Abstract
Objective
To determine the association of long-term exposure to atherosclerosis risk factors with valvular calcification.
Background
Traditional atherosclerosis risk factors have been associated with aortic and mitral valve calcium in cross-sectional studies but long-term prospective data is lacking.
Methods
Prospective community-based cohort study with 27-year follow-up (median follow-up 26.9 years; range 23.1–29.6 years). Participants from the Framingham Offspring Study (n=1323, enrolled between 1971–1975, mean age at enrollment 34±9 years, 52% women) underwent cardiac multi-detector computed tomography testing between 2002–2005. Associations between the long-term average of each cardiovascular risk factor and valve calcium were estimated using logistic regression.
Results
Aortic valve calcium was present in 39% of participants and mitral valve calcium in 20%. In multivariable models, the odds ratio for aortic valve calcium associated with every standard deviation (SD) increment in long-term mean total cholesterol was 1.74 (P<0.0001), with every SD increment in high-density lipoprotein cholesterol, 0.77 (P=0.002), and with every 9 cigarettes smoked per day, 1.23 (P=0.002). Associations of similar magnitude were seen for mitral valve calcium. The mean of three serum C-reactive protein measurements was associated with mitral valve calcium (OR 1.29 per-SD increment in CRP levels, P=0.002). A higher Framingham risk score in early adulthood (≤40 years age) was associated with increased prevalence and severity of aortic valve calcium measured three decades later.
Conclusions
Exposure to multiple atherosclerotic risk factors starting in early to mid-adulthood is associated with aortic and mitral valve calcium. Studies evaluating early risk factor modification to reduce the burden of valve disease are warranted.
Keywords: calcification, aortic valve, mitral valve, atherosclerosis, stenosis
Aortic and mitral stenosis are among the most common forms of valvular heart disease affecting the elderly. Valve calcification precedes clinical stenosis and may represent an important intermediate phenotype for valve disease.(1) Previously considered a degenerative consequence of aging, valve calcification and the resulting valvular stenosis are now recognized as “active” processes with marked histological similarities to atherosclerosis.(2–7) However, the failure of lipid-lowering strategies to prevent or slow the progression of valvular disease has raised questions about the role of atherosclerosis risk factors in valvular stenosis.(8–11) Improved understanding of the role of cardiovascular risk factors in valvular disease and the appropriate timing for their control could provide insights into the prevention of valvular disease.
Atherosclerosis risk factors such as lipoproteins, cigarette smoking and metabolic syndrome have been associated with valvular calcium in several cross-sectional studies but in only few prospective studies. Prospective studies to date have been limited by short-term follow-up(2,12,13) and single assessments of risk factors(14) which may underestimate the long-term cumulative effects of cardiovascular risk factors on valve calcium. In addition, contemporary rates of treatment for cholesterol and other risk factors may attenuate the associations of valvular disease with risk factors.
Prospective, longitudinal studies with repeated measurement of risk factors could overcome these limitations. Accordingly, using over 25 years of longitudinal data from the Framingham Offspring study, we sought to evaluate the association of long-term exposure to atherosclerosis risk factors and the prevalence of aortic valve and mitral valve calcium in a community-based sample. We also sought to establish the association between an adverse risk factor profile in early to mid-adulthood and valvular calcification measured nearly three decades later.
METHODS
Study Sample
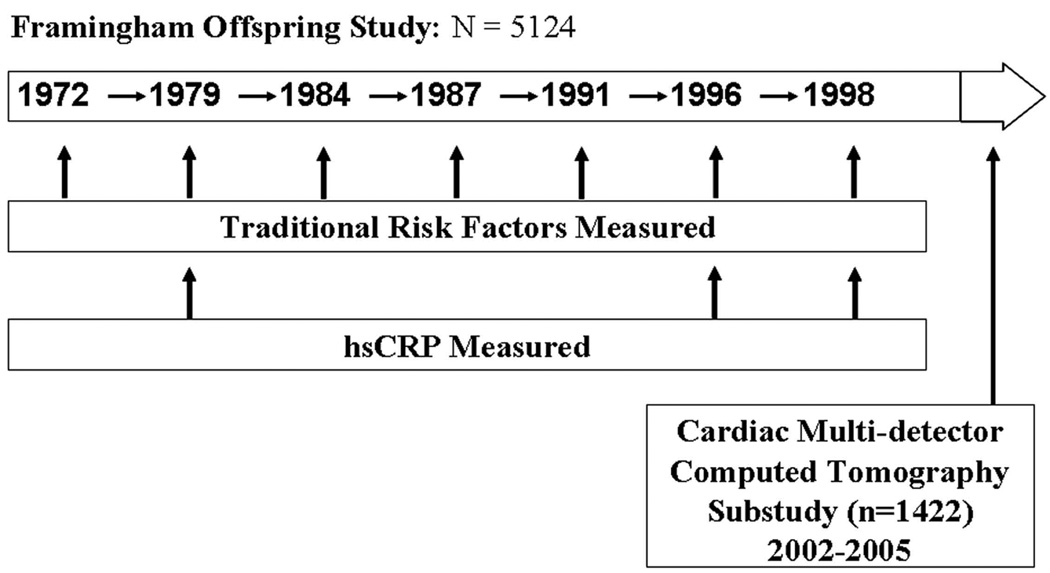
The Framingham Offspring Study was initiated in 1971 as described previously,(15,16). As part of a sub-study to measure subclinical cardiovascular disease, 1422 Offspring Study participants underwent cardiac multi-detector computed tomography (MDCT) between 2002 and 2005 (Figure 1). The MDCT study has been described previously(17). Of the 1422 participants who underwent cardiac MDCT, we excluded 28 participants due to uninterpretable CT images and/or previous valve surgery. Of the 1394 participants with interpretable images, 20 participants were excluded for not attending exam 7; 38 participants were excluded for missing covariate data at exam 7; 9 participants who attended fewer than four of the seven examinations were excluded; 4 participants who attended at least four of the previous seven examinations but had all risk factors measured at only three or fewer of the previous seven examinations were excluded. After all exclusions, 1323 participants remained eligible for the present investigation. Study protocols were approved by the Institutional Review Board of Boston University Medical Center and Massachusetts General Hospital. Written informed consent was obtained from all participants.
