Abstract
Background
Ragweed extract (RWE) contains NADPH oxidases that induce oxidative stress in the airways independent of adaptive immunity (signal 1) and augment antigen (signal 2)–induced allergic airway inflammation.
Objective
To test whether inhibiting signal 1 by administering antioxidants inhibits allergic airway inflammation in mice.
Methods
The ability of ascorbic acid (AA), N-acetyl cystenine (NAC), and tocopherol to scavenge pollen NADPH oxidase–generated reactive oxygen species (ROS) was measured. These antioxidants were administered locally to inhibit signal 1 in the airways of RWE-sensitized mice. Recruitment of inflammatory cells, mucin production, calcium-activated chloride channel 3, IL-4, and IL-13 mRNA expression was quantified in the lungs.
Results
Antioxidants inhibited ROS generation by pollen NADPH oxidases and intracellular ROS generation in cultured epithelial cells. AA in combination with NAC or Tocopherol decreased RWE-induced ROS levels in cultured bronchial epithelial cells. Coadministration of antioxidants with RWE challenge inhibited 4-hydroxynonenal adduct formation, upregulation of Clca3 and IL-4 in lungs, mucin production, recruitment of eosinophils, and total inflammatory cells into the airways. Administration of antioxidants with a second RWE challenge also inhibited airway inflammation. However, administration of AA+NAC 4 or 24 hours after RWE challenge failed to inhibit allergic inflammation.
Conclusion
Signal 1 plays a proinflammatory role during repeated exposure to pollen extract. We propose that inhibiting signal 1 by increasing antioxidant potential in the airways may be a novel therapeutic strategy to attenuate pollen-induced allergic airway inflammation.
Clinical implications
Administration of antioxidants in the airways may constitute a novel therapeutic strategy to prevent pollen induced allergic airway inflammation.
Keywords: Allergy, inflammation, antioxidants, eosinophils, TH2
Asthma is an inflammatory disease of the airways that is associated with oxidative stress. Reactive oxygen species (ROSs) that worsen symptoms of asthma are induced by environmental exposure to diesel exhaust, cigarette smoke, and ozone and from inflammatory cells in the airways.1,2 Environmental pollutants can activate cellular oxidases such as NADPH oxidase, xanthine oxidase, and p450 cytochrome oxidase and exacerbate intracellular levels of ROS.3 Excess ROS can readily damage various biomolecules like protein, DNA, and lipids and/or perpetuate chain reactions that result in cell activation cascades, leading to cell recruitment during inflammation.4 Termination of this reaction occurs in the form of stabilization of the reactive molecule or decay into a harmless product. Antioxidants work either by terminating the chain reaction or by decreasing the rate of reaction. Tocopherol (vitamin E) is a chain-breaking antioxidant in membranes and lipoproteins that scavenges peroxyl radical intermediates. 5 Water-soluble antioxidants like ascorbic acid (vitamin C) and N-acetyl cysteine (NAC) have high reducing potential and thereby alter cells’ redox state. NAC can be converted to glutathione, which is a substrate for glutathione peroxidase enzyme, a potent intracellular antioxidant enzyme.
We have recently shown that NADPH oxidases are present in pollens and proposed a 2-signal ROS-antigen model for the initiation of allergic inflammation.6 In this model, the intrinsic pollen NADPH oxidases generate ROS in the lungs independent of adaptive immune response (signal 1). Presentation of major pollen antigens to TH2 cells generates an antigen-specific signal 2. These signals work in concert to induce full-blown allergic airway inflammation. Pollen proteins that do not deliver signal 1 (Amb a 1, heat-inactivated ragweed pollen extract [RWEH]) are unable to induce robust allergic airway inflammation. In this article, we examined whether scavenging ROS by administration of antioxidants inhibits signal 1, and if so, whether this inhibition blocks allergic airway inflammation.
METHODS
Inhibitory effects of antioxidants on RWE-induced ROS
Ragweed pollen extract (Lot XP56-D18-1320; Greer Laboratories, Lenoir, NC)–mediated ROS was measured as previously described.6 Briefly, RWE (100 µg) was mixed with 50 µmol/L 2′–7′-dihydro-dichlorofluorescein diacetate (H2DCF-DA; Molecular Probes, Carlsbad, Calif) in the presence of increasing concentrations of antioxidants like ascorbic acid (AA), NAC, reduced glutathione, or tocopherol. The reaction mixture was incubated at 37°C for 15 minutes. The change in fluorescence intensity was assessed in an FLx800 microplate reader (Bio-Tek Instruments, Winooski, Vt) at 488 nm excitation and 530 nm emission. The concentration of antioxidants that reduced dichlorofluorescein fluorescence by 50% was calculated and represented as inhibitory dose 50 (ID50). Alternatively, synergistic effects of antioxidants were detected by adding 2 or more antioxidants to the reaction.
Inhibition of RWE-induced intracellular ROS by antioxidants
To determine changes in intracellular ROS, A549 cells grown to 70% confluence were loaded with 50 µmol/L H2DCF-DA at 37°C for 15 minutes. After removing excess probe, cells were treated with PBS, RWE, RWE+AA, RWE+NAC, or RWE+AA+NAC in the presence of NADPH(100 µmol/L). Glucose oxidase was used as positive control. The change in fluorescence intensity was assessed in an FLx800 microplate reader at 488 nm excitation and 530 nm emission. Each data point represents the mean fluorescence intensity ± SEM from 3 or more independent experiments.
