of the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated rats (Figure 3A; Treatment: F1,16=16.84, P<0.0008). Pretreatment with prazosin dose-dependently attenuated the mecamylamine-induced elevations in brain reward thresholds in the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated control rats (Dose × Treatment interaction: F2,32=3.95, P<0.0007). Posthoc analyses indicated that 0.0625 mg/kg of prazosin slightly attenuated the elevations in brain reward thresholds associated with nicotine withdrawal. Furthermore, 0.125 mg/kg of prazosin strongly attenuated the elevations in brain reward thresholds associated with nicotine withdrawal. Neither nicotine withdrawal nor the administration of prazosin affected the response latencies (Figure 3B).
Figure 3.
Effect of the α1-adrenergic receptor antagonist prazosin (0.0625 and 0.125 mg/kg) on the elevations in brain reward thresholds associated with mecamylamine-precipitated nicotine withdrawal (A; saline, n = 9; nicotine, n = 9) and response latencies (B). Asterisks (** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Plus signs (++ P<0.01) indicate lower brain reward thresholds compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle. Pound signs (## P<0.01) indicate lower brain reward thresholds compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle or mecamylamine and 0.0625 mg/kg of prazosin. Data are expressed as means ± SEM.
The effect of prazosin (0.0625 and 0.125 mg/kg) on somatic withdrawal signs was investigated after the ICSS study was completed. Prazosin did not affect the total number of somatic signs, abdominal constrictions, shakes, yawns, eye blinks, and other signs in the nicotine withdrawing rats (data not shown). Prazosin significantly increased the number of occurrences of ptosis (dose 0.0625, P<0.05; dose 0.125, P<0.01) and facial fasciculations (dose 0.125, P<0.05) in the nicotine withdrawing rats (data not shown).
Experiment 2: Effects of clonidine on nicotine withdrawal
There were no differences in absolute brain reward thresholds and response latencies between the saline group and the nicotine group on the test-day prior to minipump implantation or on the test-day prior to the first mecamylamine/clonidine injections (Table 2). Mecamylamine elevated the brain reward thresholds of the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated rats (Figure 4A; Treatment: F1,19=25.26, P<0.0001). Pretreatment with clonidine did not affect the brain reward thresholds of the nicotine-treated rats. Posthoc analyses indicated that 40 μg of clonidine elevated the brain reward thresholds of the saline-treated control rats. Mecamylamine increased the response latencies of the nicotine-treated rats compared to those of the saline-treated rats (Figure 4B; Treatment: F1,19=5.46, P<0.03). Posthoc comparisons did not reveal a significant difference at any specific dose. The administration of clonidine increased the response latencies of the nicotine-treated and the control rats (Dose: F3,57=4.83, P<0.005). Nicotine withdrawal increased the total number of somatic signs (Figure 5; Treatment: F1,16=57.04, P<0.0001), abdominal constrictions, eye blinks, the number of occurrences of ptosis, and facial fasciculations (Table 4). Clonidine decreased the total number of somatic signs in the nicotine withdrawing animals (Dose × Treatment: F3,48=6.86, P<0.0006). Clonidine also decreased the number of shakes, eye blinks, and the number of occurrences of ptosis in the nicotine withdrawing rats (Table 4).
Figure 4.
Effect of the α2-adrenergic/imidazoline-1 receptor agonist clonidine on the elevations in brain reward thresholds associated with mecamylamine-precipitated nicotine withdrawal (A; saline, n = 10; nicotine, n =11) and response latencies (B). Asterisks (** P<0.01) indicate elevations in brain reward thresholds compared to those of the corresponding saline-treated control group. Plus signs (+ P<0.05) indicate elevations in brain reward thresholds compared to those of rats chronically treated with saline and acutely treated with mecamylamine and vehicle. Data are expressed as means ± SEM.
Figure 5.
Effect of the α2-adrenergic/imidazoline-1 receptor agonist clonidine on overall somatic signs associated with mecamylamine-precipitated nicotine withdrawal (A; saline, n = 8; nicotine, n = 10). Asterisks (** P<0.01) indicate a greater number of somatic signs in the nicotine-treated rats than in the saline-treated control group. Plus signs (++ P<0.01) indicate fewer somatic signs compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle. Pound signs (# P<0.05) indicate fewer somatic signs compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and 10 μg/kg of clonidine. Data are expressed as means ± SEM.
Table 4.
