Abstract
Background
Leukocyte telomere length (LTL) is relatively short in individuals who have evidence of cardiovascular disease.
Objective
To examine the link between LTL and the predisposition to atherosclerosis, as determined by carotid artery intimal medial thickness (IMT) in participants of the Framingham Offspring Study.
Methods
LTL was assayed by the mean length of the terminal restriction fragments and carotid artery IMT by B-mode ultrasonography in 1062 individuals (496 men, 566 women) aged 33–86 years,
Results
In the whole sample, there was a significant association of age-and sex-adjusted LTL with internal carotid artery IMT (ICA-IMT)(r= −0.07, p= 0.02). In sex-stratified analysis, this association remained significant for men (r= −0.11, p= 0.02) but not for women (r= −0.04, p= 0.36). After further adjustment for cigarette smoking and BMI, a borderline significant association persisted in men (p= 0.06). In secondary analysis, the age-adjusted LTL was significantly (and negatively) associated with ICA-IMT (r= −0.28, p 0.0006) in obese (BMI > 30kg/m2) men but not in non-obese (BMI ≤ 30 kg/m2) men. In addition, age-adjusted LTL was significantly shorter in men (6.89 ± 0.02 kb) than women (7.02 ± 0.02 kb) (p< 0.0001) and in current cigarette smokers (6.87±0.05 kb) than never smokers (6.99±0.03 kb) (p = 0.02). Although there was no significant association of LTL with common carotid artery-IMT or with carotid artery stenosis, there was a significant inverse association of LTL with common carotid artery IMT in obese men.
Conclusion
In obese men, shortened LTL is a powerful marker of increased carotid IMT. Given the public health impact of atherosclerosis and in particular the current epidemic of obesity, the associations noted in obese men warrant further confirmation.
Keywords: Telomeres, atherosclerosis, leukocytes, obesity, sex, smoking
INTRODUCTION
Leukocyte telomere length (LTL) is shorter in individuals with atherosclerotic cardiovascular disease (CVD) than in their peers.1–5 In addition, individuals with increased CVD risk display relatively short LTL, particularly men (compared with women),4, 6–8 cigarette smokers,8, 9 and individuals with insulin resistance and high BMI.4, 9–11 These observations support the thesis that relatively short LTL is an index of both subclinical and overt atherosclerotic CVD. One measure of atherosclerosis that has been studied extensively is carotid intimal medial thickness (IMT) determined by B-mode ultrasound. The IMT of both internal carotid artery (ICA) and common carotid artery (CCA) are associated with incident myocardial infarction and stroke, after adjustment for major cardiovascular risk factors.12–14 However, increased IMT of the ICA differs from that of the CCA in that it primarily represents atherosclerotic plaques,15 while in the CCA such an increase may represent vascular hypertrophy in response to shear stresses.16, 17 Indeed, thickening of the intima of the ICA appears to be more strongly associated than that of the CCA with increased risk for incident atherosclerotic CVD.15, 18
Atherosclerosis and vascular aging in general are protracted processes in which inflammation19 and oxidative stress20 play central roles. Most indices of inflammation and oxidative stress derived from blood samples are snapshots of the metabolic status at the time of sample collection. In contrast, LTL is apparently a record of the cumulative burden of inflammation and oxidative stress over the individual’s life course.21 This is the key reason for the use of LTL as a gauge of a host of aging-related disorders, including atherosclerotic CVD.
Two studies have examined the association of LTL with carotid IMT,4, 22 but the sample sizes of these studies were relatively small and there were no significant multivariable-adjusted associations between LTL and measures of IMT in either study. Thus, a detailed, larger study is warranted to further characterize the nexus between LTL and the IMT of the ICA and the CCA. To this end, we studied participants of the Framingham Offspring Study with available LTL and carotid artery IMT parameters.
METHODS
Study Sample
The Framingham Offspring Study began in 1971 with the enrollment of 5124 men and women. It comprised offspring of the Original Cohort of the FHS and the spouses of the offspring. The members of the Framingham Offspring Study underwent repeated examinations every 4–8 years, and details of the selection of these participants have been previously described.23, 24 A total of 3532 participants of the Offspring Cohort attended examination cycle 6 (1995 to 1998) and underwent carotid ultrasonography. LTL was measured in 1244 subjects. Out of these, we excluded 105 subjects who did not have IMT measurement data. As insulin resistance is associated with LTL10, 11 and treatment of hyperglycemia may impact this association, we also excluded 77 subjects who were treated for diabetes. Thus, data from 1062 subjects were analyzed for this report.
Carotid Ultrasonography
Ultrasonographic measurements were performed using a Toshiba SSH-140A imaging unit with a 7.0 MHz transducer for the common carotid artery (CCA) and a 5.0 MHz transducer for the internal carotid artery (ICA), as previously described.25 Measurements of the right and left arteries were obtained from longitudinal views of both the distal CCA (at end-diastole and end-systole) and the ICA at end-diastole (each measured twice). Measurements performed by the sonographer were reread by a radiologist, with both individuals blinded to clinical information.