Animals, sensitization, and challenge
Animal experiments were performed according to the National Institute of Health Guide for Care and Use of Experimental Animals and approved by the University of Texas Medical Branch IACUC (#9708038-05). Female BALB/c mice 6 to 8 weeks old purchased from Harlan Sprague-Dawley (San Diego, Calif) were used for these studies. Airway inflammation was induced with RWE sensitization and challenges.7,8 Briefly, mice were sensitized on days 0 and 4 with 150 µg/mouse RWE mixed with alum, as previously described. 9,10 On day 11, parallel groups of mice were challenged intranasally with either PBS or RWE (100 µg). Alternatively, intranasal instillation of the antioxidants (NAC, 120 mg/kg; AA, 130 mg/kg; Tocopherol, 1900 U/kg; or their combination) was performed. To study the effect of antioxidants on multiple RWE challenge, we used a similar RWE challenge model with minor changes. Briefly, mice were sensitized with RWE and challenged with PBS, RWE, or RWE+AA+NAC on day 11. A similar challenge was performed a second time on either day 14 or day 21. For the ovalbumin (OVA) experiment, mice were injected with OVA (50 µg) intraperitoneally on day 0 and 14. On days 28 to 32 and 35 to 39, mice were exposed to aerosolized OVA (10 mg/mL) for 20 minutes according to a protocol described previously.11 On day 39, AA+NAC was administered intranasally in groups of mice just before OVA aerosol exposure. Mice were killed 72 hours after last allergen challenge, bronchoalveolar lavage (BAL) was performed, lungs were collected, and total and differential cell count was performed in BAL fluid. To study IL-4 mRNA transcripts, mice were killed 4 hours after challenge.
Detection of 4-hydroxynonenal protein adducts in lungs
BALB/c mice (n = 2 per group) were sensitized with RWE and challenged with PBS, RWE, or RWE+NAC. Lungs were collected 15 minutes after challenge, and lysates were subjected to SDS-PAGE. Western blots were incubated with rabbit anti–4-hydroxy-2-nonenal antibody (cat #HNE11-S; Alpha Diagnostics International, San Antonio, Tex) and detected with horseradish peroxidase–conjugated secondary antibody (cat #NA934; Amersham Biosciences, Piscataway, NJ) by using an ECL kit (Amersham Biosciences).
Morphometric quantitation of mucin production
Mucin production in airway epithelial cells was assessed in periodic acid-Schiff (PAS)–stained lung sections as previously described.6 The data were represented as mucin staining area in µm2 per mm of total peribronchial area.
Evaluation of peribronchial eosinophils
Peribronchial eosinophils 72 hours after challenge were detected in optimal cutting temperature cryosections stained with rabbit antimouse eosinophil major basic protein (a generous gift from Dr Gerald Gleich) or normal rabbit serum as previously described.6
Total RNA preparation and quantitative RT-PCR
Lung RNA was extracted by using TRIzol solution (Invitrogen, Carlsbad, Calif). RNA concentration was measured with Ribogreen RNA Estimation Kit (Molecular Probes, Carlsbad, Calif). Five micrograms total RNA was reverse-transcribed using 200 U SuperScript II Reverse Transcriptase (Invitrogen). Real-time PCR was performed on an ABI 7000 Sequence Detection System (Applied Biosystems, Foster City, Calif) using SYBR Green PCR Master Mix Kit (Applied Biosystems). The following primer sequences were used: β-actin: 5′ ccttcaacacccagccatgt, 3′ tgtggaccaccagaggcatac; Clca3: 5′ cacgggtgtctgtgttcatc, 3′ ccctagccacttccacattatc; IL-4: 5′ agatcatcggcattttgaacg, 3′ tttggcacatccatctccg; IL-13: 5′ aactgcagcaagaccgtgagt, 3′ tgcctcagttgccctgtgt. Known quantities of β-actin, Clca3, IL-4, and IL-13 served as standard curve.
Evaluation of allergic inflammation
Cellular infiltration into the airways was assessed by BAL fluid analysis at 72 hours after challenge, as previously described.12
Effect of antioxidants on surrogate ROS generator xanthine+xanthine oxidase-augmented airway inflammation
Mice were challenged with RWE mixed with surrogate O2.− generator, xanthine (0.32 mmol/L) + xanthine oxidase (50 mU; Sigma-Aldrich, St Louis, Mo). Alternatively, AA+NAC was added to challenge material that contained RWE+X+XO.
Kinetics of neutrophil recruitment
Ragweed pollen extract–sensitized mice were killed kinetically at 4 or 24 hours or immediately after RWE challenge (0 hour), and neutrophils were measured in BAL fluid. Data represent means ± SEMs (n = 5–6 animals/group) of 3 independent experiments.