Effects of clonidine on somatic nicotine withdrawal signs.
| Clonidine (μg/kg) | vehicle | 10 | 20 | 40 | |
|---|---|---|---|---|---|
| Abd. const. | Saline | 0.1 ± 0.1++ | 0.1 ± 0.1++ | 0.3 ± 0.3++ | 0.3 ± 0.2++ |
| Nicotine | 3.8 ± 1.5 | 4.3 ± 0.8 | 4.8 ± 0.8 | 4.9 ± 0.9 | |
| Eye blinks | Saline | 5.4 ± 0.8++ | 4.1 ± 0.5++ | 3.0 ± 0.7 | 4.8 ± 1.0 |
| Nicotine | 22.0 ± 3.4 | 13.0 ± 3.5** | 6.6 ± 1.8** | 6.1 ± 2.4** | |
| Ptosis | Saline | 0.3 ± 0.3++ | 0.4 ± 0.4+ | 0.8 ± 0.5 | 2.3 ± 1.0 + |
| Nicotine | 3.1 ± 0.6 | 2.1 ± 0.7 | 1.0 ± 0.5* | 0.3 ± 0.3* | |
| Facial fasc. | Saline | 0.0 ± 0.0++ | 0.0 ± 0.0++ | 0.0 ± 0.0++ | 0.0 ± 0.0++ |
| Nicotine | 5.3 ± 1.1 | 4.1 ± 1.1 | 4.4 ± 1.5 | 4.8 ± 0.8 | |
| Yawns | Saline | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Nicotine | 0.2 ± 0.1 | 1.1 ± 0.6 | 0.2 ± 0.2 | 0.2 ± 0.2 | |
| Shakes | Saline | 0.9 ± 0.5 | 0.0 ± 0.0+ | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Nicotine | 1.4 ± 0.5 | 1.0 ±0.3 | 0.2 ± 0.1 | 0.1 ± 0.1* | |
| Other signs | Saline | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.4 ± 0.3 | 0.6 ± 0.4 |
| Nicotine | 0.0 ± 0.0 | 0.4 ± 0.2 | 0.5 ± 0.2 | 0.4 ± 0.3 | |
Somatic signs in nicotine-treated (n = 10) and saline-treated rats (n = 8) as recorded during 10-minute observation periods. Abdominal constrictions include gasps and writhes; facial fasciculations include cheek tremors and teeth chattering; shakes include head shakes and body shakes; other signs include escape attempts and genital licks.
Plus signs (+ P<0.05, ++ P<0.01) indicate fewer somatic signs in the saline group than in the nicotine group.
Asterisks (* P<0.05, ** P<0.01) indicate a decrease in the number of somatic signs compared to rats in the same treatment group (nicotine) and acutely treated with vehicle. Data are expressed as means ± SEM.
Experiment 3: Effects of propranolol on nicotine withdrawal
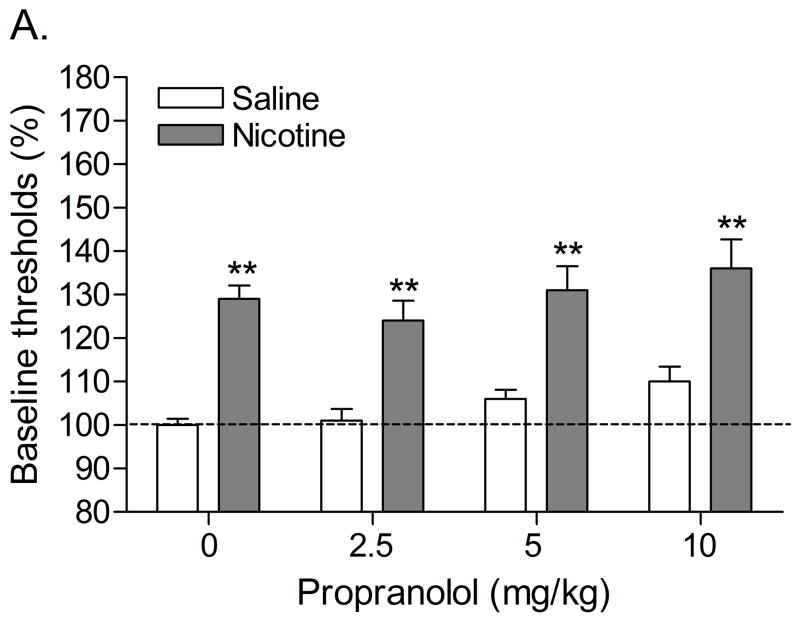
Statistical analyses indicated that there were no differences in absolute brain reward thresholds and response latencies between the saline group and the nicotine group on the test-day prior to minipump implantation or on the test-day prior to the first mecamylamine/propranolol injections (Table 2). The administration of 3 mg/kg of mecamylamine elevated the brain reward thresholds of the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated rats (Figure 6A; Treatment: F1,21=39.61, P<0.0001). Pretreatment with propranolol increased the brain reward thresholds of the nicotine-treated rats and the control rats (Dose; F3,63=3.68, P<0.02). Mecamylamine and propranolol did not affect the response latencies (Figure 6B). Nicotine withdrawal increased the total number of somatic signs (Figure 7; Treatment: F1,16=196.53, P<0.0001), facial fasciculations, shakes, abdominal constrictions, and eye blinks. Propranolol decreased the total number of somatic signs in the nicotine withdrawing animals (Dose × Treatment: F3,48=2.89, P<0.05). Propranolol also decreased the number of shakes and eye blinks in the nicotine withdrawing rats (Table 5).
