Abstract
Objective
This project was designed to follow-up prior evidence that demonstrated a significant association between vitamin B12 transport and metabolism and the frailty syndrome in community-dwelling older women. The cross-sectional relationship between genetic variants within six candidate genes along this pathway with serum methylmalonic acid (MMA) levels and frailty was evaluated in this same population of older women.
Methods
Baseline measures were collected prior to folate fortification from 326 women in the Women’s Health and Aging Studies I and II. Odds ratios and statistical tests were estimated for single SNP and haplotype via linear regression models for serum MMA, a marker for available vitamin B12, and in logistic regression models for frailty.
Results
Fifty-six SNPs from CBS, MTHFR, MTR, MTRR, TCN1 and TCN2 genes were genotyped. Several SNPs in MTHFR, MTR and MTRR demonstrated a modest association to elevated MMA, while SNPs in TCN2 showed significant association to the frailty syndrome. TCN2 polymorphisms, particularly one SNP reported to be in perfect LD with functional variant Pro259Arg, were significantly associated with increased odds of frailty, after adjustment for age, presence of cardiovascular disease and elevated MMA (OR = 2.25, p-value =0.009).
Conclusions
Using MMA as a marker for vitamin B12, these results suggest that TCN2 gene variants may lead to decreased vitamin B12 availability, leading to reduced energy metabolism, ultimately contributing to frailty pathology. Further studies to determine the biological role of functional TCN2 polymorphisms in frailty are needed.
Keywords: Transcobalamin-II, vitamin B12, genetic association
Introduction
The geriatric syndrome of frailty poses a critical public health concern, conferring a significant vulnerability to adverse health outcomes in older persons (1–4). Age-related declines in energy metabolism, neuroendocrine, musculoskeletal, and immunological systems are likely to contribute to the clinical characteristics of frailty and to the increases in disability, disease and death observed in frail older adults (2, 5). Reduced mitochondrial function and DNA damage have been hypothesized to contribute to these multisystem declines (6). Similarly, dysregulation of one-carbon transfer triggers activation of inflammatory pathways via mitochondrial dysfunction and increased oxidative stress with resulting DNA damage, making these vitamins important targets for studying frailty (7).
One-carbon transfer pathways rely on sufficient stores of vitamin B12 for efficiency of folate-dependent one-carbon transfers. Reduced vitamin availability along with decreased enzyme activity due to genetic variants disrupt these processes, causing increased serum homocysteine (tHcy), DNA, RNA, protein hypomethylation and decreased glutathione production (7). Genes coding for proteins regulating one-carbon transfer have been associated with reduced methylation, increased DNA damage, and increased risk of cardiovascular disease, osteoporosis and a number of cancers (8–12). For example, sequence variation in the gene encoding vitamin B12 transport protein has been associated with markers of functional vitamin B12, such as methylmalonic acid (MMA) and may influence vitamin availability to these one-carbon pathways (13, 14).
We have previously shown a cross-sectional relationship between increased risk of frailty syndrome and insufficient stores of functional vitamin B12, as measured by elevated MMA levels (OR = 2.33, p-value = 0.02) (15). Genes that influence one-carbon transfer pathways may help to explain this relationship, possibly by preventing appropriate transport and use of vitamin B12 in one-carbon transfer and propionate metabolism. We examined six genes related to this pathway (MTHFR, MTR, MTRR, CBS, TCN1, and TCN2) for their relationship to serum MMA levels and frailty syndrome in community-dwelling women aged 70–79. We hypothesize that gene variation in vitamin B-dependent one-carbon transfer pathway genes contributes to variation in MMA levels and to risk of the frailty syndrome.