Assay for total antioxidant potential
Ascorbic acid and NAC were administered intranasally in BALB/c mice, and BAL fluids were collected at different time points. The total antioxidant potential in BAL samples was measured spectrophotometrically at 490 nm by using an AOP-490 assay kit (OXIS International Inc., Foster City, Calif). A standard of known uric acid concentration was used to create calibration curve, and the results of the assay were expressed as mmol/L uric acid equivalents.
Statistics
Data from different treatment groups were analyzed by ANOVA, followed by Bonferroni post hoc analyses for least significant difference. Differences were considered significant at P < .05 (*P < .05, **P < .01, ***P < .001, ****P < .0001).
RESULTS
Antioxidants inhibit ROS generated by RWE pollen NADPH oxidase
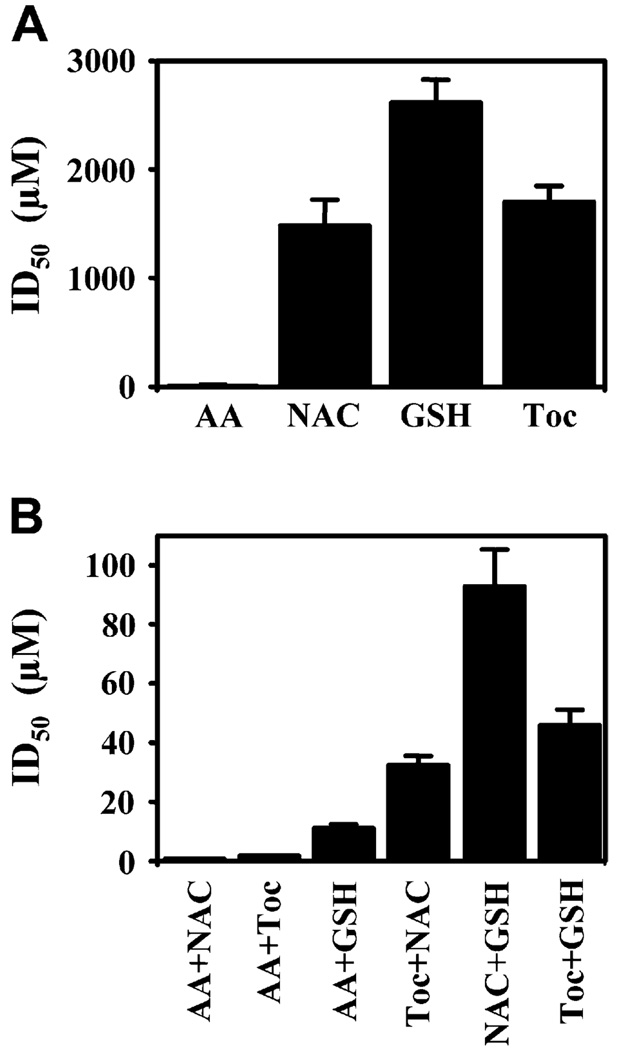
We have previously shown that ROS generated by RWE can be monitored by the change of redox sensitive nonfluorescent H2DCF-DA dye to fluorescent dichlorofluorescein. We compared the efficiency of antioxidants (AA, NAC, and tocopherol) to decrease ROS levels generated by RWE in solution by calculating their ID50. AA potently eliminated ROS (ID50, 5 µmol/L), whereas the ID50 of NAC, glutathione, and tocopherol was much higher (ID50, 1500, 2700, and 1900 µmol/L, respectively; Fig 1, A). These data are consistent with the fact that NAC is a glutathione precursor and alone has a low antioxidant potential. Glutathione is used as an electron acceptor by glutathione peroxidase and was used as a negative control. The combination of AA+NAC and AA+tocopherol had ID50 of 1 and 2 µmol/L, respectively (Fig 1, B). These data indicate that the combination of AA with either NAC or tocopherol synergistically (P = .003 and P = .001) scavenge RWE-induced ROS.
FIG 1.
Antioxidants inhibit RWE mediated oxidative stress in vitro. ID50 of individual (A) and combination (B) of antioxidants. GSH, Glutathione; Toc, tocopherol.