Figure 1. Description of the Framingham Offspring Study and Cardiac Multi-Detector Computed Tomography Sub-study.
Atherosclerosis Risk Factors
Risk factor information was collected from routine medical history, physical examination, and laboratory assessment. Total cholesterol and high-density lipoprotein cholesterol (HDL-C) were determined on fasting blood samples, using conventional biochemical methods. C-reactive protein level was measured for each participant with a Dade Behring BN100 nephelometer (Deerfield, IL) on fasting morning serum samples. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. A physician ascertained the number of cigarettes smoked daily and medication history during an interview. Blood pressure was determined in the left arm by a sphygmomanometer in subjects who had been seated for at least five minutes. Hypertension was defined as a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, or antihypertensive treatment.
Cardiac Multidetector Computed Tomography
Each participant underwent cardiac imaging using an eight-slice MDCT (LightSpeed Ultra, GE, Milwaukee, WI, USA) as previously described.(18) Two scans were performed for each participant using a sequential scan protocol with prospective gating of image acquisition. Calcium measurements were performed offline on an Aquarius workstation (TeraRecon, San Matteo, CA, USA).
Valve Calcium Measurement
Calcium was defined as an area of ≥ 3 connected pixels with an attenuation of ≥ 130 Hounsfield units. A modified Agatston scoring protocol was used to quantify the extent of calcium in the aortic and mitral valves. Aortic valve calcium was defined as calcium deposits of the aortic cusps or nodular deposits at the coaptation points of the aortic cusps. We excluded calcium deposits restricted to the aortic wall. Mitral valve calcium was defined as calcium deposits in the region of the annulus and/or the mitral valve leaflets.
Each set of two scans for each participant was initially read for the presence or absence of valve calcium by either of two observers [a cardiologist (SK) and a trained technician (EM)]. If valve calcium was present on at least one of the two scans, the scan underwent an independent read, blinded to the results of the first read, by a second observer [a radiologist (RC)]. Disagreement on the presence or absence of calcium was resolved by a consensus read involving the cardiologist and the radiologist. To determine a participant’s valve calcium score, we first averaged the results from each of two scans read by the first observer and then averaged the results of the two observers. For scans requiring a consensus read to adjudicate valve calcium, we used the calcium score determined by consensus between the two observers. To determine inter-observer variability, two observers independently read a random sample of 112 scans. Inter-observer agreement for the presence or absence of aortic valve calcium and mitral valve calcium was kappa=0.95 and kappa=1.00, respectively. Intra-class correlations for aortic and mitral valve calcium scores were 0.98 and 0.99, respectively.
Statistical Analyses
Spearman correlation coefficients were calculated for the relation between aortic and mitral valve calcium. We assessed agreement between aortic and mitral valve calcium scores by cross-classifying the aortic and mitral calcium scores into a 5 × 5 matrix (the first category consisted of scores of 0, and the other four categories consisted of scores within the first, second, third and fourth quartiles of valve calcium scores, for those with detectable calcium). A weighted kappa statistic was then calculated to summarize agreement between scores.
To examine the association of long-term average risk factors with valve calcium, we constructed multivariable logistic regression models using p<0.10 as the significance criterion for covariates to be retained in the forward stepwise model selection process. The outcome variable was presence or absence of valve calcium. Separate logistic regression models were fit with aortic valve calcium and mitral valve calcium as the outcome measures. In addition, we separately modeled blood pressure as a continuous variable (systolic and diastolic blood pressure seperately) and as a dichotomous variable based on our definition of hypertension (i.e. blood pressure ≥140/90 mmHg or antihypertensive treatment). Age (at examination 7) and sex were forced in as covariates in all models. The candidate predictor variables were total cholesterol, HDL-C, BMI, fasting glucose, C-reactive protein level, cigarettes smoked daily, lipid lowering therapy, hypertension and anti-hypertensive therapy. For continuous risk factors, we modeled the exposure to be the average of available values from the seven examination cycle time points (three examinations were available for C-reactive protein). For categorical variables (lipid lowering therapy, hypertension), we modeled the exposure as the proportion of examinations (out of a maximum of seven) where hypertension or lipid lowering therapy was present.
In a secondary analysis, to determine if atherosclerosis risk factors present in early adulthood were related with valve calcium, we restricted the risk factor determinations to examination cycles 1, 2, and 3, and studied the association of risk factors measured at these early examinations with the presence of valve calcium. For these analyses, participants were required to have attended at least 2 of the 3 examinations. For continuous risk factors, we considered the average of available values and for categorical risk factors, we modeled the proportion of examinations (out of maximum 3) where the risk factor was present.
Lastly, we evaluated the relations between risk factors measured at the baseline examination (1971–1975) and valve calcium. Specifically, we estimated the prevalence and severity of aortic and mitral valve calcium across categories of Framingham coronary heart disease (CHD) risk scores(19) based upon baseline risk factors (i.e. examination cycle 1). Trend tests across cardiovascular risk categories were adjusted for age and sex. In a sensitivity analysis, we repeated this analysis in a sample restricted to participants ≤40 years of age (n=871 participants). All analyses were conducted in SAS 9.0. Odds ratios and 95% confidence intervals (CI) are reported for a 1-standard deviation (SD) change in the predictor variable. A two-sided P value of less than 0.05 was considered significant.
RESULTS
Participant Characteristics
Participant characteristics for the baseline examination (1971–1975) and for all seven examinations (1971–2001) are shown in Table 1. The mean age of the participants at the baseline examination was 34±9 years and at the time of CT scan was 64±9 years. Median follow-up time from baseline exam was 26.8 years (range 23.1–29.6 years). On MDCT scans performed from 2002–2005, aortic valve calcium was detectable in 39% (95% CI 37–42%) of participants and mitral valve calcium in 20% (95% CI 18 – 23%) of participants. The correlation between aortic and mitral valve calcium was 0.45 (p<0.0001). The agreement between aortic and mitral valve calcium scores was low (weighted kappa=0.34).