Figure 6.
Effect of the nonselective β-adrenergic receptor antagonist propranolol on the elevations in brain reward thresholds associated with mecamylamine-precipitated nicotine withdrawal (A; saline, n = 11; nicotine, n =12) and response latencies (B). Brain reward thresholds and response latencies are expressed as a percentage of the pre-test day values. Asterisks (** P<0.01) indicate elevations in brain reward thresholds compared with those of the corresponding saline-treated control group. Data are expressed as means ± SEM.
Figure 7.
Effect of the nonselective β-adrenergic receptor antagonist propranolol on overall somatic signs associated with mecamylamine-precipitated nicotine withdrawal (A; saline, n = 11; nicotine, n = 12). Asterisks (** P<0.01) indicate a greater number of somatic signs in the nicotine-treated rats than in the saline-treated control group. Plus signs (++ P<0.01) indicate fewer somatic signs compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle. Data are expressed as means ± SEM.
Table 5.
Effects of propranolol on somatic nicotine withdrawal signs.
| Propranolol (mg/kg) | vehicle | 2.5 | 5 | 10 | |
|---|---|---|---|---|---|
| Abd. const. | Saline | 0.0 ± 0.0++ | 0.0 ± 0.0++ | 0.0 ± 0.0++ | 0.1 ± 0.1++ |
| Nicotine | 7.4 ± 2.1 | 9.8 ± 2.0 | 9.2 ± 2.4 | 7.0 ± 2.2 | |
| Eye blinks | Saline | 6.9 ± 1.1++ | 6.6 ± 1.1++ | 7.3 ± 1.3++ | 5.5 ± 0.7 |
| Nicotine | 25.0 ± 2.9 | 18.0 ± 2.0* | 21.0 ± 2.9 | 12.0 ± 3.8* | |
| Ptosis | Saline | 0.8 ± 0.5 | 0.6 ± 0.3 | 0.0 ± 0.0 | 2.1 ± 0.9 |
| Nicotine | 5.4 ± 1.0 | 6.2 ± 1.2 | 5.1 ± 1.0 | 6.5 ± 1.3 | |
| Facial fasc. | Saline | 0.1 ± 0.1++ | 0.0 ± 0.0++ | 0.0 ± 0.0++ | 0.0 ± 0.0+ |
| Nicotine | 5.8 ± 1.5 | 4.4 ± 1.0 | 3.7 ± 1.4 | 3.1 ± 1.3 | |
| Yawns | Saline | 0.8 ± 0.8 | 0.5 ± 0.4 | 0.0 ± 0.0 | 0.3 ± 0.2 |
| Nicotine | 1.0 ± 0.7 | 0.4 ± 0.3 | 0.2 ± 0.2 | 1.1 ± 0.7 | |
| Shakes | Saline | 0.4 ± 0.3++ | 0.5 ± 0.4 | 0.1 ± 0.1++ | 0.1 ± 0.1 |
| Nicotine | 3.7 ± 1.0 | 1.0 ± 0.4* | 2.1 ± 0.4 | 0.9 ± 0.4 | |
| Other signs | Saline | 0.4 ± 0.2 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 |
| Nicotine | 0.7 ± 0.4 | 0.4 ± 0.3 | 1.0 ± 0.5 | 0.4 ± 0.3 | |
Somatic signs in nicotine-treated (n = 10) and saline-treated rats (n = 8) as recorded during 10-minute observation periods. Abdominal constrictions include gasps and writhes; facial fasciculations include cheek tremors and teeth chattering; shakes include head shakes and body shakes; other signs include escape attempts and genital licks.
Plus signs (+ P<0.05, ++ P<0.01) indicate fewer somatic signs in the saline group than in the nicotine group.
Asterisks (* P<0.05, ** P<0.01) indicate a decrease in the number of somatic signs compared to rats in the same treatment group (nicotine) and acutely treated with vehicle. Data are expressed as means ± SEM.
DISCUSSION
The aim of the present studies was to investigate the effects of prazosin, clonidine, and propranolol on the deficit in brain reward function (i.e., elevations in brain reward thresholds) and somatic signs associated with precipitated nicotine withdrawal in rats. The nAChR antagonist mecamylamine (3 mg/kg) elevated the brain reward thresholds of the nicotine-treated rats and did not affect the brain reward thresholds of the control rats. In addition, mecamylamine (2 mg/kg) induced more somatic signs in the nicotine-treated rats than in the saline-treated rats. The present results also indicated that the α1-adrenergic receptor antagonist prazosin attenuates the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. Prazosin had a U-shaped dose-dependent effect on the brain reward thresholds of the nicotine withdrawing rats. Pretreatment with 0.125 mg/kg of prazosin was more effective in preventing the mecamylamine-precipitated elevations in brain reward thresholds in the nicotine group than lower or higher doses of this compound. In addition, it was shown that neither the α2-adrenergic/I1 receptor agonist clonidine nor the nonselective β-adrenergic receptor antagonist propranolol attenuated the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. Furthermore, clonidine and propranolol decreased the total number of somatic signs associated with precipitated nicotine withdrawal while prazosin did not decrease the total number of somatic signs associated with nicotine withdrawal. Prazosin increased the number of occurrences of ptosis in the control rats. This is in line with previous studies which reported that blockade of peripheral α1-adrenergic receptors induces ptosis (Honda et al. 1985; Millan et al. 1994).