Antioxidants inhibit intracellular ROS in airway epithelial cells and 4-hydroxynonenal adduct formation in lungs
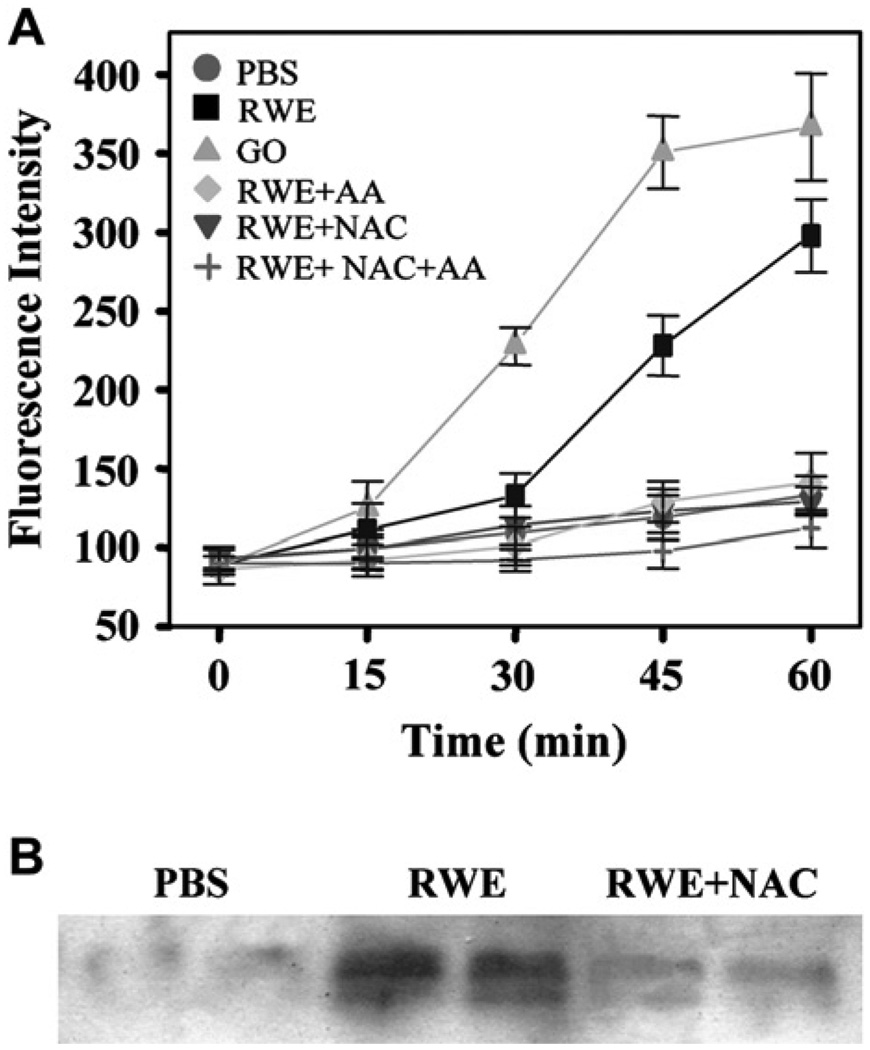
Building on our observation that antioxidants can scavenge ROS generated by RWE NADPH oxidase, we next determined whether they could decrease intracellular ROS levels. RWE exposure induced oxidative stress in A549 cells, and this was significantly decreased by addition of AA and NAC (Fig 2, A). In our previous study, we showed that RWE NADPH oxidase increases levels of 4-hydroxynonenal (4-HNE) in the BAL fluids.6 Here we extend our observations by demonstrating that RWE challenge increased 4-HNE protein adduct formation in murine lungs (Fig 2, B), and coadministration of AA+NAC inhibited 4-HNE protein adduct formation (Fig 2, B).
FIG 2.
Effect of antioxidants on RWE-induced intracellular ROS and 4-HNE protein adduct formation. A, Effect of increasing concentration of antioxidants on RWE-induced intracellular ROS in A549 cells. B, Effect of NAC on RWE-induced 4-HNE adduct formation in lungs. Each lane represents a different mouse. GO, Glucose oxidase.
Antioxidants decrease RWE-induced mucus hypersecretion
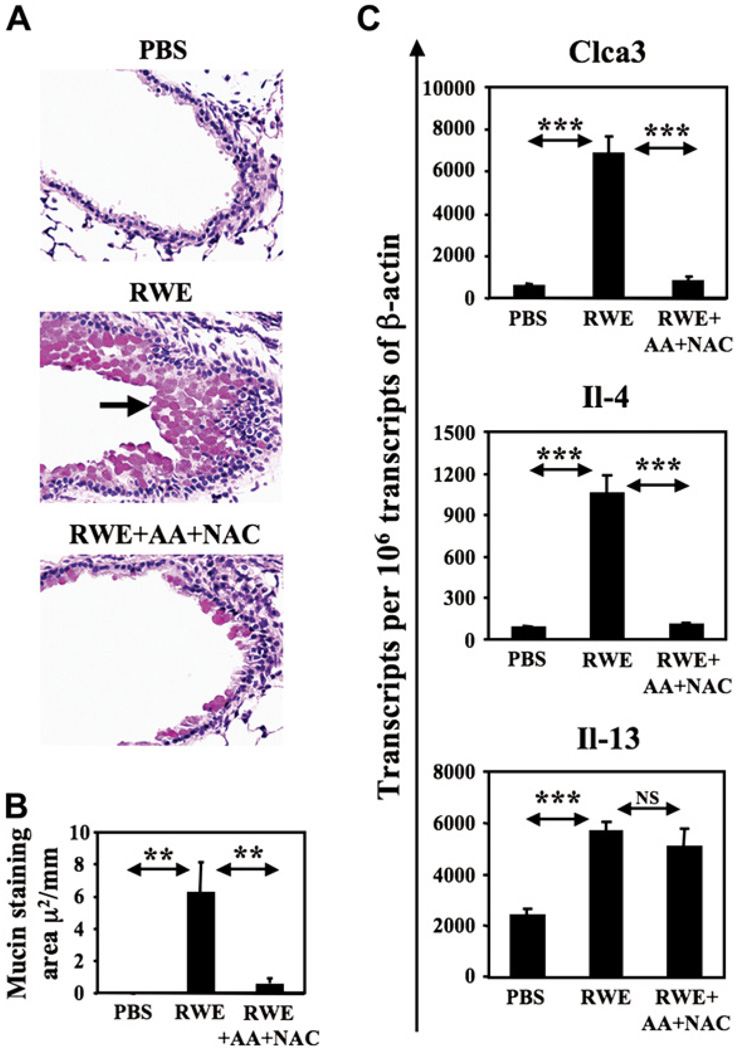
Mucus hypersecretion by goblet cells is an important hallmark of allergic asthma.13 PBS challenge failed to induce mucin production (Fig 3, A) with total mucin staining area being 0.0002 µm2 per mm of bronchial perimeter (Fig 3, B). RWE challenge significantly upregulated mucin production by 33,000-fold increase in mucin staining area (P < .01) (Fig 3, A and B). Coadministration of AA+NAC reduced RWE challenge–induced mucin production by 10-fold (P < .01) (Fig 3, A and B).