Table 1.
Participant Characteristics at Baseline Exam and Longitudinal Risk Factors According to the Presence or Absence of Aortic and Mitral Valve Calcium
| Characteristic | Aortic Valve Calcium | Mitral Valve Calcium | |||
|---|---|---|---|---|---|
| Entire Sample (N=1323) |
Absent (N=804) |
Present (Score>0) (N=519) |
Absent (N=1053) |
Present (Score>0) (N=270) |
|
| Baseline Data (1971–1975) | |||||
| Age (yr) | 34±9 | 30±8 | 39±8 | 32±9 | 41±8 |
| Female sex (%) | 52 | 59 | 42 | 52 | 52 |
| Body mass index, kg/m2 | 24.7±4.1 | 23.9±4.1 | 25.8±3.9 | 24.4±4.1 | 25.8±3.7 |
| Hypertension (%) | 14 | 10 | 20 | 12 | 21 |
| Systolic Blood Pressure (mmHg) | 119±14 | 117±14 | 122±14 | 119±14 | 122±14 |
| Diastolic Blood Pressure (mmHg) | 78±10 | 76±10 | 80±9 | 77±10 | 80±9 |
| Total cholesterol, mg/dL | 191±35 | 180±32 | 207±34 | 187±34 | 207±35 |
| High-density lipoprotein cholesterol, mg/dL | 52±16 | 53±15 | 51±16 | 52±16 | 52±16 |
| Lipid lowering therapy (%) | 0.3 | 0.3 | 0.4 | 0.1 | 1.1 |
| Cigarette smoking (%) | 35 | 33 | 39 | 35 | 36 |
| Cigarettes smoked daily | 19±12 | 18±11 | 21±12 | 19±11 | 22±13 |
| Diabetes (%) | 0.3 | 0.1 | 0.6 | 0.0 | 1.5 |
| Fasting glucose, mg/dL | 100±9 | 99±8 | 101±9 | 99±8 | 101±10 |
| C-reactive protein, mg/dL | 2.0±4.2 | 1.9±4.6 | 2.1±3.5 | 1.9±4.4 | 2.3±3.3 |
| Longitudinal Data†(Exams 1 to 7) | |||||
| Body mass index, kg/m2 | 26.6±4.3 | 26.0±4.4 | 27.5±4.1 | 26.4±4.4 | 27.5±4.0 |
| Hypertension (%) | 49 | 45 | 53 | 47 | 54 |
| Systolic blood pressure (mmHg) | 122±12 | 120±12 | 126±12 | 121±13 | 128±12 |
| Diastolic blood pressure (mmHg) | 77±7 | 76±7 | 78±7 | 76±7 | 78±7 |
| Total cholesterol, mg/dL | 200±29 | 193±27 | 211±28 | 198±28 | 211±27 |
| High-density lipoprotein cholesterol, mg/dL | 51±13 | 53±13 | 48±13 | 51±13 | 49±13 |
| Lipid lowering therapy (%) | 28 | 27 | 29 | 27 | 30 |
| Cigarette Smoking (%) | 50 | 48 | 52 | 50 | 50 |
| Cigarettes smoked daily | 10±9 | 9±9 | 11±10 | 10±9 | 11±10 |
| Diabetes (%) | 37 | 36 | 37 | 35 | 40 |
| Fasting glucose, mg/dL | 97±14 | 95±11 | 101±16 | 96±12 | 102±18 |
| C-reactive protein‡, mg/dL | 2.6±2.9 | 2.4±2.9 | 2.9±2.9 | 2.4±2.7 | 3.4±3.5 |
|
Valve calcium score median (1st and 3rd Quartiles) |
0 |
46 |
0 |
57 |
|
| -- | (13, 140) | -- | (15, 297) | ||
Continuous variables presented as mean±SD. To convert values for cholesterol to millimoles per liter, multiply by 0.02586.
For longitudinal data, continuous variables are reported as mean±SD of available values during follow-up (exams 1 through 7), whereas categorical variables are reported as proportion of participants with presence of risk factor at any exam during follow-up (exams 1 through 7).
C-reactive protein values are the mean of measurement at a maximum of three time points (examinations 2, 6, and 7)
Multivariable associations of long-term risk factors and aortic valve calcium
In multivariable models, older age at baseline and higher values of the long-term average of several risk factors (mean total cholesterol, BMI, and number of cigarettes smoked daily) were significantly associated with increased odds of aortic valve calcium, whereas female sex and higher values of the long-term average of HDL-C were associated with reduced odds of aortic valve calcium (Table 2). The average of C-reactive protein from three examinations (spanning ~22 years) was not associated with aortic valve calcium.
Table 2.
Association of Long-term Average of Individual Atherosclerosis Risk Factors (Examinations 1 – 7) with the Presence of Aortic and Mitral Valve Calcium
| Aortic Valve Calcium | Mitral Valve Calcium | |||||
|---|---|---|---|---|---|---|
| Adjusted Odds Ratio |
95% Confidence Interval |
P | Adjusted Odds Ratio |
95% Confidence Interval |
P | |
| Age, per SD | 3.25 | 2.76 – 3.82 | <0.0001 | 3.11 | 2.58 – 3.75 | <0.0001 |
| Female sex (versus men) | 0.56 | 0.41 – 0.76 | 0.0003 | - | - | - |
| Mean total cholesterol, per SD | 1.74 | 1.50 – 2.01 | <0.0001 | 1.26 | 1.08 – 1.48 | 0.004 |
| Mean high-density lipoprotein cholesterol, per SD | 0.77 | 0.66 – 0.91 | 0.002 | 0.85 | 0.71 – 1.02 | 0.07 |
| Body mass index, per SD | 1.21 | 1.05 – 1.40 | 0.008 | - | - | - |
| Cigarettes smoked daily, per SD | 1.23 | 1.08 – 1.41 | 0.002 | 1.18 | 1.02 – 1.36 | 0.03 |
| Mean C-reactive protein, per SD | - | - | 1.29 | 1.10 – 1.52 | 0.002 | |
Stepwise logistic regression models were constructed with outcome variable of presence of aortic valve calcium and the following candidate independent variables as long-term average (examinations 1 – 7): age, sex, total cholesterol, high-density lipoprotein cholesterol, hypertension, body mass index, number of cigarettes smoked per day, C-reactive protein, fasting glucose and lipid lowering therapy.