The observation that the nAChR antagonist mecamylamine elevated the brain reward thresholds of the nicotine-treated rats, but not of the saline-treated rats, is in agreement with studies that investigated the effects of antagonism of nAChRs on brain reward thresholds (Bruijnzeel et al. 2007; Bruijnzeel et al. 2009; Epping-Jordan et al. 1998). It has been suggested that elevations in brain reward thresholds reflect a negative emotional state that resembles the dysphoria experienced by drug dependent patients after the discontinuation of drug use (Barr and Markou 2005). Previous research has shown that the activation of brain stress systems at least partly mediates the negative mood state associated with nicotine withdrawal. Blockade of corticotropin-releasing factor receptors prevents the elevations in brain reward thresholds associated with precipitated nicotine withdrawal (Bruijnzeel et al. 2007; Bruijnzeel et al. 2009). The present findings demonstrate that blockade of α1-adrenergic receptors also attenuates the elevations in brain reward thresholds associated with nicotine withdrawal. Experimental evidence indicates that blockade of α1-adrenergic receptors decreases drug-intake in animals that have a history of long-access to drugs of abuse and attenuates the behavioral effects of exposure to stressors (Greenwell et al. 2009; Wee et al. 2008). Animals that have long-access (6 hrs/day) to cocaine, but not animal with short-access (1 hr/day), display an escalation of cocaine intake and a gradual elevation in brain reward thresholds (Ahmed et al. 2002). It has been suggested that these animals increase their drug intake in an attempt to counter their deficit in brain reward function (Ahmed et al. 2002; Koob 2008). The observation that prazosin diminishes the negative mood state associated with drug withdrawal (Fig. 3A) would suggest that α1-adrenergic receptor antagonists may decrease drug intake in the long-access rats by diminishing their deficit in brain reward function. Additional studies are warranted to test this hypothesis. Blockade of α1-adrenergic may also attenuate the behavioral effects of exposure to stressors. Restraint stress has been shown to increase the latency before rats emerge from a dark chamber in a novel environment and increases the total amount of time spent in the chamber. Pretreatment with prazosin prevents the restraint stress-induced increase in emergence latency and time spent in the chamber (Yang et al. 1990). Furthermore, restraint or inescapable tail shocks leads to a long-term potentiation of the acoustic startle response. The administration of prazosin prior to the restraint-stress or the inescapable tail shocks inhibits the stress-induced potentiation of the acoustic startle response (Manion et al. 2007). Taken together, these findings support the hypothesis that stress-induced behavioral changes such as increased anxiety-like behavior and the negative mood state associated with nicotine withdrawal are at least partly mediated via the activation of α1-adrenergic receptors. Additional studies are warranted to investigate if α1-adrenergic receptor antagonists decrease anxiety-like behavior associated with nicotine withdrawal (Irvine et al. 1999).
The first experiment investigated the effect of pretreatment with prazosin on the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. This experiment was conducted in 2 steps, first the effect of relatively high doses (0.25–1 mg/kg) on nicotine withdrawal was investigated and then the effect of lower doses (0.0625 and 0.125 mg/kg) was investigated. It was shown that 0.125 mg/kg of prazosin was more effective in attenuating the elevations in brain reward thresholds associated with nicotine withdrawal than lower (0.0625 mg/kg) or higher (0.25 and 0.5 mg/kg) doses of prazosin. This pattern of results suggest that suboptimal, either too low or too high, levels of noradrenergic transmission lead to elevations in brain reward thresholds. The present study suggests that nicotine withdrawal leads to an increase in noradrenergic transmission which causes excessive stimulation of α1-adrenergic receptors and elevations in brain reward thresholds. This effect is partly antagonized with a low dose of prazosin (0.0625 mg/kg) and more completely antagonized with a slightly higher dose of prazosin (0.125 mg/kg). The 0.125 mg/kg of prazosin is the optimal dose as higher doses are also less effective in attenuating the elevations in brain reward thresholds associated with nicotine withdrawal. This is most likely due to the fact that a strong blockade of α1-adrenergic receptors also leads to an elevation in brain reward thresholds. This is supported by previous studies that have shown that α1-adrenergic receptor antagonists dose-dependently inhibit responding in rate dependent intracranial self-stimulation procedures (Fenton and Liebman 1982; Liebman et al. 1982; Lin et al. 2007).