FIG 3.
Effect of AA+NAC on RWE-induced mucin production. A, RWE-sensitized BALB/c mice were challenged with PBS, RWE, or RWE+AA+NAC and killed at 72 hours. The PAS-stained lung sections show mucin content of airway epithelial cells. Arrow shows PAS-positive mucin globules. Magnification ×200. B, Morphometric quantification of mucin in airway epithelial cells. Results are means ± SEMs (n = 3–6 mice per group). C, Measurement of Clca3, IL-4, and IL-13 transcripts in the lungs by real-time quantitative PCR. **P < .01; ***P < .005.
Clca3 is a calcium-activated chloride channel 3 gene that regulates mucin production. Initially identified as gob-5, Clca3 is selectively expressed in airway goblet cells of allergen sensitized mice in response to challenge. 14 RWE challenge induced a 10-fold increase in Clca3 transcripts in the lungs compared with PBS challenge at 72 hours postchallenge (Fig 3, C). Coadministration of AA+NAC with RWE reduced Clca3 transcripts by 7-fold (Fig 3, C). IL-4 has also been shown to induce mucin gene expression and goblet cell metaplasia in vitro and in vivo.15 We have observed that the highest upregulation of IL-4 occurs 4 hours after allergen challenge (N. Dharajiya, B. Choudhury, R. Alan, B. Clancy, and S. Sur, unpublished data, March 2003). Building on this observation, we measured IL-4 expression in the lungs 4 hours postchallenge. RWE challenge induced an approximately 12-fold increase in IL-4 transcripts in the lungs, and coadministration of AA+NAC decreased this upregulation by 10-fold (Fig 3, C). Because IL-13 induces mucin production,16 we measured the effect of antioxidants on IL-13 expression in the lungs. RWE induced 2-fold increase in IL-13 transcripts in the lungs at 4 hours postchallenge compared with PBS. In our model, AA+NAC failed to downregulate this increase in IL-13 mRNA levels (Fig 3, C). These data suggest that inhibiting signal 1 by administering antioxidants suppresses mucin production, IL-4, and Clca3 transcripts in the lungs.
Scavenging the ROS generated by RWE NADPH oxidase attenuates allergic airway inflammation
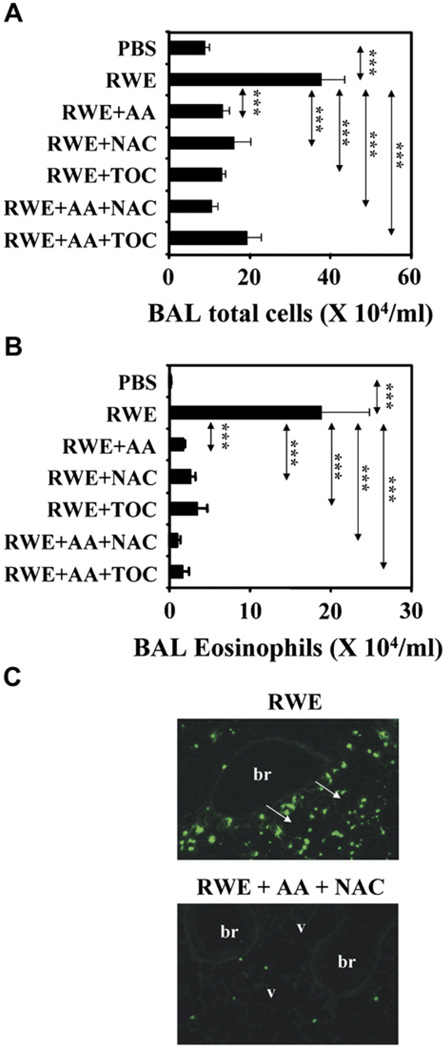
To study the effects of antioxidants on RWE-mediated allergic inflammation, we coadministered antioxidants with RWE in various combination in RWE-sensitized mice. PBS challenge in RWE-sensitized mice induced minimal inflammatory cell recruitment in the lungs, whereas RWE challenge increased total cells and eosinophils by 4-fold and 100-fold, respectively (Fig 4, A and B). Scavenging ROS produced by RWE pollen NADPH oxidase by coadministration of AA, tocopherol, and NAC with RWE decreased recruitment of total inflammatory cells by 2-fold to 3-fold and eosinophils by 5-fold to 10-fold (Fig 4, A and B). Combination of AA+NAC also decreased peribronchial recruitment of eosinophils (Fig 4, C). These findings indicate that inhibiting signal 1 generated by pollen NADPH oxidase by antioxidants significantly decreases allergic airway inflammation.