Predictor variables with P<0.10 are presented.
CI denotes confidence interval.
Multivariable associations of long-term risk factors and mitral valve calcium
Similar risk factors were associated with mitral valve calcium including age and the long-term averages of total cholesterol, HDL-C, and number of cigarettes smoked daily (Table 2). In contrast to our findings with aortic valve calcium, long-term average C-reactive protein level was associated with increased odds of mitral valve calcium (odds ratio 1.29 per SD increment of CRP level, 95% confidence interval 1.10–1.52, P=0.002).
Risk factors in early adulthood and valve calcium
As shown in Table 3, early risk factors obtained in the first three examinations (1971–1982) predicted the future presence of valve calcium in a manner largely similar to the long-term average across all seven examinations. Exceptions included the presence of a relation between early BMI to mitral valve calcium and the absence of a relation between mitral valve calcium and either sex or HDL-C.
Table 3.
Association of Early Atherosclerosis Risk Factors (Examinations 1 – 3) with the Presence of Aortic and Mitral Valve Calcium
| Aortic Valve Calcium | Mitral Valve Calcium | |||||
|---|---|---|---|---|---|---|
| Adjusted Odds Ratio |
95% Confidence Interval |
P | Adjusted Odds Ratio |
95% Confidence Interval |
P | |
| Age, per SD | 2.81 | 2.39 – 3.31 | <0.0001 | 2.89 | 2.39 – 3.50 | <0.0001 |
| Female sex | 0.64 | 0.47 – 0.87 | 0.005 | - | - | - |
| Mean total cholesterol, per SD | 1.81 | 1.55 – 2.11 | <0.0001 | 1.33 | 1.13 – 1.57 | 0.0005 |
| Mean high-density lipoprotein cholesterol, per SD | 0.84 | 0.72 – 0.99 | 0.03 | - | - | - |
| Body mass index, per SD | 1.15 | 0.99 – 1.33 | 0.06 | 1.26 | 1.08 – 1.47 | 0.003 |
| Cigarettes smoked daily, per SD | 1.22 | 1.06 – 1.39 | 0.005 | 1.21 | 1.05 – 1.39 | 0.009 |
| C-reactive protein, per SD (from exam 2) | - | - | - | - | - | - |