The data depicted in Figure 1A indicate that 1 mg/kg of prazosin induces a non-significant, 20%, elevation in brain reward thresholds in rats that are prepared with saline pumps and acutely treated with mecamylamine. The same dose of prazosin did not affect the brain reward thresholds in a separate experiment in which the effect of prazosin alone on brain rewards thresholds was investigated (Table 1). These findings suggest that prazosin alone, up to 2 mg/kg, does not affect brain reward thresholds but the co-administration of mecamylamine (3 mg/kg) leads to a small elevation in brain reward thresholds. As indicated in the present study and other studies, the administration of 3 mg/kg of mecamylamine alone does not affect brain reward thresholds in the ICSS procedure (Bruijnzeel et al. 2009; Watkins et al. 2000). However, it has been reported that a slightly higher dose of mecamylamine, 5 mg/kg, suppresses the rate of lever pressing in the ICSS procedure (Olds and Domino 1969). Therefore, in the present study the administration of prazosin alone or mecamylamine alone did not affect the brain reward thresholds of the rats. However, the additive effects of the subthreshold doses of mecamylamine and prazosin may have mediated the non-significant, 20%, elevation in brain brain reward thresholds in the control group.
Clonidine is a α2-adrenergic receptor and I1 receptor agonist (Buccafusco et al. 1995; Ernsberger et al. 1987). Previous research suggests that clonidine prevents naloxone-induced conditioned place aversion in morphine dependent animals at least partly by stimulating I1 receptors (Georges et al. 2005). Furthermore, naloxone-precipitated morphine withdrawal leads to a hypoactivity of dopaminergic neurons in the ventral tegmental area and this effect is prevented by clonidine acting upon I1 receptors (Georges and Aston-Jones 2003). In the present study, clonidine did not prevent the elevations in brain reward thresholds associated with nicotine withdrawal. This suggests that neither stimulation of α2-adrenergic receptors nor stimulation of I1 receptors prevents the negative mood state associated with nicotine withdrawal. In contrast, clonidine dose-dependently decreased the somatic signs associated with nicotine withdrawal. Coupar investigated the effects of various α2-adrenergic receptor agonists (clonidine, azepexole, guanfacine) on naloxone-precipitated somatic morphine withdrawal signs (Coupar 1992). Clonidine has a high affinity for the α2-adrenergic receptor and the I1 receptor (clonidine: Ki α2-site 3.8, Ki I1-site 1.0) and azepexole and guanfacine have a relatively high affinity for the α2-adrenergic receptor and a low affinity for the I1 receptor (azepexole: Ki α2-site 31, Ki I1-site 1140; guanfacine: Ki α2-site 2.3, Ki I1-site 2500) (Buccafusco et al. 1995). Pretreatment with azepexole attenuates all precipitated somatic morphine withdrawal signs and pretreatment with clonidine and guanfacine attenuate all somatic withdrawal signs with the exception of paw shakes (clonidine) and jumping and diarrhea (guanfacine). This suggests that compounds that have an extremely low affinity for the I1 receptor are more effective than or at least as effective as clonidine in blocking somatic morphine withdrawal signs. This would suggest that clonidine reduces somatic withdrawal signs by stimulating the α2-adrenergic receptor and not the I1 receptor.
The results of these studies demonstrated that the nonselective β-adrenergic receptor antagonist propranolol slightly attenuates the somatic nicotine withdrawal signs and does not prevent the elevations in brain reward thresholds associated with nicotine withdrawal. We are not aware of any studies that investigated the effects of propranolol or other nonselective β-adrenergic receptor antagonists on the negative mood state associated with nicotine withdrawal or somatic nicotine withdrawal signs. However, the effect of propranolol on morphine withdrawal has been investigated. These studies reported that propranolol attenuates the somatic morphine withdrawal signs and reduces morphine withdrawal-induced place aversion (Delfs et al. 2000; Harris and Aston-Jones 1993; Watanabe et al. 2003). These findings suggest that β-adrenergic receptor activation plays a critical role in mediating the somatic nicotine and opioid withdrawal signs. These findings would also suggest that β-adrenergic receptors play a critical role in the negative mood state associated with morphine withdrawal but do not play a role in the negative mood state associated with nicotine withdrawal. It should be noted, however, that the negative affective state of morphine withdrawal was assessed with the conditioned place aversion procedure and the ICSS procedure was used to investigate the negative affective state associated with nicotine withdrawal. Although both the ICSS procedure and conditioned place aversion tests are used to assess the negative mood state associated with drug withdrawal there is a major difference between these tests. In the ICSS procedure the acute effects of a drug are assessed (i.e., animal is under the influence of the drug) and in the place aversion procedure the rats are tested in a drug-free state and the outcome of the test is dependent on a previously formed association between the subjective effects of the drug and the environment (Carlezon, Jr. 2003). Extensive evidence indicates that propranolol and other β-adrenergic receptor antagonists block the consolidation of aversive memories in humans and experimental animals (Cahill et al. 1994; McGaugh 2000; van Stegeren et al. 1998). Therefore, in the morphine withdrawal experiments, propranolol might not have prevented the negative mood state but avoided the formation of an association between the withdrawal-induced negative emotional state and the test chamber. We are not aware of any studies that reported that propranolol prevents the elevations in brain reward thresholds associated with morphine withdrawal or withdrawal from other drugs of abuse.