FIG 4.
Scavenging pollen NADPH oxidase–generated ROS inhibits allergic lung inflammation. Total inflammatory cells (A) and eosinophils (B) in BAL fluids of RWE-challenged mice. Results are means ± SEMs (n = 7–9 mice per group). ***P < .001. C, Effect of antioxidants on recruitment of eosinophils in peribronchial (br) and perivascular (v) regions (arrows). Magnification ×100. TOC, Tocopherol.
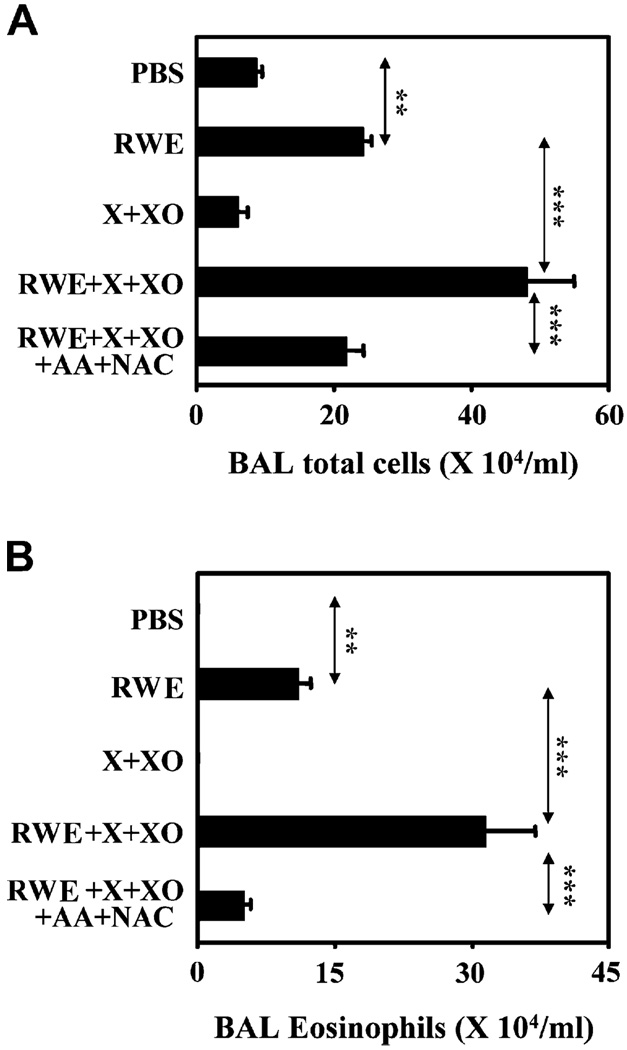
Xanthine oxidase generates superoxides similar to RWE pollen NADPH oxidase.6 Generating signal 1 by intrapulmonary administration of XO in RWE-sensitized mice failed to induce inflammation. As expected, the combination of signals 1 and 2 delivered by RWE increased inflammatory cell and eosinophil recruitment in the airways (Fig 5, A and B). Consistent with our 2-signal model, boosting signal 1 by coadministering X+XO with RWE induced robust allergic inflammation with 2-fold and 3-fold increase in total cells and eosinophils in the airways compared with RWE alone. When signal 1 was eliminated by coadministration of AA+NAC, it prevented XO-mediated increase in airway inflammation (Fig 5, A and B). These data provide additional evidence that antioxidants can prevent allergic inflammation by inhibiting signal 1 generated by pollen NADPH oxidase.
FIG 5.
Effect of antioxidants on X+XO-mediated augmentation of allergic airway inflammation. Effect of antioxidants on X+XO-augmented total inflammatory cell (A) and eosinophil (B) recruitment in BAL fluids. Results are means ± SEMs (n = 7–9 mice per group). **P < .01; ***P < .001.