Stepwise logistic regression models were constructed with outcome variables of the presence of either aortic or mitral valve calcium and the following candidate predictor variables averaged over examination 1 through 3: age, sex, total cholesterol, high-density lipoprotein cholesterol, body mass index, number of cigarettes smoked per day, fasting glucose, lipid lowering therapy and anti-hypertensive therapy.
Predictor variables with P<0.10 are presented.
CI denotes confidence interval.
Framingham coronary heart disease risk score and valve calcium
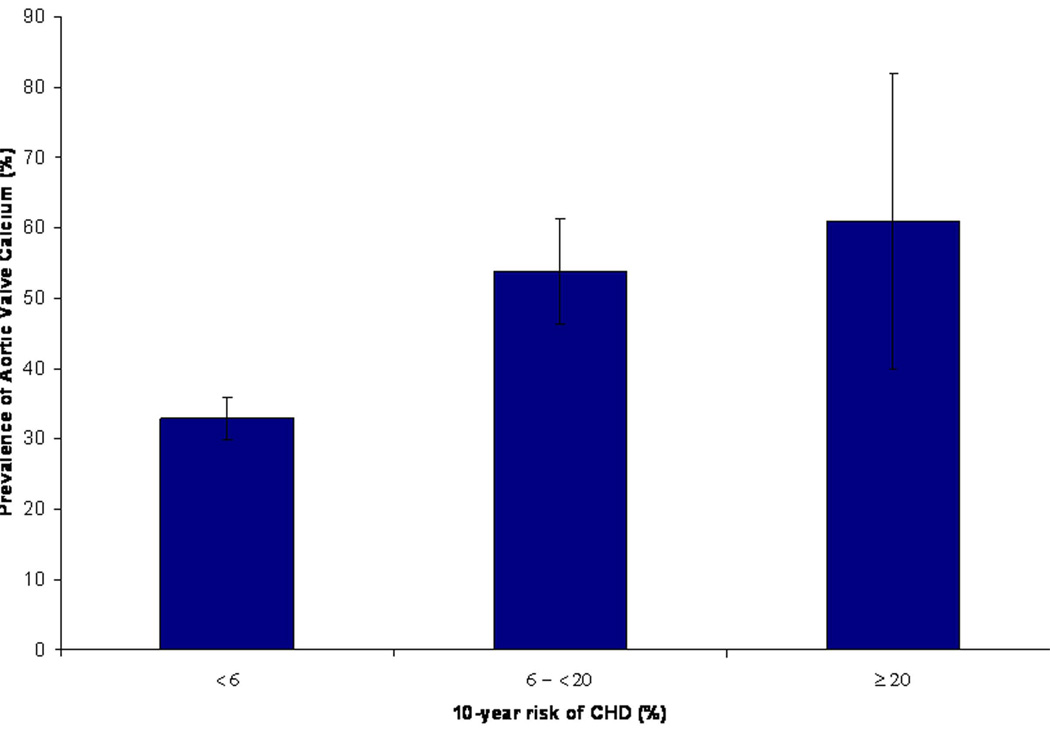
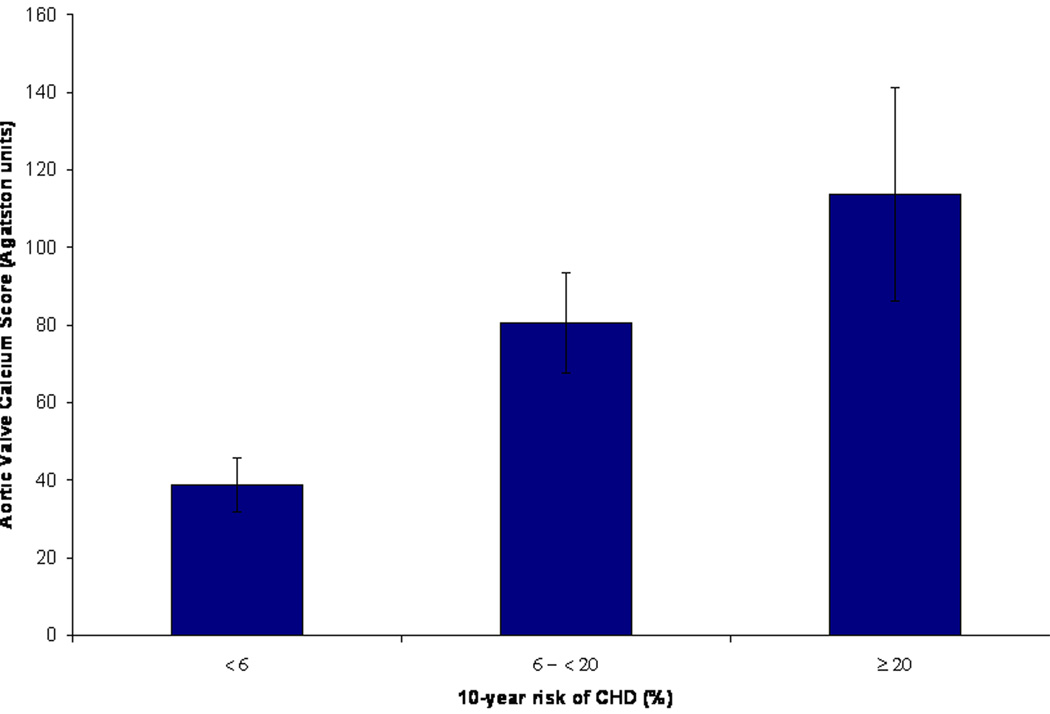
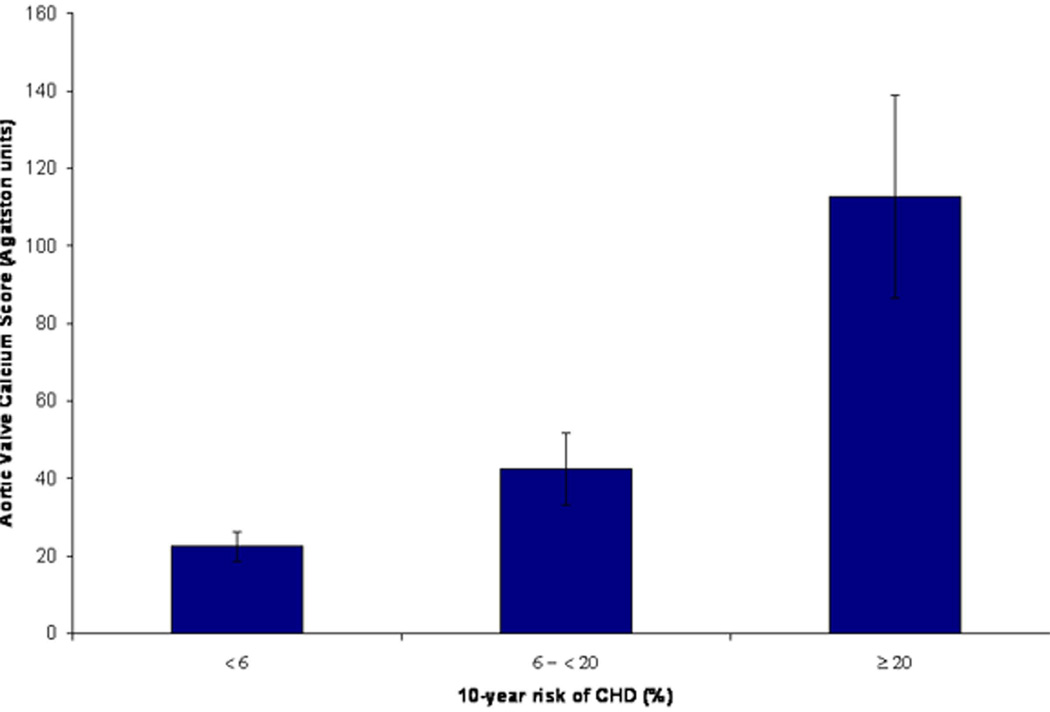
To evaluate the combined effect of multiple atherosclerotic risk factors in early adulthood on valvular calcification later in adulthood, we examined the prevalence of valve calcification across categories of CHD risk based on the Framingham risk score at the baseline exam (1971–1975). The prevalence of aortic valve calcium was 33.0%, 53.8% and 61.1%, for low, intermediate and high Framingham CHD risk score categories, respectively (p<0.0001 for trend across risk categories, Figure 2). A similar relationship was also seen when we evaluated the severity of aortic valve calcium (determined by mean aortic valve calcium score) across increasing risk score categories (p=0.006 for trend, Figure 3). We did not observe any significant trend for the presence or severity of mitral valve calcium across risk score categories.
Figure 2. Prevalence of Aortic Valve Calcium Stratified by Framingham Risk Score Categories in Early Adulthood.
Low, intermediate and high cardiovascular risk correspond to a <6%, 6 - <20% and ≥20% 10-year risk of CHD, respectively. Error bars represent 95% confidence intervals. *Adjusted for age and sex
Figure 3. Mean Aortic Valve Calcium Score Stratified by Framingham Risk Score Categories in Early Adulthood.
Low, intermediate and high cardiovascular risk correspond to a <6%, 6 - <20% and ≥20% 10-year risk of CHD, respectively. Error bars represent standard errors for calcium score. *Adjusted for age and sex.