The present studies focused on investigating the effects of relatively specific adrenergic receptor agonist and antagonists on the affective and somatic signs of nicotine withdrawal. Other studies have explored the effects of drugs that block the reuptake of norepinephrine on nicotine withdrawal and smoking cessation. One of the compounds that has been investigated as a smoking cessation aid is the antidepressant and relative selective norepinephrine reuptake inhibitor nortriptyline (5-HT, 570; NE, 3.4; DA, 3500, IC50, reuptake inhibition in vivo) (Hyttel 1994). Nortriptyline has been shown to improve relapse rates in subjects who attempt to quit smoking and diminish anxiety, anger, irritability, difficulty concentrating, restlessness, and impatience associated with smoking cessation (Hall et al. 1998; Prochazka et al. 1998; Wagena et al. 2005). Nortriptyline also decreases the number of somatic withdrawal signs in nicotine withdrawing rats, but only at doses that also decrease locomotor activity (Wing and Shoaib 2007). It cannot be completely ruled out that nortriptyline’s effect on the serotonin transporter contribute to its effectiveness as a smoking cessation aid (Hyttel 1994). However, selective serotonin reuptake inhibitors such as fluoxetine, paroxetine, and sertraline do not improve smoking cessation rates (Hughes et al. 2007). Therefore, it is more likely that the effects of nortriptyline on smoking cessation are mediated by its actions on the norepinephrine transporter than on the serotonin transporter. Nortriptyline also decreases the expression of α2 and β-adrenergic receptors which may contribute to the clinical effects of this compound (Garcia-Sevilla et al. 1981; Sellinger-Barnette et al. 1980; Sugrue 1983). The antidepressant drug bupropion has also been shown to improve smoking cessation rates and decrease tobacco withdrawal symptoms such as depression, difficulty concentrating, and irritability (Hurt et al. 1997; Jorenby et al. 1999; Shiffman et al. 2000). In addition, bupropion decreases affective and somatic nicotine withdrawal signs in rats (Cryan et al. 2003; Wing and Shoaib 2007). Although there is evidence that bupropion and its metabolites affect noradrenergic transmission, bupropion has also been shown to affect other brain systems and therefore it has been difficult to pinpoint its clinical effects to a specific neurotransmitter system. Bupropion has been shown to be a relatively weak inhibitor of the reuptake of dopamine and norepinephrine, stimulates norepinephrine release, and blocks nAChRs (Damaj et al. 2004; Dong and Blier 2001; Ferris et al. 1982; Fryer and Lukas 1999). Taken together, nortriptyline and bupropion inhibit the reuptake of norepinephrine and have been shown to improve smoking cessation rats and attenuate nicotine withdrawal. However, because these compounds affect a variety of neurotransmitter systems and receptors their specific mode of action remains to be elucidated.
Taken together, these findings indicate that blockade of α1-adrenergic receptors attenuates the deficit in brain reward function associated with nicotine withdrawal and blockade of β-adrenergic receptors or stimulation of α2-adrenergic receptors reduces the somatic nicotine withdrawal signs. These studies have significant clinical implications for the treatment of tobacco addiction. The present findings suggest that a cocktail of an α1-adrenergic receptor antagonist and an α2-adrenergic receptor agonist or a nonspecific β-adrenergic receptor antagonist might diminish the anhedonic state as well as the somatic symptoms associated with smoking cessation. Drugs that attenuate the negative affective and somatic withdrawal signs might improve smoking cessation rates.