Subjects and methods
Study Population
The women included in this study were participants in the Women’s Health and Aging Study (WHAS) I and II, companion studies of community-dwelling older women. WHAS I was a prospective cohort of women age 65 years and older designed to sample the 1/3 most disabled women in a 12 zip code area of Baltimore, MD (16). After screening, 1,002 of 1,409 eligible women consented to participate, 78% of whom consented to baseline blood draw. WHAS II sampled the 2/3 least disabled women, age 70–79, in the WHAS I catchment area using the same sampling frame (17). There were 436 who consented to participate, 90% of whom consented to baseline anthropometry. Standardized data collected upon interview included demographics, self-report on physical function and prevalent diseases, and performance-based functional measures. Due to the complementary nature of the research designs, the WHAS cohorts can be joined for analysis. Differences in inclusion standards between the cohorts restrict the combined analyses to women between the ages of 70–79 who had a Mini-Mental State Exam (MMSE) above 23 and consented to a blood draw at baseline, resulting in a final sample size of 416 women, 326 Caucasian and 90 African American (18). All blood was obtained prior to food folate fortification in the United States. Characteristics of these women have been previously presented (15).
Blood Measurements
Non-fasting blood samples were collected, serum prepared and frozen at the Johns Hopkins Core Facility and shipped frozen to the University of Colorado Health Sciences Center for measurement. Serum methylmalonic acid (MMA) was assayed through stable isotope dilution capillary gas chromatography/mass spectrometry with selected ion monitoring (19). Normal range, previously established using 60 ‘normal’ blood donors age 18–65 with an equal number of males and females, was defined within two standard deviations of the mean after log normalization (73–271 nmol/L) (20). Serum folate levels were measured at Corning Clinical Laboratories (Ciba-Corning Diagnostics Corporation, Medfield, MA.). The normal range of folate was reported as 6.8–36.0 nmol/liter. Serum creatinine was also assayed at Corning Clinical Labs by routine methods. The normal range for creatinine was reported as 0.6–1.1 mg/dL for females. Log transformation was carried out to normalize MMA. Biomarkers tested as potential confounders were dichotomized as normal versus abnormal based on reported normal ranges: low serum folate (< 5.7 nmol/L) and elevated creatinine (> 1.2 mg/dL) (19, 21).
Phenotype Measurements
Frailty was defined by previously validated five-component criteria that include weight, grip strength, endurance, physical activity and walking speed (2, 5). Women were evaluated at baseline for the following frailty measures: 1) shrinking, defined as either body mass index less than 18.5 kg/m2 or greater than 10% loss of body weight since age 60; 2) weakness, defined as the lowest quintile of grip strength of the dominant hand assessed with a hand-held JAMAR dynamometer (model BK-7498; Fred Sammons Inc, Burr Ridge, IL); 3) poor endurance and energy, defined as self-report of being either more tired or weaker than usual in the past 30 days; 4) slowness, defined as the lowest quintile in time to complete a 4 meter walk; and 5) low activity level, defined by the lowest quintile of self-report of weekly activity determined by a subset of questions from the Minnesota Leisure Activity Questionnaire. Lowest quintile cutoffs were established using the female population of the Cardiovascular Health Study (CHS) cohort (2). Frailty was defined as the presence of three or more of these five criteria, while women with one or two criteria were considered prefrail, or intermediate, and women with no criteria were labeled not frail, or robust.
Gene Variant Measurement and Statistical Analysis
Six candidate genes related to one-carbon metabolism were included in this study: MTHFR, MTR, MTRR, CBS, TCN1 and TCN2. Single nucleotide polymorphisms (SNPs) within each gene were selected using information from available databases at the time of design (August, 2005) (22, 23). A final list of SNPs was determined based on high probability of being successfully genotyped (> 0.80), heterozygosity (> 0.10), and dense coverage across gene (5 kb density in regions of low linkage disequilibrium (LD) (solid spine of LD D′ < 0.80) and 20 kb density in regions of high LD (D′ > 0.80)).
DNA samples were extracted from whole blood using Puregene isolation kits (Gentra Systems, Inc.) and stored in a −80°C freezer. Sample plating and genotyping were carried out as part of a larger frailty candidate gene SNP project of the Claude B. Pepper Older Americans Independence Center at Johns Hopkins University using Illumina BeadArray technology (24, 25).