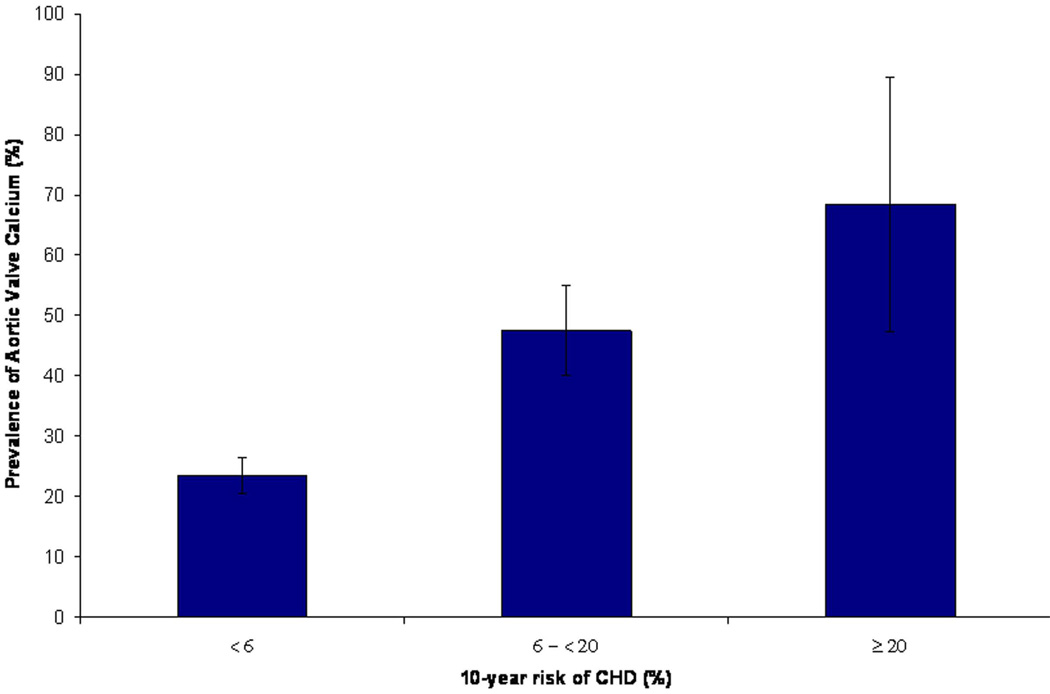
In a sensitivity analysis among participants ≤40 years of age, we noted a similar graded increase in the prevalence and severity of aortic valve calcium across risk score categories (Figure 4).
Figure 4. Prevalence of Aortic Valve Calcium (A) and Mean Aortic Valve Calcium Score (B) Stratified by Framingham Risk Score in Early Adulthood Among Participants ≤ 40 Years of Age.
Low, intermediate and high cardiovascular risk correspond to <6%, 6 - <20% and ≥20% 10-year risk of CHD, respectively. Error bars represent 95% confidence intervals for prevalence and standard errors for calcium score. *Adjusted for age and sex.
COMMENT
Principal Findings
In our study of over 1300 participants with over 25 years of follow-up, we identified several traditional atherosclerosis risk factors that are associated with the presence of aortic and mitral valve calcium as detected by cardiac MDCT. Of the modifiable risk factors, we found that cigarette smoking and total cholesterol were strongly associated with valve calcium. We also noted important associations for HDL-C and body mass index with valvular calcification. The observed associations were consistent whether we examined the risk factors averaged over the entire 27 years of observation or over the first 12 years of observation. Our study with over 25 years of follow-up, represents the longest community-based cohort to examine associations between atherosclerosis risk factors and valve calcium, and provides new evidence that long-term exposure to an adverse risk factor profile starting in early adulthood is associated with an increased prevalence of valvular calcification measured nearly three decades later.
In the Context of the Current Literature
Aortic Valve Calcium
We found that 39% of participants had aortic valve calcium on CT. Our estimate of aortic valve calcium is higher than previous reports of aortic sclerosis by echocardiography which have ranged from 26–29%(7,14) and aortic valve calcium by CT which was reported to be 14%.(20) This may be due be to differences in baseline participant characteristics, a predominantly white sample, lower use of lipid lowering therapies, differences in the imaging protocol or differences in the CT reading methodology between our study and others.
Several previous studies have reported associations with cholesterol and aortic valve disease. (7,21–25) Our longitudinal data with serial serum cholesterol measurements over a 27 year period support the association between lipids and aortic valve calcium demonstrating that for each 29 mg/dL increase in the long-term average of total cholesterol there was a marked 74% increase in the odds of aortic valve calcium. Associations with valve calcium have also been reported for other cardiovascular risk factors including cigarette smoking,(13,18,19,23,24) BMI,(23,24) metabolic syndrome,(12,17) and hypertension.(7,18,19,22,23) Our results confirm that cigarette smoking is a potent risk factor for aortic valve calcification. In addition, we found a weak association between increased BMI and aortic valve calcium. Contrary to several prior reports,(7,21,22,25,26) we did not find any association with hypertension or blood pressure; this may have been due to the lower age of our sample or other differences in baseline characteristics. In addition, despite histologic evidence for inflammation in aortic calcification,(27) we found no association with plasma CRP and aortic valve calcium, which is in agreement with a previous study evaluating CRP and progression of aortic stenosis.(28)
We are unaware of any prior studies that have evaluated the presence of aortic valve calcification based on CHD risk factor profiles in early adulthood. However, our findings that an early adverse risk factor profile in the 4th decade of life is associated with increased prevalence of aortic valve disease later in life are supported by a recent study by Owens et al., who reported that the association between low-density lipoprotein levels and prevalent valvular calcification was significant only among younger subjects <65 years of age.(29) These observations suggest that risk factors early in life, as opposed to later in life, may be more important for future valvular calcification. Whether risk factor modification at an earlier stage in life could arrest valvular damage and prevent valvular disease in old age requires further study.