Acknowledgments
This research was funded by a National Institute on Drug Abuse grant (DA023575) and a Flight Attendant Medical Research Institute Young Clinical Scientist Award (Grant nr. 52312) to A. Bruijnzeel. Irma van Tuijl received salary support from the Dr. Saal van Zwanenberg Foundation.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Press; Washington, DC: 2000. text revision edn. [Google Scholar]
- Barr AM, Markou A. Psychostimulant withdrawal as an inducing condition in animal models of depression. Neurosci Biobehav Rev. 2005;29:675–706. doi: 10.1016/j.neubiorev.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Lewis B, Bajpai LK, Morey TE, Dennis DM, Gold M. Severe deficit in brain reward function associated with fentanyl withdrawal in rats. Biol Psychiatry. 2006;59:477–480. doi: 10.1016/j.biopsych.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-Releasing Factor-1 Receptor Activation Mediates Nicotine Withdrawal-Induced Deficit in Brain Reward Function and Stress-Induced Relapse. Biol Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Lapp CA, Westbrooks KL, Ernsberger P. Role of medullary I1-imidazoline and alpha 2-adrenergic receptors in the antihypertensive responses evoked by central administration of clonidine analogs in conscious spontaneously hypertensive rats. J Pharmacol Exp Ther. 1995;273:1162–1171. [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Carlezon WA., Jr Place conditioning to study drug reward and aversion. Methods Mol Med. 2003;84:243–249. doi: 10.1385/1-59259-379-8:243. [DOI] [PubMed] [Google Scholar]
- Coupar IM. Effect of alpha 2-adrenoceptor agonists on the expression of morphine-withdrawal in rats. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:553–557. doi: 10.1007/BF00168948. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Dong J, Blier P. Modification of norepinephrine and serotonin, but not dopamine, neuron firing by sustained bupropion treatment. Psychopharmacology (Berl) 2001;155:52–57. doi: 10.1007/s002130000665. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, De KM, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984–1995) Anesthesiology. 1996;85:655–674. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Ernsberger P, Meeley MP, Mann JJ, Reis DJ. Clonidine binds to imidazole binding sites as well as alpha 2-adrenoceptors in the ventrolateral medulla. Eur J Pharmacol. 1987;134:1–13. doi: 10.1016/0014-2999(87)90125-7. [DOI] [PubMed] [Google Scholar]
- Fenton HM, Liebman JM. Self-stimulation response decrement patterns differentiate clonidine, baclofen and dopamine antagonists from drugs causing performance deficit. Pharmacol Biochem Behav. 1982;17:1207–1212. doi: 10.1016/0091-3057(82)90122-8. [DOI] [PubMed] [Google Scholar]
- Ferris RM, Maxwell RA, Cooper BR, Soroko FE. Neurochemical and neuropharmacological investigations into the mechanisms of action of bupropion. HCl--a new atypical antidepressant agent. Adv Biochem Psychopharmacol. 1982;31:277–286. [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exp Ther. 1999;288:88–92. [PubMed] [Google Scholar]
- Fuentealba JA, Forray MI, Gysling K. Chronic morphine treatment and withdrawal increase extracellular levels of norepinephrine in the rat bed nucleus of the stria terminalis. J Neurochem. 2000;75:741–748. doi: 10.1046/j.1471-4159.2000.0750741.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Sevilla JA, Zis AP, Hollingsworth PJ, Greden JF, Smith CB. Platelet alpha 2-adrenergic receptors in major depressive disorder. Binding of tritiated clonidine before and after tricyclic antidepressant drug treatment. Arch Gen Psychiatry. 1981;38:1327–1333. doi: 10.1001/archpsyc.1981.01780370029003. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Prolonged activation of mesolimbic dopaminergic neurons by morphine withdrawal following clonidine: participation of imidazoline and norepinephrine receptors. Neuropsychopharmacology. 2003;28:1140–1149. doi: 10.1038/sj.npp.1300161. [DOI] [PubMed] [Google Scholar]
- Georges F, Caille S, Vouillac C, Le MC, Stinus L. Role of imidazoline receptors in the anti-aversive properties of clonidine during opiate withdrawal in rats. Eur J Neurosci. 2005;22:1812–1816. doi: 10.1111/j.1460-9568.2005.04356.x. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Stetner F, Walsh BT, Raizman PS, Fleiss JL, Cooper TB, Covey LS. Heavy smokers, smoking cessation, and clonidine. Results of a double-blind, randomized trial. JAMA. 1988;259:2863–2866. [PubMed] [Google Scholar]
- Gold MS, Redmond DE, Jr, Kleber HD. Clonidine blocks acute opiate-withdrawal symptoms. Lancet. 1978;2:599–602. doi: 10.1016/s0140-6736(78)92823-4. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Stead LF, Benowitz NL. Clonidine for smoking cessation. Cochrane Database Syst Rev. 2004:CD000058. doi: 10.1002/14651858.CD000058.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF. The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol Biochem Behav. 2009;91:295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, Frederick S, Triffleman E. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55:683–690. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Beta-adrenergic antagonists attenuate somatic and aversive signs of opiate withdrawal. Neuropsychopharmacology. 1993;9:303–311. doi: 10.1038/npp.1993.66. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Honda K, Takenaka T, Shiono K, Miyata-Osawa A, Nakagawa C. Autonomic and antihypertensive activity of oral amosulalol (YM-09538), a combined alpha- and beta-adrenoceptor blocking agent in conscious rats. Jpn J Pharmacol. 1985;38:31–41. doi: 10.1254/jjp.38.31. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007:CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs) Int Clin Psychopharmacol. 1994;9(Suppl 1):19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Time-course of changes in the social interaction test of anxiety following acute and chronic administration of nicotine. Behav Pharmacol. 1999;10:691–697. doi: 10.1097/00008877-199911000-00016. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Koob GF. Hedonic Homeostatic Dysregulation as a Driver of Drug-Seeking Behavior. Drug Discov Today Dis Models. 2008;5:207–215. doi: 10.1016/j.ddmod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Kosten TA. Clonidine attenuates conditioned aversion produced by naloxone-precipitated opiate withdrawal. Eur J Pharmacol. 1994;254:59–63. doi: 10.1016/0014-2999(94)90370-0. [DOI] [PubMed] [Google Scholar]