Since SNP allele frequencies and LD characteristics may differ by self-reported race, all statistical analyses in this report were stratified by race. Allele and genotype frequencies were calculated and tests of Hardy-Weinberg equilibrium were performed for each SNP per race. Pairwise LD values (D′ and r2 statistics) were calculated for each pair of SNPs within a particular gene.
Multivariate linear regression models were used to assess the relationship between individual SNPs and ln(MMA), adjusted for age and low serum folate. Multinomial logistic regression models evaluated the association between SNP and frailty (3 levels: robust, pre-frail, frail), adjusting for age, presence of CVD and elevated MMA. Inheritance model-independent analyses were first tested in which heterozygous genotypes and homozygous rare allele genotypes were separately compared to homozygous common allele genotypes. Statistical significance was assessed via 2-df likelihood ratio tests (LRT) comparing models with both heterozygous and homozygous indicator variables to those where genotype effects were constrained to zero. Regression estimates from statistically significant tests were used to infer and refit appropriate genetic inheritance models such as dominant, recessive or additive inheritance. Effect modification by relevant vitamin deficiencies per candidate gene was also assessed. Single SNP tests were carried out using STATA version 9.0 (26).
Haplotype block structure for each gene was estimated from the observed data using the Haploview program (27). Haplotype blocks were defined according to the solid spine of LD model (D′ > 0.80), and haplotypes within each block with estimated frequencies < 1% were pooled for analysis. Adjusted haplotype association tests were performed using the Haplo.Stats (haplo.glm) software in the R statistical environment (28, 29) which employs generalized linear models to estimate regression coefficients of serum MMA or frailty on haplotype pair status. Global score statistics were calculated using the haplo.score function. Since multinomial logistic models are not accommodated in current haplotype software, frailty was evaluated via two independent binary logistic regression models, the first testing the odds of being prefrail compared to robust and the second testing the odds of being frail compared to robust.
Results
Given the extremely small set of African American participants (n=90), this report focuses on results from 326 Caucasian women. On average the WHAS Caucasian women were 74.1 years of age, were diagnosed with 2 chronic conditions, and had mean serum MMA of 238.7 nmol/L (SD = 157.1, range of values= 79.0–1,427.0). Elevated MMA (> 271 nmol/L) was observed in 25.2% of women, while low serum folate and elevated creatinine were present in 17.5% and 11.0% of subjects, respectively.
There were 55 SNPs successfully genotyped in the six candidate genes. The average distance between SNPs was 3.75 kb per gene. Two MTR SNPs (rs10925250, rs10925257) were slightly out of Hardy-Weinberg equilibrium (p-values = 0.03), but were included in subsequent analyses. Haplotype block length was highly variable, ranging from 0.2 to 106.6 kb.
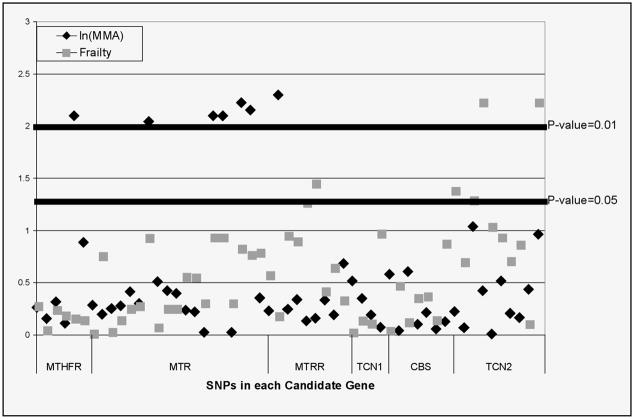
Significant associations between ln(MMA) and SNPs in genes MTHFR, MTR and MTRR were observed in our multiple linear regression models (Figure 1), adjusted for age and low serum folate. The common functional variant C677T SNP of MTHFR best fit a recessive model, with the TT genotype significantly associated with 56.9 nmol/L lower MMA compared to CT and CC individuals (p-value = 0.005). Likewise, the G allele of MTRR functional variant A66G carried a 55.7 nmol/L increase in MMA compared to AA individuals under a dominant model (p-value = 0.009). Across the MTR gene, heterozygotes at multiple SNPs high LD with each other had a 38–42 nmol/L decrease in serum MMA, compared to either type of homozygote.