Mitral Valve Calcium
Mitral calcification also shares many risk factors with atherosclerosis and aortic calcification. Our findings of relations between mitral calcification with cigarette smoking and lipid levels are in agreement with previous studies evaluating mitral annular calcification by echocardiography.(22,23,30–32) We also observed an important relationship between serum CRP and mitral valve calcification, independent of other risk factors, which represents to our knowledge, a novel association not previously reported in the literature. In our analysis, it remains unclear why CRP was associated only with mitral valve calcium and not aortic valve calcium. Whether this observation represents differences in the pathogenesis of mitral and aortic calcification or is a fortuitous finding requires further study.
Potential Mechanisms
Valvular sclerosis and calcification appears to have marked similarities with coronary atherosclerosis. Histologically, early valve lesions are characterized by a disruption of valvular endothelium at areas of mechanical stress,(33,34) accumulation of oxidized lipids and foam cells and a prominent inflammatory infiltrate.(3–6,35) Changes in the valvular microenvironment promote differentiation of valvular cells to adopt an osteoblast-like phenotype leading to calcification, which characterizes the pathological process.(36,37) Numerous lines of experimental evidence have demonstrated the importance of plasma cholesterol in this process(2,3). Once LDL is oxidized due to cigarette smoking or other factors, it has been shown to be a major stimulus for the deposition of extracellular matrix required for calcium deposition.(36,38)
Animal models of hyperlipidemia have also provided additional evidence for the integral role of lipids in valvular calcification.(39–41) In a recent study, using hyperlipidemic transgenic mice with a “genetic switch” which allowed reversal of the hyperlipidemic phenotype, Miller et al. demonstrated reductions in oxidative stress, lipid deposition and calcium deposition with arrest of further valvular sclerosis after hyperlipidemia was reversed.(42) These results provide new evidence for the role of lipids in the initiation and early stages of valvular disease.
Clinical Implications
Randomized controlled trials of lipid lowering therapy to reduce the development or progression of aortic valve disease have been generally disappointing.(8–10) These trials were limited to elderly patients (mean age across trials was 67 years) with established moderate or severe aortic valve stenosis (mean valve area across these trials was 1.3 cm2 with a peak transvalvular gradient of 40 mmHg). Our findings of strong and consistent associations between valve calcium and cardiovascular risk factors determined decades earlier raises the hypothesis that aggressive risk factor modification at an earlier age, among predisposed individuals, may be required to reduce the incidence of future valvular disease.(29,42) However, the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) trial(11) which randomized patients with less severe aortic valve disease also failed to demonstrate efficacy for statins to slow aortic valve disease. Although participants in this trial were younger than in prior trials, their mean age was still 58 years, therefore, it remains possible that earlier lipid lowering drug therapy and risk factor modification using alternative strategies (e.g., exercise),(43) prior to the initiation of valvular remodeling, may be necessary to reduce progression to aortic stenosis.
Limitations
Our study has a number of limitations that deserve comment. First, we used valve calcification, a subclinical surrogate marker for aortic or mitral valve sclerosis, as our outcome. We therefore could not assess the impact on physiological correlates (i.e., valve gradients) or on “hard outcomes” such as critical valve stenosis or valve replacement. However, valvular calcification is known to be an important risk factor for the development of clinical valvular disease and represents the same pathophysiological process involved in clinical disease.(1) Second, participants did not have a CT scan on enrollment and therefore, any baseline calcification could not be quantified. However, given the young age at enrollment, it is unlikely that participants had any calcification at this early age. Third, due to the observational nature of our study, reported associations with atherosclerosis risk factors cannot imply causal relations. Lastly, our sample was derived from a predominantly white population of European descent and as such, our results may not apply to other races or ethnicities.
Conclusion
We found that long-term exposure to atherosclerosis risk factors, such as plasma lipids and cigarette smoking, starting in early to mid-adulthood are associated with both aortic and mitral valvular calcification. Further study is warranted to evaluate whether early modification of cardiovascular risk factors may also reduce the development of valvular disease in the elderly.
Acknowledgments
From the Framingham Heart Study of the National Heart, Lung, and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This work was supported by the National Heart, Lung, and Blood Institute's Framingham Heart Study (contract No. N01-HC-25195). Dr. Thanassoulis is supported by a Research Fellowship by the Canadian Institute of Health Research and the Fonds de Recherche en Sante du Quebec. Dr. Kathiresan’s efforts were supported by the American College of Cardiology Foundation/Merck Adult Cardiology Research Fellowship Award and the GlaxoSmithKline Research & Education Foundation for Cardiovascular Disease Young Investigator Award. None of the sponsors had any role in the design and conduct of this study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review and approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Kathiresan serves on a scientific advisory board for Merck and has received research funding from Pfizer and Alnylam Pharmaceuticals. None of the other authors have any disclosures.