Interpretation and quantitative measurement of imaging studies was performed using a standardized protocol. Near- and far-wall IMT, lumen diameter, and vessel width were calculated at the ICA and CCA using high-resolution images uploaded into a specialized computer software analysis package as previously described.14, 25 IMT of the CCA and the ICA were defined as the mean of the mean IMT measurements for the right and left sides, as reported previously. Replicate readings (n=25) by two independent interpreters showed intra-class correlation coefficients for mean maximum ICA and CCA IMT of 0.74 and 0.90, respectively.26, 27
Determination of LTL
LTL was derived from the mean of the terminal restriction fragment length (TRFL), measured by Southern blot analysis. Samples were digested overnight with restriction enzymes digest set, HinfI (5.2 U)/Rsa I (5.2 U) (Roche). DNA samples (2 μg each) and DNA ladders (1 kb DNA ladder plus 23.1kb fragment of λ DNA/Hind III fragments (Invitrogen, Carlsbad, CA)) were resolved on a 0.5% agarose gel (20 cm × 20 cm) at 50 V (GNA-200 Pharmacia Biotech). After 16 hr, the DNA was depurinated for 15 min in 0.25 N HCl, denatured 30 min in 0.5 mol/L NaOH/1.5 mol/L NaCl and neutralized for 30 min in 0.5 mol/L Tris, pH 8/1.5 mol/L NaCl. The DNA was transferred for 1 hr to a positively charged nylon membrane (Roche) using a vacuum blotter (Boeckel Scientific, Feasterville, PA). The membranes were spotted at 4 sites with diluted telomeric probe [digoxigenin 3′-end labeled 5′-(CCTAAA)3] and then hybridized at 65°C with the probe overnight in 5 × SSC, 0.1% Sarkosyl, 0.02% SDS and 1% blocking reagent (Roche). The membranes were washed 3 times at room temperature in 2 × SSC, 0.1% SDS each for 15 min and once in 2 × SSC for 15 min. The digoxigenin-labeled probe was detected by the digoxigenin luminescent detection procedure (Roche) and exposed on X-ray film. After scanning the terminal restriction fragment signal by densitometry, the membrane was stripped and re-probed with a molecular weight marker probe. The merging of the two x-ray films, using the 4 spotted sites of telomeric probe yields minimized variation in DNA migration in different lanes. The coefficient of variation (CV) for this approach (for samples measured in duplicate or triplicate on different gels and occasions and by two researchers) was 2.4%. All measurements of LTL were performed ‘blindly.’ Upon completion of the measurements, the LTL data were electronically transmitted to the FHS and merged with relevant parameters.
Clinical and Environmental Measurements and Definitions
At examination 6, offspring participants underwent a physician-administered history and physical examination that included standardized measurements of blood pressure in an upright seated position, after a period of rest, using a mercury sphygmomanometer. Systolic and diastolic blood pressures were the average of two separate readings performed by a physician. Pulse pressure (mm Hg) was calculated as the differences between the systolic and diastolic blood pressures. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or the use of anti-hypertensive medications. Fasting blood was used for measurements of lipids, including total cholesterol, HDL cholesterol, and serum triglyceride concentration. Body mass index (BMI) was calculated as the weight in kilograms measured in light clothing, divided by the height in meters squared. Cigarette smoking status was defined as current (having smoked at least one cigarette per day over the past year prior to the exam), past (past smokers), and never (subjects who had never smoked cigarettes). Diabetes was defined as a fasting blood glucose ≥126 mg/dL or treatment with oral hypoglycemic agents or insulin.
Statistical Analysis
The values of mean ICA and CCA thicknesses were log-transformed to normalize their distributions. Sex-specific and sex-pooled analyses were performed to describe the data and to test the association of LTL with log CCA or log ICA IMT thickness. Means ± SD, for continuous variables, and proportions, for categorical variables, were computed for all study subjects and for men and women, separately. Variables were compared between men and women using two-sample t-tests for continuous variables and chi-square tests for categorical variables. To evaluate the relationships between LTL and IMT measures we performed linear regression analyses using LTL as a dependent variable; log CCA or log ICA IMT thickness as predictor variables; and age, sex (in combined analyses), body mass index (BMI), cigarette smoking status, pulse pressure, diabetic status, total cholesterol, high density lipoprotein cholesterol, triglycerides, cholesterol treatment, C-reactive protein, and hormone replacement therapy and oral contraceptive use in women as candidate covariates. To describe these associations we also computed partial correlation coefficients. Our final regression models included the following covariates: 1) age and sex adjusted; 2) age, sex, smoking status; 3) age, sex, smoking status, and BMI; 4) age, sex, smoking status, BMI and pulse pressure. The other covariates were excluded because they were not significant contributors independent of the other covariates in the multivariable model. Given the existence of marginally significant associations of LTL with IMT measures, secondary stratified analyses were performed by clinically important variables hypothesized to be potentially important modifiers of the association between LTL and carotid IMT based upon prior literature, in particular, CVD status,1, 4 current smoking and obesity status.9 Results with p-values less than 0.05 were considered to be statistically significant. Analyses were performed using SAS version 8.12 (SAS Institute, Inc., Cary, NC).