Figure 1.
Summary of results of single SNPs for all candidate genes for ln(MMA) and frailty. The −log10(p-value) values are based on likelihood ratio tests comparing regression models with confounders and genetic variables to model with confounders. Candidate genes include 5,10-methylenetetrahydrolfolate reductase (MTHFR), methionine synthase (MTR), methionine synthase reductase (MTRR), transcobalamin I (TCN1), cystathionine-beta-synthase (CBS) and transcobalamin II (TCN2)
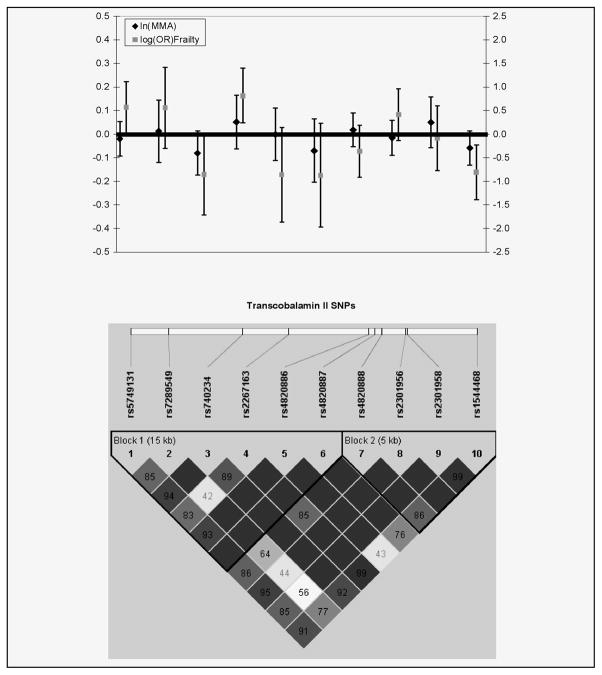
We next examined whether variation in these vitamin B-dependent one-carbon pathways genes contributes to risk for frailty. SNPs in the TCN2 gene showed significant association with frailty syndrome, after adjustment for age, presence of cardiovascular disease and elevated MMA (Figure 1). In particular, in an additive genetic model rs2267163 demonstrated a 2.29 increased odds of being frail compared to being robust (p-value= 0.006) (Figure 2). Other typed SNPs in high LD with rs2267163 were observed to have similar effect sizes (Figure 2). A non-significant decrease in odds of being prefrail compared to robust was also observed for SNP rs22671634 (OR = 0.76, p-value = 0.13). Two SNPs in the MTRR gene also showed 2–4 times greater odds of being frail compared to robust, although these associated SNPs appeared to be unrelated to the MTRR SNP associated with MMA levels. No evidence of effect modification by MMA or low serum folate was observed with TCN2 or MTRR gene SNPs. Results of global and haplotype specific regression models for ln(MMA) and frailty were consistent with single SNP tests (results not shown).
Figure 2.
Beta estimates with 95% confidence intervals for TCN2 for change in ln(MMA) and ln(odds ratio) of frailty with corresponding LD plot
Discussion
We have shown association between genetic variation in genes that influence one-carbon metabolism and vitamin B12 availability, as measured by MMA. Polymorphisms in the MTR gene and the G allele of MTRR’s functional A66G variant were associated with higher MMA, representing poorer vitamin B12 status and consistent with previous findings (30, 31). However, the TT genotype of MTHFR’s functional C677T polymorphism was associated with decreased MMA, interpreted as better B12 status, a finding contrary to previous findings (8, 10). None of these polymorphisms appear to be related to frailty, suggesting that the folate-dependent one-carbon metabolism does not play a role in frailty pathogenesis.