References
- 1.Cosmi JE, Kort S, Tunick PA, et al. The Risk of the Development of Aortic Stenosis in Patients With "Benign" Aortic Valve Thickening. Arch Intern Med. 2002;162:2345–2347. doi: 10.1001/archinte.162.20.2345. [DOI] [PubMed] [Google Scholar]
- 2.Chan KL. Is aortic stenosis a preventable disease? J Am Coll Cardiol. 2003;42:593–599. doi: 10.1016/s0735-1097(03)00786-1. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of 'degenerative' valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- 4.Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Ryden L, Nilsson J. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol. 1994;23:1162–1170. doi: 10.1016/0735-1097(94)90606-8. [DOI] [PubMed] [Google Scholar]
- 5.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218–1222. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 6.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of 'degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 7.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 8.Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 9.Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 11.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J, Investigators A. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 12.Agmon Y, Khandheria BK, Meissner I, et al. Aortic valve sclerosis and aortic atherosclerosis: different manifestations of the same disease? Insights from a population-based study. J Am Coll Cardiol. 2001;38:827–834. doi: 10.1016/s0735-1097(01)01422-x. [DOI] [PubMed] [Google Scholar]
- 13.Katz R, Budoff MJ, Takasu J, et al. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes. 2009;58:813–819. doi: 10.2337/db08-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stritzke J, Linsel-Nitschke P, Markus MR, et al. Association between degenerative aortic valve disease and long-term exposure to cardiovascular risk factors: results of the longitudinal population-based KORA/MONICA survey. Eur Heart J. 2009;30:2044–2053. doi: 10.1093/eurheartj/ehp287. [DOI] [PubMed] [Google Scholar]
- 15.Dawber TR, Meadors GF, Moore FEJ. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 17.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial Fat, Visceral Abdominal Fat, Cardiovascular Disease Risk Factors, and Vascular Calcification in a Community-Based Sample: The Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann U, Siebert U, Bull-Stewart A, et al. Evidence for lower variability of coronary artery calcium mineral mass measurements by multi-detector computed tomography in a community-based cohort--consequences for progression studies. Eur J Radiol. 2006;57:396–402. doi: 10.1016/j.ejrad.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 20.Katz R, Wong ND, Kronmal R, et al. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 21.Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001;88:693–695. doi: 10.1016/s0002-9149(01)01821-5. [DOI] [PubMed] [Google Scholar]
- 22.Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987;59:998–999. doi: 10.1016/0002-9149(87)91144-1. [DOI] [PubMed] [Google Scholar]
- 23.Boon A, Cheriex E, Lodder J, Kessels F. Cardiac valve calcification: characteristics of patients with calcification of the mitral annulus or aortic valve. Heart. 1997;78:472–474. doi: 10.1136/hrt.78.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoagland PM, Cook EF, Flatley M, Walker C, Goldman L. Case-control analysis of risk factors for presence of aortic stenosis in adults (age 50 years or older) Am J Cardiol. 1985;55:744–747. doi: 10.1016/0002-9149(85)90149-3. [DOI] [PubMed] [Google Scholar]
- 25.Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkila J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15:865–870. doi: 10.1093/oxfordjournals.eurheartj.a060602. [DOI] [PubMed] [Google Scholar]
- 26.Peltier M, Trojette F, Sarano ME, Grigioni F, Slama MA, Tribouilloy CM. Relation between cardiovascular risk factors and nonrheumatic severe calcific aortic stenosis among patients with a three-cuspid aortic valve. Am J Cardiol. 2003;91:97–99. doi: 10.1016/s0002-9149(02)03010-2. [DOI] [PubMed] [Google Scholar]
- 27.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 28.Novaro GM, Katz R, Aviles RJ, et al. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;50:1992–1998. doi: 10.1016/j.jacc.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 29.Owens DS, Katz R, Johnson E, et al. Interaction of Age With Lipoproteins as Predictors of Aortic Valve Calcification in the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:1200–1207. doi: 10.1001/archinte.168.11.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair CK, Sudhakaran C, Aronow WS, Thomson W, Woodruff MP, Sketch MH. Clinical characteristics of patients younger than 60 years with mitral anular calcium: comparison with age- and sex-matched control subjects. Am J Cardiol. 1984;54:1286–1287. doi: 10.1016/s0002-9149(84)80082-x. [DOI] [PubMed] [Google Scholar]
- 31.Savage DD, Garrison RJ, Castelli WP, et al. Prevalence of submitral (anular) calcium and its correlates in a general population-based sample (the Framingham Study) Am J Cardiol. 1983;51:1375–1378. doi: 10.1016/0002-9149(83)90315-6. [DOI] [PubMed] [Google Scholar]
- 32.Fox CS, Guo CY, Larson MG, et al. Relations of inflammation and novel risk factors to valvular calcification. Am J Cardiol. 2006;97:1502–1505. doi: 10.1016/j.amjcard.2005.11.086. [DOI] [PubMed] [Google Scholar]
- 33.Thubrikar MJ, Aouad J, Nolan SP. Patterns of calcific deposits in operatively excised stenotic or purely regurgitant aortic valves and their relation to mechanical stress. Am J Cardiol. 1986;58:304–308. doi: 10.1016/0002-9149(86)90067-6. [DOI] [PubMed] [Google Scholar]
- 34.Zand T, Majno G, Nunnari JJ, et al. Lipid deposition and intimal stress and strain. A study in rats with aortic stenosis. Am J Pathol. 1991;139:101–113. [PMC free article] [PubMed] [Google Scholar]
- 35.Warren BA, Yong JL. Calcification of the aortic valve: its progression and grading. Pathology. 1997;29:360–368. doi: 10.1080/00313029700169315. [DOI] [PubMed] [Google Scholar]
- 36.Mohler ER, 3rd, Chawla MK, Chang AW, et al. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8:254–260. [PubMed] [Google Scholar]
- 37.Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien KD, Kuusisto J, Reichenbach DD, et al. Osteopontin Is Expressed in Human Aortic Valvular Lesions. Circulation. 1995;92:2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 39.Fazio S, Sanan DA, Lee YL, Ji ZS, Mahley RW, Rall SC., Jr Susceptibility to diet-induced atherosclerosis in transgenic mice expressing a dysfunctional human apolipoprotein E(Arg 112,Cys142) Arterioscler Thromb. 1994;14:1873–1879. doi: 10.1161/01.atv.14.11.1873. [DOI] [PubMed] [Google Scholar]
- 40.Sarphie TG. Surface responses of aortic valve endothelia from diet-induced, hypercholesterolemic rabbits. Atherosclerosis. 1985;54:283–299. doi: 10.1016/0021-9150(85)90122-4. [DOI] [PubMed] [Google Scholar]
- 41.Zahor Z, Czabanova V. Experimental atherosclerosis of the heart valves in rats following a long-term atherogenic regimen. Atherosclerosis. 1977;27:49–57. doi: 10.1016/0021-9150(77)90023-5. [DOI] [PubMed] [Google Scholar]
- 42.Miller JD, Weiss RM, Serrano KM, et al. Lowering Plasma Cholesterol Levels Halts Progression of Aortic Valve Disease in Mice. Circulation. 2009;119:2693–2701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto Y, Adams V, Jacob S, Mangner N, Schuler G, Linke A. Regular Exercise Training Prevents Aortic Valve Disease in Low-Density Lipoprotein-Receptor-Deficient Mice. Circulation. 121:759–767. doi: 10.1161/CIRCULATIONAHA.109.892224. [DOI] [PubMed] [Google Scholar]