Results
General Characteristics
The baseline characteristics of the 1062 FHS Offspring men (n=496) and women (n=566) in the current study are described in Table 1. Men in the cohort were slightly older than women (mean age 59.2 years for men and 58.6 years for women, respectively). Current cigarette smoking was observed in 14% of the overall cohort and there were significant sex differences: current smoking was slightly lower in men compared with women (13% versus 15%, respectively), but rates of past smoking were substantially higher in men versus women (56% versus 47%, respectively). In addition, men had a modest but significantly higher BMI than women (28.5 versus 27.4 kg/m2, respectively).
Table 1.
General Characteristics of Study Subjects
| Men and Women (n =1062) | Men (n =496) | Women (n =566) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean ± SD or N (%) | Min | Max | n | Mean ± SD or N(%) | n | Mean ± SD or N(%) | n | ** P-Value |
| Age (years)a | 59.1± 9.4 | 33 | 86 | 1062 | 59.6 ± 9.6 | 496 | 58.6 ± 9.2 | 566 | 0.08 |
| Smoking | 0.01 | ||||||||
| Currentb | 143 (13.5) | 1062 | 63 (12.7) | 496 | 80 (14.1) | 566 | |||
| Pastb | 545 (51.3) | 1062 | 280 (56.5) | 496 | 265 (46.8) | 566 | |||
| Neverb | 374 (35.2) | 1062 | 153 (30.8) | 496 | 221 (39) | 566 | |||
| Body Mass Index (kg/m2)a | 27.7 ± 5.1 | 17.2 | 53 | 1061 | 28.2 ± 4.4 | 496 | 27.2 ± 5.5 | 565 | <0.001 |
| Obese (BMI≥ 30 kg/m2)b | 285 (26.9) | 1061 | 144 (29) | 496 | 141 (25) | 565 | 0.14 | ||
| Systolic blood pressure (mm Hg)a | 129.2 ± 18.9 | 77 | 212 | 1062 | 130.5 ± 17.7 | 496 | 128.1 ± 19.9 | 566 | 0.04 |
| Diastolic blood pressure (mm Hg)a | 75.5 ± 9.3 | 49 | 106 | 1062 | 77.5 ± 9 | 496 | 73.7 ± 9.3 | 566 | <0.001 |
| Pulse pressure (mm Hg)a | 53.7 ± 16 | 16 | 132 | 1062 | 53 ± 15.3 | 496 | 54.4 ± 16.6 | 566 | 0.16 |
| Hypertensionb | 436 (41.2) | 1058 | 220 (44.6) | 493 | 216 (38.2) | 565 | 0.04 | ||
| Hypertension treatmentb | 287 (27.1) | 1059 | 146 (29.6) | 494 | 141 (25) | 565 | 0.09 | ||
| Diabetesb | 79 (7.4) | 1062 | 42 (8.5) | 496 | 37 (6.5) | 566 | 0.23 | ||
| Total cholesterol (mg/dL)a | 205.8 ± 36.9 | 93 | 330 | 1062 | 199.7 ± 35.2 | 496 | 211.1 ± 37.5 | 566 | <0.001 |
| High-density lipoprotein cholesterol (mg/dL)a | 51.7 ± 15.9 | 11 | 122 | 1060 | 44.5 ± 12.3 | 494 | 58 ± 16.1 | 566 | <0.001 |
| Triglycerides (mg/dL)a | 135.3 ± 84.8 | 29 | 822 | 1062 | 143.8 ± 101.4 | 496 | 127.8 ± 66.2 | 566 | 0.002 |
| Log(Triglycerides)a | 4.8 ± 0.5 | 3.4 | 6.7 | 1062 | 4.8 ± 0.6 | 496 | 4.7 ± 0.5 | 566 | 0.04 |
| Cholesterol treatmentb | 132 (12.4) | 1062 | 74 (14.9) | 496 | 58 (10.2) | 566 | 0.02 | ||
| Prevalent Cardiovascular Diseaseb | 106 (10) | 1062 | 68 (13.7) | 496 | 38 (6.7) | 566 | <0.001 | ||
| C-Reactive proteina | 4.5 ± 9.7 | 0.2 | 250.5 | 1029 | 4.3 ± 12.6 | 481 | 4.7 ± 6.2 | 548 | 0.49 |
| Hormone replacement therapya | 175 (31) | 565 | |||||||
| Oral Contraceptivesa | 6 (1.1) | 564 | |||||||
Mean ± SD,
N (%),
Comparing men and women.
Carotid Artery Characteristics
As shown in Table 2, men had higher ICA-IMT and CCA-IMT than women. In addition, men displayed a higher prevalence of carotid artery stenosis than women.
Table 2.