MTRR and TCN2 polymorphisms exhibited significant association with odds of frailty, after adjusting for age elevated MNA and presence of CVD. TCN2 SNP genotypes that were associated with increased odds of frailty also demonstrated a 15–22 nmol/L increase in MMA levels (Figure 2), although these associations did not reach statistical significance (p-values = 0.10). However, our study is likely to be underpowered to detect a modest effect difference among genotype groups. The trend in the data may suggest that vitamin B12 transport and absorption mechanisms contribute decreased vitamin B12 availability and to increased risk to the frailty syndrome. MTRR polymorphisms did not seem to demonstrate any consistent relationship with MMA levels.
The transcobalamin-II (TC) protein plays a critical role in vitamin B12 transport and absorption and TC deficiency has been linked to megaloblastic anemia and neurological disorders (7). Failure to bind to and utilize available vitamin B12 could impair the conversion of methylmalonyl-CoA to succinyl-CoA in propiate metabolism, leading to increased levels of circulating MMA, particularly in skeletal muscle (32). In a methylmalonyl-CoA mutase knock-out mouse model, Chandler et al observed significant increases in MMA over time in methylmalonyl-CoA mutase knock-out mice compared to wild-type mice (32). This increase of MMA in skeletal muscle could represent a considerable decrease in energy metabolism, ultimately leading to the activation of inflammatory pathways that contribute to frailty syndrome.
Interestingly, previous work has observed an association between a coding SNP of TCN2 (G775C) that substitutes arginine (R) for a proline (P) at codon 259 and lower serum MMA, higher TC and higher tHcy concentrations in older adults (13, 33). In those studies, proline (P) carriers had lower serum MMA and TC and higher tHcy concentrations, suggesting more efficient vitamin B12 transport and binding mechanisms versus R allele homozygotes who may be more susceptible to vitamin B12 deficiency. This coding SNP is completely correlated (r2=1) with our SNP (rs2267163) most strongly associated with frailty, suggesting this coding SNP may be the functional variant in our study. While the P259R variant is not in a region hypothesized to form the hydrophobic pocket for vitamin B12 binding, protein structure algorithms predict that it may affect binding through alteration of the secondary structure of TC (34, 35). Additionally, replacement of neutral amino acid, proline, by positively charged arginine may inhibit TC expression (36). Further research should focus on genotyping this variant.
The cross-sectional findings of this study were observed in a cohort of community-dwelling Caucasian women between the ages of 70 and 79 and should be carefully interpreted. While serum MMA has been shown to be a good marker of vitamin B12 status, we did not have cobalamin-related dietary data or holo-transcobalamin II (holoTC) complex measurements, which could have improved measurement further. Since candidate genes selection was based on a priori scientific evidence regarding one-carbon metabolism and pathophysiology of the frailty syndrome, interpretation of each statistical test must allow for qualitative differences among tests, based on causal reasoning (37). Therefore, we did not correct further for multiple testing. Nonetheless, independent replication of the association between TCN2 gene variation and frailty syndrome in another cohort is necessary. Structural equation modeling could also be used to test the relationships among TCN2 gene variation, serum MMA and frailty. Lastly, a larger sample is needed to evaluate these candidate genes in African Americans. Despite these limitations, our results suggest genes of vitamin B12 transport may be important in the availability of vitamin B12 and can ultimately contribute to the risk of frailty via mechanisms of reduced energy metabolism. Furthermore, the results of this study may help identify valuable targets for pharmacogenetic interventions in frailty.
Acknowledgments
Supported by: National Institute on Aging, Claude D. Pepper Older American Independence Centers, Grant P30 AG021334, and NIH-NIA grants AG-09834 (to SPS) and T32-AG-000–247 and T32-AG-000–120 (to AMM). The Women’s Health and Aging Study was supported by contract N01-AG-1–2112 from the National Institute on Aging. Quest Diagnostics provided funding for phlebotomy and general laboratory analysis.