- Liebman JM, Hall N, Prowse J. Effects of various catecholamine receptor antagonists, muscle relaxation and physical hindrance on shuttlebox self-stimulation. Pharmacol Biochem Behav. 1982;16:785–790. doi: 10.1016/0091-3057(82)90235-0. [DOI] [PubMed] [Google Scholar]
- Lin Y, de Vaca SC, Carr KD, Stone EA. Role of alpha(1)-adrenoceptors of the locus coeruleus in self-stimulation of the medial forebrain bundle. Neuropsychopharmacology. 2007;32:835–841. doi: 10.1038/sj.npp.1301145. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Carter VA, Cunningham JS, Hebert KM, Conrad DL, Wilson OB. The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 1994;115:180–184. doi: 10.1007/BF02244770. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Manion ST, Gamble EH, Li H. Prazosin administered prior to inescapable stressor blocks subsequent exaggeration of acoustic startle response in rats. Pharmacol Biochem Behav. 2007;86:559–565. doi: 10.1016/j.pbb.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Rivet JM, Gobert A, Canton H, Veiga S, Bervoets K. 5-HT1A receptors and the tail-flick response. VI. Intrinsic alpha 1A-adrenoceptor antagonist properties can mask the actions of 5-HT1A receptor agonists in the spontaneous tail-flick paradigm. J Pharmacol Exp Ther. 1994;269:121–131. [PubMed] [Google Scholar]
- Olds ME, Domino EF. Comparison of muscarinic and nicotinic cholinergic agonists on self-stimulation behavior. J Pharmacol Exp Ther. 1969;166:189–204. [PubMed] [Google Scholar]
- Ozdogan UK, Lahdesmaki J, Scheinin M. Influence of prazosin and clonidine on morphine analgesia, tolerance and withdrawal in mice. Eur J Pharmacol. 2003;460:127–134. doi: 10.1016/s0014-2999(02)02961-8. [DOI] [PubMed] [Google Scholar]
- Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Arch Intern Med. 1998;158:2035–2039. doi: 10.1001/archinte.158.18.2035. [DOI] [PubMed] [Google Scholar]
- Rylkova D, Boissoneault J, Isaac S, Prado M, Shah HP, Bruijnzeel AW. Effects of NPY and the specific Y1 receptor agonist [D-His(26)]-NPY on the deficit in brain reward function and somatic signs associated with nicotine withdrawal in rats. Neuropeptides. 2008;42:215–227. doi: 10.1016/j.npep.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther. 1994;271:1391–1398. [PubMed] [Google Scholar]
- Sellinger-Barnette MM, Mendels J, Frazer A. The effect of psychoactive drugs on beta-adrenergic receptor binding sites in rat brain. Neuropharmacology. 1980;19:447–454. doi: 10.1016/0028-3908(80)90052-0. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Gnys M, Evoniuk G, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl) 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Sparber SB, Meyer DR. Clonidine antagonizes naloxone-induced suppression of conditioned behavior and body weight loss in morphine-dependent rats. Pharmacol Biochem Behav. 1978;9:319–325. doi: 10.1016/0091-3057(78)90292-7. [DOI] [PubMed] [Google Scholar]
- Sugrue MF. Chronic antidepressant therapy and associated changes in central monoaminergic receptor functioning. Pharmacol Ther. 1983;21:1–33. doi: 10.1016/0163-7258(83)90065-7. [DOI] [PubMed] [Google Scholar]
- Timmermans PB, Schoop AM, Kwa HY, Van Zwieten PA. Characterization of alpha-adrenoceptors participating in the central hypotensive and sedative effects of clonidine using yohimbine, rauwolscine and corynanthine. Eur J Pharmacol. 1981;70:7–15. doi: 10.1016/0014-2999(81)90426-x. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Everaerd W, Cahill L, McGaugh JL, Gooren LJ. Memory for emotional events: differential effects of centrally versus peripherally acting beta-blocking agents. Psychopharmacology (Berl) 1998;138:305–310. doi: 10.1007/s002130050675. [DOI] [PubMed] [Google Scholar]
- Wagena EJ, Knipschild P, Zeegers MP. Should nortriptyline be used as a first-line aid to help smokers quit? Results from a systematic review and meta-analysis. Addiction. 2005;100:317–326. doi: 10.1111/j.1360-0443.2005.00998.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of noradrenergic system within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Psychopharmacology (Berl) 2003;170:80–88. doi: 10.1007/s00213-003-1504-0. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. Examining the clinical efficacy of bupropion and nortriptyline as smoking cessation agents in a rodent model of nicotine withdrawal. Psychopharmacology (Berl) 2007;195:303–313. doi: 10.1007/s00213-007-0902-0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Munn E. Withdrawal from chronic amphetamine elevates baseline intracranial self-stimulation thresholds. Psychopharmacology (Berl) 1995;117:130–136. doi: 10.1007/BF02245178. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ. The involvement of central noradrenergic systems and corticotropin-releasing factor in defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1990;255:1064–1070. [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;58:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]