Distribution of Primary Variables of Study
| Men and Women (n =1062) | Men(n =496) | Women (n =566) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean ± SD or N (%) | Min | Max | N | Mean ± SD or N (%) | N | Mean ± SD or N (%) | N | ** P-Value |
| Age-adjusted TRFLa, in bp | 6.97 ± 0.55 | 5.58 | 8.51 | 1062 | 6.89 ± 0.02* | 496 | 7.04 ± 0.022* | 566 | <0.001 |
| Stenosisb | 221 (20.8) | 1062 | 130 (26.2) | 496 | 91 (16.1) | 566 | <0.001 | ||
| ICA-IMT a, in mm | 0.56 ± 0.36 | 0.21 | 3.94 | 1062 | 0.63 ± 0.39 | 496 | 0.49 ± 0.33 | 566 | <0.001 |
| Log(ICA-IMT)c | 0.49 ± 0.47 | −0.48 | 0.50 | 1062 | 0.55 ± 0.49 | 496 | 0.44 ± 0.42 | 566 | <0.001 |
| CCA-IMT a, in mm | 0.61 ± 0.14 | 0.35 | 1.79 | 1058 | 0.64 ± 0.13 | 493 | 0.58 ± 0.13 | 565 | <0.001 |
| Log(CCA-IMT)c | 0.59 ± 0.20 | 0.59 | 0.63 | 1058 | 0.62 ± 0.20 | 493 | 0.57 ± 0.20 | 565 | <0.001 |
Mean ± SD
N (%)
Mean value = geometric mean and Min and Max values = 95% confidence bounds
Standard error
Comparing men and women.
Abbreviations: TRFL=terminal restriction fragment length, bp=base pair, ICA=internal carotid artery, CCA=common carotid artery, IMT=intimal medial thickness.
Relations of Leukocyte Telomere Length with Risk Factors
Sex-adjusted LTL was inversely correlated with age (r= −0.34, p< 0.0001), displaying attrition at a rate of 21.1 ± 1.78 (SE) bp/year. There was no statistically significant difference in age-dependent LTL attrition between women (21.5±2.5 bp/year) and men (20.7±2.5 bp/year) (sex-age interaction p= 0.815). However, age-adjusted LTL was significantly shorter in men (6.89 ± 0.02 kb) than in women (7.02 ± 0.02 kb) p< 0.0001) and in current cigarette smokers (6.87±0.05 kb, p = 0.02), but not past smokers 6.96±0.02 kb, p= 0.34), compared with never smokers (6.99±0.03 kb) (Figure 1). Additionally, an inverse association was observed for age- and sex-adjusted LTL with BMI (r= −0.08, p= 0.01). There were no significant associations of LTL with pulse pressure, total cholesterol, HDL cholesterol or triglycerides (data not shown).
Figure 1.
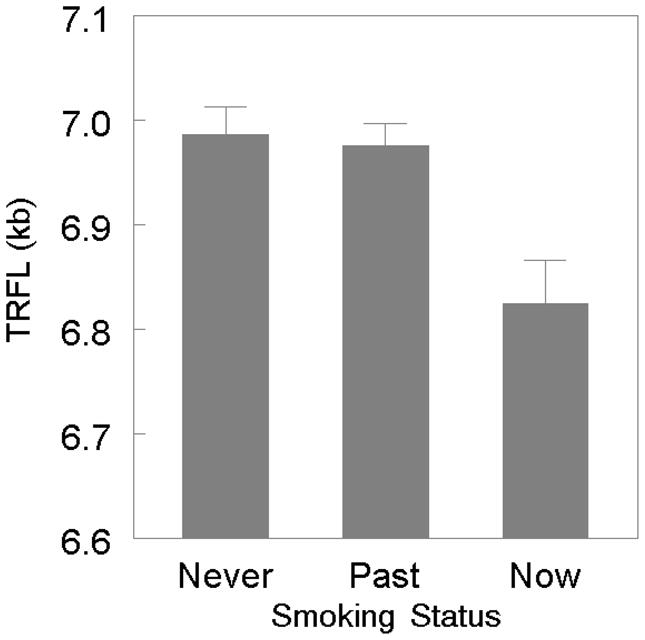
Leukocyte Telomere Length in Never-Smokers, Past-Smokers and Present-Smokers
Associations between Leukocyte Telomere Length and Carotid Artery Parameters
In the whole sample, age-adjusted LTL displayed significant association with ICA-IMT (r= −0.09, p= 0.003). As women have longer LTL and lower ICA-IMT, we adjusted LTL for both age and sex. Age- and sex-adjusted LTL also showed significant association with ICA-IMT (r= −0.07, p= 0.02). In further analyses, conducted separately by sex, this association remained significant for men (r= −0.12, p= 0.02) but not for women (r= −0.04, p= 0.36).
The associations of LTL with ICA-IMT after further multivariable-adjustment are presented in Table 3. For the entire sample, in a model adjusted for age, sex, and cigarette smoking, the association between LTL and ICA-IMT was of borderline significance (r= −0.06, p= 0.07). This association was borderline significant in men (r= −0.09, p= 0.05) but not women (r= −0.02, p= 0.58). When BMI was added to the model, the association between LTL and ICA-IMT was unchanged in men (r= −0.09, p= 0.05) and women (r= −0.01, p= 0.84). There was further attenuation of the association by the further adjustment for pulse pressure (Table 3), and the overall association was similarly attenuated in separate models adjusting for hypertension drug treatment, total or HDL cholesterol or triglycerides (data not shown).
Table 3.