References
- 1.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8(1):1–17. [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Walston J. Frailty and Failure to Thrive. In: Hazzard WR, et al., editors. Principles of Geriatric Medicine and Gerontology. McGraw Hill; New York: 1999. pp. 1387–1402. [Google Scholar]
- 4.Studenski S, Hayes RP, Leibowitz RQ, et al. Clinical Global Impression of Change in Physical Frailty: development of a measure based on clinical judgment. J Am Geriatr Soc. 2004;52(9):1560–6. doi: 10.1111/j.1532-5415.2004.52423.x. [DOI] [PubMed] [Google Scholar]
- 5.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 6.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 7.Depeint F, Bruce WR, Shangari N, Mehta R, O’Brien PJ. Mitochondrial function and toxicity: role of B vitamins on the one-carbon transfer pathways. Chem Biol Interact. 2006;163(1–2):113–32. doi: 10.1016/j.cbi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Kluijtmans LA, I, Young S, Boreham CA, et al. Genetic and nutritional factors contributing to hyperhomocysteinemia in young adults. Blood. 2003;101(7):2483–8. doi: 10.1182/blood.V101.7.2483. [DOI] [PubMed] [Google Scholar]
- 9.Botto N, Andreassi MG, Manfredi S, et al. Genetic polymorphisms in folate and homocysteine metabolism as risk factors for DNA damage. Eur J Hum Genet. 2003;11(9):671–8. doi: 10.1038/sj.ejhg.5201024. [DOI] [PubMed] [Google Scholar]
- 10.Andreassi MG, Botto N, Cocci F, et al. Methylenetetrahydrofolate reductase gene C677T polymorphism, homocysteine, vitamin B12, and DNA damage in coronary artery disease. Hum Genet. 2003;112(2):171–7. doi: 10.1007/s00439-002-0859-3. [DOI] [PubMed] [Google Scholar]
- 11.Ma J, Stampfer MJ, Christensen B, et al. A polymorphism of the methionine synthase gene: association with plasma folate, vitamin B12, homocyst(e)ine, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 1999;8(9):825–9. [PubMed] [Google Scholar]
- 12.Aras O, Hanson NQ, Yang F, Tsai MY. Influence of 699C-->T and 1080C-->T polymorphisms of the cystathionine beta-synthase gene on plasma homocysteine levels. Clin Genet. 2000;58(6):455–9. doi: 10.1034/j.1399-0004.2000.580605.x. [DOI] [PubMed] [Google Scholar]
- 13.Miller JW, Ramos MI, Garrod MG, Flynn MA, Green R. Transcobalamin II 775G>C polymorphism and indices of vitamin B12 status in healthy older adults. Blood. 2002;100(2):718–20. doi: 10.1182/blood-2002-01-0209. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann W, Obeid R, Schorr H, Geisel J. Functional vitamin B12 deficiency and determination of holotranscobalamin in populations at risk. Clin Chem Lab Med. 2003;41(11):1478–88. doi: 10.1515/CCLM.2003.227. [DOI] [PubMed] [Google Scholar]
- 15.Matteini AM, Walston JD, Fallin MD, et al. Markers of B-vitamin deficiency and frailty in older women. J Nutr Health Aging. 2008;12(5):303–8. doi: 10.1007/BF02982659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guralnik JM, Fried LP, Simonsick EM, Kasper D, Lafferty ME. NIo Aging. NIH Publication No. 95–4009. National Institute on Aging; Bethesda, MD: 1995. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. [Google Scholar]
- 17.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55(1):M43–52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Elevation of 2-methylcitric acid I and II levels in serum, urine, and cerebrospinal fluid of patients with cobalamin deficiency. Metabolism. 1993;42(8):978–88. doi: 10.1016/0026-0495(93)90010-l. [DOI] [PubMed] [Google Scholar]