Inhibiting signal 1 derived from pollen NADPH oxidase and not from inflammatory cells prevents airway inflammation
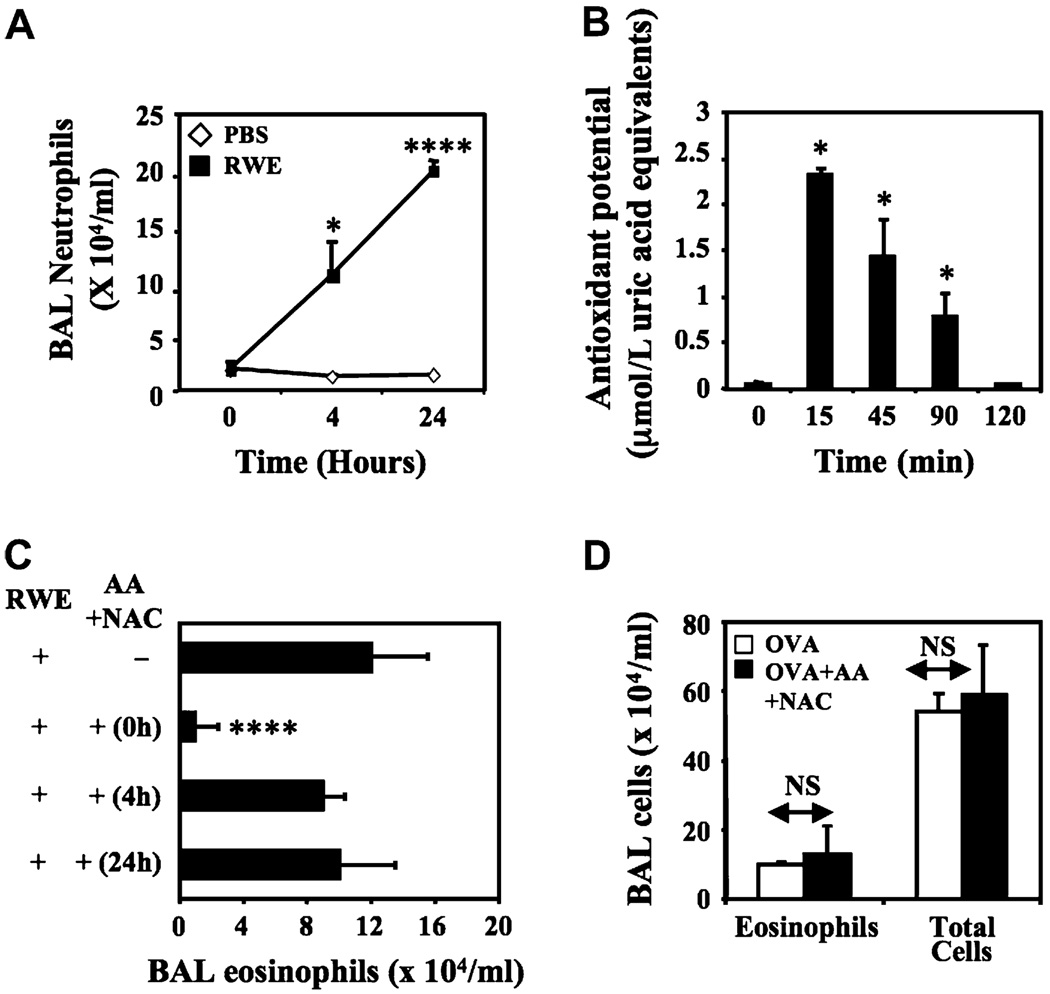
Allergen exposure induces an initial neutrophil recruitment that is followed by eosinophil recruitment in the airways,17 and these cells contribute to oxidative stress in the airways during allergic inflammation.18 Neutrophils and eosinophils contain membrane-associated NADPH oxidases that generate respiratory burst.19 Hence we examined whether the inhibitory effects of antioxidants on RWE-induced allergic airway inflammation is due to suppression of ROS derived from neutrophils and eosinophils recruited by allergen challenge. RWE challenge in sensitized mice recruited neutrophils starting at 4 hours and peak levels at 24 hours (Fig 6, A). Intrapulmonary administration of a single dose of AA increased antioxidant potential of airways for 90 minutes, and this returned to basal levels by 2 hours (Fig 6, B). We used this short-lasting effect of AA on antioxidant potential in the airways to compare the effect of scavenging ROSs induced by pollen NADPH oxidases from those induced by recruited neutrophils. Neutralization of RWE generated ROS by coadministering antioxidants prevented eosinophil recruitment in the airways (Fig 6, C). However, scavenging ROS generated by neutrophil recruited at 4 and 24 hours failed to inhibit allergic inflammation (Fig 6, A and C). Administration of AA+NAC failed to block allergic airway inflammation mediated by OVA, an allergen that does not induce oxidative stress in the airways (Fig 6, D).20 These findings indicate that antioxidants specifically prevent initiation of allergic airway inflammation by RWE NADPH oxidase–generated signal 1, and inhibiting ROS derived from recruited inflammatory cells has little effect on allergic airway inflammation.
FIG 6.
Scavenging ROS generated by RWE pollen NADPH oxidase and not by inflammatory cells inhibits allergic airway inflammation. A, Kinetics of neutrophil recruitment in the BAL fluid. RWE-sensitized mice were challenged with either PBS or RWE and killed kinetically, and BAL fluid was examined for cellular content. B, AA+NAC increased total antioxidant potential in the airways for 90 minutes. X-axis represents time after administration of antioxidants. Results are means ± SEMs (n = 3–6 mice per group). C, Analysis of eosinophil recruitment in the airways after administration of antioxidants at various time points. Results are means ± SEMs (n = 7–9 mice per group). *P < .05; ****P < .0001. D, BALB/c mice were sensitized with OVA and challenged with nebulized OVA. Mice were treated with either PBS or AA+NAC immediately before and just after nebulized OVA challenge. NS, Not significant.