Linear Regression Analysis of LTL on R2 Individual Covariates and on ICA-IMT
| ALL | MEN | WOMEN | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta Coefficient | P- value | Cumulative R2 | N | Beta Coefficient | P- value | Cumulative R2 | N | Beta Coefficient | P- value | Cumulative R2 | N | |
| Intercept | 8.004 | <0.001 | 0.00 | 1062 | 7.903 | <0.001 | 0.00 | 496 | 8.195 | <0.001 | 0.00 | 566 |
| Age(years) | −0.020 | <0.001 | 0.12 | −0.018 | 0.001 | 0.12 | −0.021 | <0.001 | 0.12 | |||
| Female | 0.093 | 0.01 | 0.13 | |||||||||
| Log(mean ICA IMT) | −0.090 | 0.02 | 0.13 | −0.125 | 0.02 | 0.13 | −0.052 | 0.36 | 0.12 | |||
| Intercept | 8.096 | <0.001 | 0.00 | 1062 | 8.044 | <0.001 | 0.00 | 496 | 8.281 | <0.001 | 0.00 | 566 |
| Age(years) | −0.021 | <0.001 | 0.12 | −0.020 | 0.00 | 0.12 | −0.021 | <0.001 | 0.12 | |||
| Female | 0.099 | 0.004 | 0.13 | |||||||||
| Current smoking status | −0.162 | 0.003 | 0.14 | −0.219 | 0.01 | 0.14 | −0.121 | 0.10 | 0.12 | |||
| Past smoking status | 0.004 | 0.91 | 0.14 | 0.037 | 0.50 | 0.14 | −0.028 | 0.58 | 0.12 | |||
| Log(mean ICA IMT) | −0.071 | 0.07 | 0.14 | −0.103 | 0.05 | 0.15 | −0.032 | 0.58 | 0.12 | |||
| Intercept | 8.335 | <0.001 | 0.00 | 1061 | 8.048 | <0.001 | 0.00 | 496 | 8.636 | <0.001 | 0.00 | 565 |
| Age(years) | −0.021 | <0.001 | 0.12 | −0.020 | <0.001 | 0.12 | −0.022 | <0.001 | 0.12 | |||
| Female | 0.092 | 0.01 | 0.13 | |||||||||
| Current smoking status | −0.162 | 0.003 | 0.14 | −0.219 | 0.01 | 0.14 | −0.119 | 0.10 | 0.12 | |||
| Past smoking status | 0.003 | 0.94 | 0.14 | 0.037 | 0.50 | 0.14 | −0.032 | 0.53 | 0.12 | |||
| BMI (kg/m2) | −0.007 | 0.03 | 0.14 | 0.000 | 0.98 | 0.14 | −0.012 | 0.01 | 0.13 | |||
| Log(mean ICA IMT) | −0.059 | 0.14 | 0.15 | −0.103 | 0.06 | 0.15 | −0.012 | 0.84 | 0.13 | |||
| Intercept | 8.450 | <0.001 | 0.00 | 772 | 8.336 | <0.001 | 0.00 | 348 | 8.671 | <0.001 | 0.00 | 424 |
| Age(years) | −0.021 | <0.001 | 0.12 | −0.018 | <0.001 | 0.11 | −0.023 | <0.001 | 0.12 | |||
| Female | 0.105 | 0.01 | 0.13 | |||||||||
| Current smoking status | −0.162 | 0.01 | 0.14 | −0.248 | 0.01 | 0.14 | −0.083 | 0.32 | 0.12 | |||
| Past smoking status | −0.014 | 0.75 | 0.14 | −0.013 | 0.84 | 0.14 | −0.016 | 0.78 | 0.12 | |||
| BMI (kg/m2) | −0.010 | 0.01 | 0.15 | −0.005 | 0.39 | 0.14 | −0.015 | 0.01 | 0.14 | |||
| Pulse Pressure | −0.001 | 0.37 | 0.15 | −0.004 | 0.07 | 0.15 | 0.001 | 0.60 | 0.14 | |||
| Log(mean ICA IMT) | −0.055 | 0.29 | 0.15 | −0.054 | 0.44 | 0.15 | −0.055 | 0.479 | 0.14 | |||
Abbreviations: ICA=internal carotid artery, IMT=intimal medial thickness, BMI=body mass index.