- 20.Stabler SP, Allen RH, Fried LP, et al. Racial differences in prevalence of cobalamin and folate deficiencies in disabled elderly women. Am J Clin Nutr. 1999;70(5):911–9. doi: 10.1093/ajcn/70.5.911. [DOI] [PubMed] [Google Scholar]
- 21.Stabler SP, Lindenbaum J, Savage DG, Allen RH. Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood. 1993;81(12):3404–13. [PubMed] [Google Scholar]
- 22.HapMap IC. A haplotype map of the human genome. 2005;437(7063):1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.HapMap IC. The International HapMap Project. Nature. 2003;426(6968):789–96. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 24.Oliphant A, Barker DL, Stuelpnagel JR, Chee MS. BeadArray technology: enabling an accurate, cost-effective approach to high-throughput genotyping. Biotechniques. 2002;(Suppl):56–8. 60–1. [PubMed] [Google Scholar]
- 25.Gunderson KL, Kruglyak S, Graige MS, et al. Decoding randomly ordered DNA arrays. Genome Res. 2004;14(5):870–7. doi: 10.1101/gr.2255804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stata C. StataCorp. Intercooled Stata 9.0 for Windows. StataCorp LP; College Station, TX: 1984. [Google Scholar]
- 27.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 28.Lake SL, Lyon H, Tantisira K, et al. Estimation and tests of haplotype-environment interaction when linkage phase is ambiguous. Hum Hered. 2003;55(1):56–65. doi: 10.1159/000071811. [DOI] [PubMed] [Google Scholar]
- 29.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70(2):425–34. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yates Z, Lucock M. Methionine synthase polymorphism A2756G is associated with susceptibility for thromboembolic events and altered B vitamin/thiol metabolism. Haematologica. 2002;87(7):751–6. discussion 756. [PubMed] [Google Scholar]
- 31.Laraqui A, Allami A, Carrie A, et al. Influence of methionine synthase (A2756G) and methionine synthase reductase (A66G) polymorphisms on plasma homocysteine levels and relation to risk of coronary artery disease. Acta Cardiol. 2006;61(1):51–61. doi: 10.2143/AC.61.1.2005140. [DOI] [PubMed] [Google Scholar]
- 32.Chandler RJ, Sloan J, Fu H, et al. Metabolic phenotype of methylmalonic acidemia in mice and humans: the role of skeletal muscle. BMC Med Genet. 2007;8:64. doi: 10.1186/1471-2350-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namour F, Olivier J, Abdelmouttaleb I, et al. Transcobalamin codon 259 polymorphism in HT-29 and Caco-2 cells and in Caucasians: relation to transcobalamin and homocysteine concentration in blood. Blood. 2001;97(4):1092–8. doi: 10.1182/blood.v97.4.1092. [DOI] [PubMed] [Google Scholar]
- 34.Li N, Seetharam S, Lindemans J, et al. Isolation and sequence analysis of variant forms of human transcobalamin II. Biochim Biophys Acta. 1993;1172(1–2):21–30. doi: 10.1016/0167-4781(93)90264-e. [DOI] [PubMed] [Google Scholar]
- 35.Afman LA, Lievers KJ, van der Put NM, Trijbels FJ, Blom HJ. Single nucleotide polymorphisms in the transcobalamin gene: relationship with transcobalamin concentrations and risk for neural tube defects. Eur J Hum Genet. 2002;10(7):433–8. doi: 10.1038/sj.ejhg.5200830. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Sood GK, Seetharam S, Seetharam B. Polymorphism of human transcobalamin II: substitution of proline and/or glutamine residues by arginine. Biochim Biophys Acta. 1994;1219(2):515–20. doi: 10.1016/0167-4781(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 37.Goodman SN. Multiple comparisons, explained. Am J Epidemiol. 1998;147(9):807–12. doi: 10.1093/oxfordjournals.aje.a009531. discussion 815. [DOI] [PubMed] [Google Scholar]