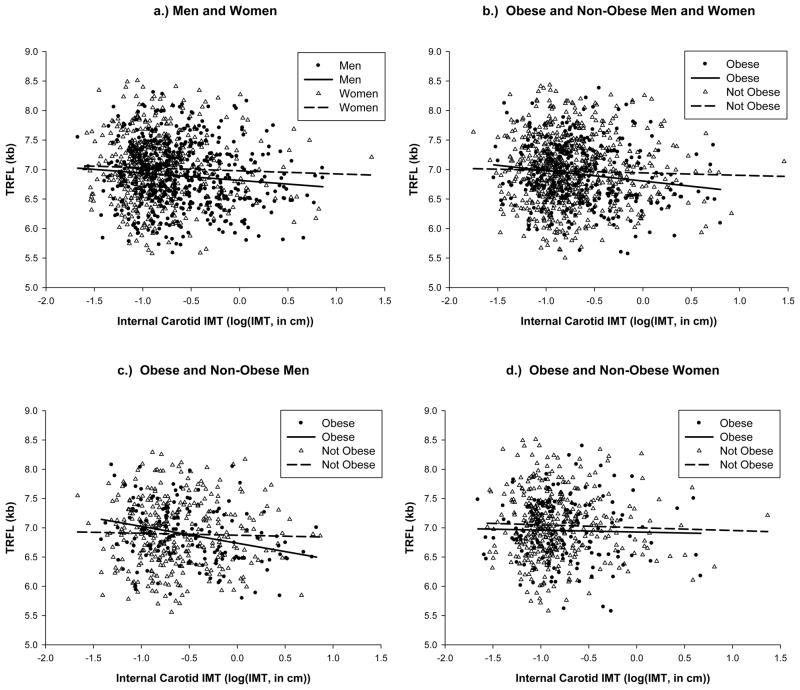
Given the consistent, albeit borderline significant association between LTL and ICA-IMT in men but not women, we undertook secondary analyses to examine for associations in subjects with obesity, cigarette smoking or prevalent CVD and for and evidence of effect modification by these parameters. For obese (BMI > 30kg/m2) compared with non-obese (BMI ≤ 30 kg/m2) men, age-adjusted LTL displayed a highly significant, negative association with ICA-IMT (r= −0.28, p= 0.0006). This association remained highly significant after adjustment for smoking (r= −0.24, p= 0.004). Further adjustment for other variables did not significantly alter the association. No significant associations between LTL and the ICA-IMT were observed in non-obese men or in women (obese and non-obese). However, in a test of interaction between obesity and ICA-IMT in men (see Figure 2c), there was no significant interaction (beta coefficient = −0.152; p= 0.14). Figure 2 displays the results of these associations as scatter plots in the combined sample, men alone and women alone, by obese and non-obese subjects. There was no evidence of strongly increased associations between LTL and ICA-IMT in those with CVD or current cigarette smoking.
Figure 2.
Age-adjusted LTL and ICA-IMT in Men and Women (2a), Obese versus Non-Obese Subjects (2b), Obese versus Non-Obese Men only (2c), and Obese versus Non-Obese Women only (2d)
There was no significant association of LTL with the CCA-IMT or with carotid artery stenosis in multivariable-adjusted models in men or women. In men but not women, there was a significant association with CCA-IMT in obese but not non-obese subjects, and the association remained significant in multivariable-adjusted models (r= −0.53, p= 0.03).
Discussion
In our community-based cohort, we found that men and current smokers had shorter age-adjusted LTL than women and never smokers, respectively. These findings are in line with previous research reporting sex4, 7, 8 and smoking8, 9 effects on LTL. In the entire cohort, a modest inverse association was noted between LTL and ICA-IMT. Upon secondary analysis for evidence of effect modification by obesity status, we found that the inverse association between LTL and ICA-IMT was highly significant in obese men, even after adjustment for BMI and cigarette smoking. The strength of such an association in obese men is underscored by the fact that age–the main determinant of LTL shortening–accounted for 12.9% of the inter-individual variation in LTL in the entire cohort and age and ICA-IMT combined accounted for 13.3% of LTL. Thus, ICA-IMT explained only 0.4% over and above age the inter-individual variation in LTL (Table 3). In obese men, however, age accounted for only 8.9%, while age and ICA-IMT combined accounted for 16.4% of the inter-individual variation in LTL (Table 4). Accordingly, increased ICA-IMT explained a substantial 7.5% over and above age of the inter-individual variation in LTL.
Table 4.
Results of Linear Regression Analysis of LTL on Individual Covariates and on ICA-IMT for Obese Men
| Beta Coefficient | P-value | Cumulative R2 | N | |
|---|---|---|---|---|
| Intercept | 7.393 | <0.001 | 0.00 | 144 |
| Age(years) | −0.011 | 0.02 | 0.09 | |
| Log(mean ICA IMT) | −0.287 | 0.001 | 0.16 | |
| Intercept | 7.606 | <0.001 | 0.00 | 144 |
| Age(years) | −0.014 | 0.01 | 0.09 | |
| Current smoking status | −0.196 | 0.15 | 0.14 | |
| Past smoking status | 0.042 | 0.65 | 0.14 | |
| Log(mean ICA IMT) | −0.240 | 0.01 | 0.19 | |
| Intercept | 7.360 | <0.001 | 0.00 | 144 |
| Age(years) | −0.014 | 0.01 | 0.09 | |
| Current smoking status | −0.193 | 0.15 | 0.14 | |
| Past smoking status | 0.044 | 0.64 | 0.14 | |
| BMI (kg/m2) | 0.006 | 0.60 | 0.14 | |
| Log(mean ICA IMT) | −0.240 | 0.01 | 0.19 | |
| Intercept | 7.522 | <0.001 | 0.00 | 91 |
| Age(years) | −0.014 | 0.06 | 0.11 | |
| Current smoking status | −0.196 | 0.24 | 0.17 | |
| Past smoking status | −0.022 | 0.86 | 0.17 | |
| BMI (kg/m2) | 0.006 | 0.64 | 0.17 | |
| Pulse Pressure | −0.003 | 0.53 | 0.18 | |
| Log(mean ICA IMT) | −0.206 | 0.12 | 0.20 | |
Abbreviations: ICA=internal carotid artery, IMT=intimal medial thickness, BMI=body mass index.
At any given time, LTL reflects birth LTL, which is highly variable among newborns,28, 29 and age-dependent LTL attrition afterward. Cross-sectional evaluation of LTL at a single point in time would, therefore, understate the effect of a given variable on LTL attrition rate. The link between insulin resistance and LTL dynamics illustrates this concept. Insulin resistance explains 28% of the variation in LTL attrition rate in a longitudinal evaluation,11 but it accounts for only 2.5% of the variation in LTL in a cross-sectional study.10 Accordingly, factors that increase the predilection of obese men to atherosclerosis, as expressed in the ICA-IMT, apparently exert a profound effect on LTL attrition rate.
Two studies have examined the association of LTL with carotid artery IMT, but they were limited to either a small number of hypertensive men or a small to modest sized elderly cohort of both men and women. In their study of 166 hypertensive men, Benetos et al observed shorter LTL in men who displayed atherosclerotic plaques in the CCA and ICA compared with those without detectable plaques.22 Fitzpatrick et al examined 419 randomly selected elderly participants of the Cardiovascular Health Study (average age 74.2 years), observing shortened LTL in individuals with increased ICA-IMT (p= 0.07).4 However, these cohorts are distinct from the Framingham Offspring Study cohort with respect to age distribution and demography. Nonetheless, our findings further extend those of these smaller studies in indicating that after age-adjustment shortened LTL is associated with increasing carotid IMT in men, particularly obese men.
The effect of sex on the association between LTL and the ICA-IMT is poorly understood. Sexual dimorphism is displayed in cardiovascular risk factors and CVD.6, 30–32 More importantly, in women, a host of variables, including menopause, might impact the association between LTL and cardiovascular risk factors.33
The mechanism underlying the association between age-adjusted LTL and ICA-IMT in obese men is not understood. However, given the growing prevalence of overweight and obesity in the United States27 and the significant proportion of deaths attributable to obesity,34 further research is warranted to confirm and decipher the link between LTL and atherosclerosis in obese persons. It is possible that the metabolic consequences of obesity provoke parallel changes in processes that are engaged in atherosclerosis and LTL dynamics to the extent that they are detectable in the shortening of LTL in obese individuals. Obesity is marked by insulin resistance, inflammation and oxidative stress.35–37 Insulin resistance,10, 11 oxidative stress,10, 38 and inflammation4, 39 are associated (inversely) with LTL. In obese men, the combined input of these factors on LTL dynamics might be of a magnitude that is reflected by the shortening of LTL in concert with increased ICA-IMT.
The same considerations might apply with regard to the shortened LTL in current smokers than in never smokers, as smoking increases the oxidative stress and inflammatory burden in the body.40–44 Interestingly, we did not observe shortened LTL in past smokers as opposed to never smokers. Several possibilities may account for this finding. First, survivorship bias may play some role, since past smokers who survive may be genetically different from those who have died as a result of smoking. Second, the characterization of individuals as current smokers, past smokers and never smokers is often imprecise and highly subjective, as it relies on the accounts of smoking provided by the subjects, which are often inaccurate. This is particularly applicable to past smokers. Third, it may be that cessation of smoking leads to LTL attrition that is markedly slower than the rate in non-smokers because the anti-oxidant and anti-inflammatory pathways were up-regulated by the chronic exposure to cigarette smoke.
The strengths of this work include its conduct in a well characterized community-based sample, the highly accurate measurements of the IMT and LTL and their blinded assessments. However, because of the cross-sectional design, evidence for association may not be equated with evidence for causality, and these findings warrant follow-up confirmation in longitudinal studies of both LTL dynamics and indices of atherosclerosis. In addition, the FHS comprises predominantly middle-class Caucasians that may or may not be generalizable to other ethnicities of different socioeconomic backgrounds.
In conclusion, we observed an (inverse) association between LTL and the ICA-IMT that in secondary analyses appeared particularly strong in obese men but not in women or non-obese men. In addition, we confirmed previous findings of shortened LTL in men versus women and in cigarette smokers versus never smokers. Our findings support further investigation of the relationships of LTL dynamics and two major risk factors, obesity and cigarette smoking, in the development of atherosclerosis.
Acknowledgments
This project was supported by a NIA grant AG021593; and by the NHLBI’s Framingham Heart Study (NIH/NHLBI Contract N01-HC-25195).
Reference List
- 1.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003 May 1;23(5):842–6. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 2.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007 January 13;369(9556):107–14. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 3.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003 February 1;361(9355):393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007 January 1;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 5.van der HP, van der SG, de Boer RA, Voors AA, Hall AS, Mulder MJ, van Gilst WH, van Veldhuisen DJ. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007 April 3;49(13):1459–64. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A. Telomere length as an indicator of biological aging: The gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001 February;37(2):381–5. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 7.Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000 August;36(2):195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 8.Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004 February 14;363(9408):507–10. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- 9.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005 August;366(9486):662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 10.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006 August;5(4):325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 11.Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005 May 3;111(17):2171–7. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 12.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997 September 2;96(5):1432–7. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007 January 30;115(4):459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 14.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999 January 7;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 15.O’Leary DH, Polak JF, Kronmal RA, Savage PJ, Borhani NO, Kittner SJ, Tracy R, Gardin JM, Price TR, Furberg CD. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke. 1996 February;27(2):224–31. doi: 10.1161/01.str.27.2.224. [DOI] [PubMed] [Google Scholar]
- 16.Carallo C, Irace C, Pujia A, De Franceschi MS, Crescenzo A, Motti C, Cortese C, Mattioli PL, Gnasso A. Evaluation of common carotid hemodynamic forces. Relations with wall thickening. Hypertension. 1999 August;34(2):217–21. doi: 10.1161/01.hyp.34.2.217. [DOI] [PubMed] [Google Scholar]
- 17.Gnasso A, Carallo C, Irace C, Spagnuolo V, De Novara G, Mattioli PL, Pujia A. Association between intima-media thickness and wall shear stress in common carotid arteries in healthy male subjects. Circulation. 1996 December 15;94(12):3257–62. doi: 10.1161/01.cir.94.12.3257. [DOI] [PubMed] [Google Scholar]
- 18.Psaty BM, Furberg CD, Kuller LH, Bild DE, Rautaharju PM, Polak JF, Bovill E, Gottdiener JS. Traditional risk factors and subclinical disease measures as predictors of first myocardial infarction in older adults: the Cardiovascular Health Study. Arch Intern Med. 1999 June 28;159(12):1339–47. doi: 10.1001/archinte.159.12.1339. [DOI] [PubMed] [Google Scholar]
- 19.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006 July;6(7):508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 20.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003 February 6;91(3A):7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 21.Aviv A. Telomeres and human somatic fitness. J Gerontol A Biol Sci Med Sci. 2006 August;61(8):871–3. doi: 10.1093/gerona/61.8.871. [DOI] [PubMed] [Google Scholar]
- 22.Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L, Adamopoulos C, Temmar M, Bean KE, Thomas F, Aviv A. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004 February;43(2):182–5. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 23.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975 December;4(4):518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979 September;110(3):281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 25.Fox CS, Polak JF, Chazaro I, Cupples A, Wolf PA, D’Agostino RA, O’Donnell CJ. Genetic and environmental contributions to atherosclerosis phenotypes in men and women: heritability of carotid intima-media thickness in the Framingham Heart Study. Stroke. 2003;34(2):397–401. doi: 10.1161/01.str.0000048214.56981.6f. [DOI] [PubMed] [Google Scholar]
- 26.Fox CS, Cupples LA, Chazaro I, Polak JF, Wolf PA, D’Agostino RB, Ordovas JM, O’Donnell CJ. Genomewide linkage analysis for internal carotid artery intimal medial thickness: evidence for linkage to chromosome 12. Am J Hum Genet. 2004 February;74(2):253–61. doi: 10.1086/381559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006 April 5;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 28.Akkad A, Hastings R, Konje JC, Bell SC, Thurston H, Williams B. Telomere length in small-for-gestational-age babies. BJOG. 2006 March;113(3):318–23. doi: 10.1111/j.1471-0528.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 29.Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G, Aviv A. Telomere length in the newborn. Pediatr Res. 2002 September;52(3):377–81. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Benetos A, Rudnichi A, Safar M, Guize L. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension. 1998 September;32(3):560–4. doi: 10.1161/01.hyp.32.3.560. [DOI] [PubMed] [Google Scholar]
- 31.Fisher ND, Ferri C, Bellini C, Santucci A, Gleason R, Williams GH, Hollenberg NK, Seely EW. Age, gender, and non-modulation. A sexual dimorphism in essential hypertension. Hypertension. 1997 April;29(4):980–5. doi: 10.1161/01.hyp.29.4.980. [DOI] [PubMed] [Google Scholar]
- 32.Chen YF. Sexual dimorphism of hypertension. Curr Opin Nephrol Hypertens. 1996 March;5(2):181–5. doi: 10.1097/00041552-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab. 2006 February;91(2):635–40. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- 34.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005 April 20;293(15):1861–7. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 35.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006 December 14;444(7121):881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 36.Frisard M, Ravussin E. Energy metabolism and oxidative stress: impact on the metabolic syndrome and the aging process. Endocrine. 2006 February;29(1):27–32. doi: 10.1385/ENDO:29:1:27. [DOI] [PubMed] [Google Scholar]
- 37.Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007 March;120(3 Suppl 1):S12–S18. doi: 10.1016/j.amjmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004 December 7;101(49):17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab. 2006 February;91(2):635–40. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- 40.Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation. 2003 May 13;107(18):2342–7. doi: 10.1161/01.CIR.0000066691.52789.BE. [DOI] [PubMed] [Google Scholar]
- 41.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002 May 1;89(9):1117–9. doi: 10.1016/s0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- 42.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985 December;64:111–26. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller EA, Pankow JS, Millikan RC, Bray MS, Ballantyne CM, Bell DA, Heiss G, Li R. Glutathione-S-transferase genotypes, smoking, and their association with markers of inflammation, hemostasis, and endothelial function: the atherosclerosis risk in communities (ARIC) study. Atherosclerosis. 2003 December;171(2):265–72. doi: 10.1016/j.atherosclerosis.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993 May 28;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]