Scavenging ROS generated during second RWE challenge inhibits allergic airway inflammation
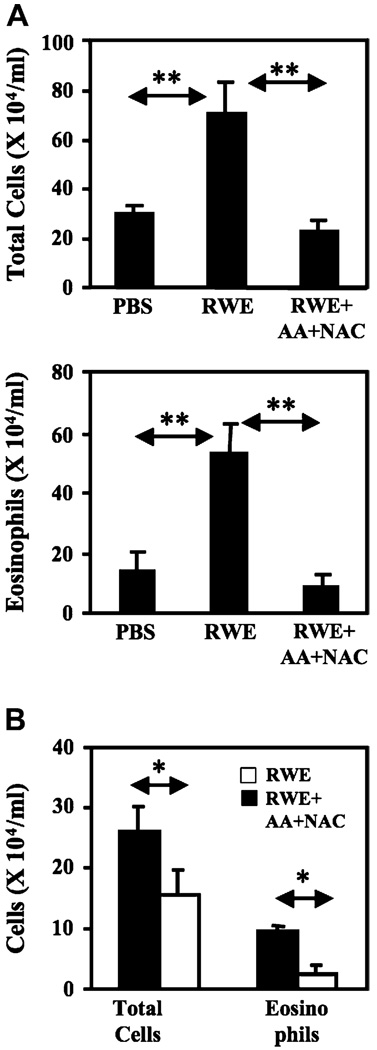
In our single RWE challenge model, recruitment of inflammatory cells peaks at 3 days (peak phase) after challenge and returns to baseline by 10 days (resolution phase). Because antioxidants blocked RWE-induced allergic inflammation in the single-challenge model, we tested their effects on a second RWE challenge performed during peak or resolution phase. RWE challenge during peak phase induced massive inflammatory cell recruitment to the lungs with a 2.4-fold and 3.6-fold increase in total cell and eosinophils, respectively (Fig 7, A). Coadministration of AA+NAC with RWE during peak phase decreased total cell and eosinophil recruitment by 3-fold and 6-fold, respectively (Fig 7, A). Similarly, coadministration of AA+NAC with RWE during resolution phase decreased total cell and eosinophil recruitment by 1.7-fold and 3.7-fold, respectively (Fig 7, B). These data indicate that ROS generated by RWE NADPH oxidase continues to play a proinflammatory role during repeated pollen exposure.
FIG 7.
Antioxidants inhibit second RWE challenge induced augmentation of allergic airway inflammation. A, AA+NAC prevents further increase in airway inflammation induced by second RWE challenge performed 72 hours after initial challenge. RWE-sensitized mice were challenged with PBS, RWE, or RWE+AA+NAC. A second similar challenge was performed 72 hours after the initial challenge; mice were killed 72 hours after last challenge, and BAL fluid was examined for cellular content. B, AA+NAC effectively inhibits RWE-induced airway inflammation during resolution phase. Mice were sensitized and challenged as described except the second challenge was performed 10 days after the first RWE challenge. *P < .05; **P < .01.
DISCUSSION
We recently reported that RWE contains intrinsic NADPH oxidases that induce robust oxidative insult to cultured epithelial cells,6,21 and in vivo in the airway epithelium independent of adaptive immune response (signal 1). Signal 1 boosts antigen-induced (signal 2) inflammatory process in the airways.6 An unanswered question was whether elimination of signal 1 by scavengers of ROS could inhibit allergic airway inflammation. In the current study, we demonstrate that AA, NAC, and tocopherol block RWE NADPH oxidase–induced intracellular oxidative stress in A549 bronchial epithelial cells. We further show that intrapulmonary administration of these antioxidants with RWE challenge inhibits RWE-induced allergic airway inflammation. These experiments provide additional evidence for our 2-signal hypothesis of initiation of allergic airway inflammation.6
We have previously shown that RWE NADPH oxidase increases malondialdehyde and 4-HNE levels in the BAL fluids.6 We also showed that 4-HNE induces phosphorylation of p38 mitogen-activated protein kinase,6 a redox-sensitive enzyme that regulates multiple downstream signaling pathways and generates a proinflammatory response.22,23 4-HNE administration in vivo along with antigen boosted antigen-induced allergic inflammation. 6 In the current study, we show that coadministration of antioxidants with RWE reverses RWE-induced 4-HNE adduct formation. Inhibition of 4-HNE adduct formation may be one of the mechanisms by which antioxidants decrease intensity of signal 1 and prevent airway inflammation.
One of the genes that regulates mucin production in the airway epithelial cells is Clca3, a member of the Ca2+-dependent chloride channel family.24 Clca3 and its human counterpart, Ca2+-dependent chloride channel 1, are increased in allergic airway inflammation and promote mucin production in the airways.14,25 Because the Clca family of proteins is activated by high cytosolic Ca2+26 and ROS can stimulate transient or sustained increase in cytosolic Ca2+,27 it is possible that antioxidant decreases Clca3 transcripts and subsequent mucin production by controlling ROS-mediated Ca2+ signaling.
Similar to the murine model of allergic airway inflammation shown here, oxidative stress occurs in human allergic asthma.28 Levels of hydrogen peroxide and nitric oxide are increased in exhaled gases of patients with asthma compared with controls.29 Also, the level of ROS in the airways is inversely correlated to FEV1 prediction in patients with asthma.30 Previous studies have shown that the antioxidant status of patients with asthma may be compromised. 31 In a study of plasma levels of antioxidants such as Trolox equivalent, acute attacks of asthma and chronic obstructive pulmonary disease significantly decreased antioxidant levels in serum.32 The concentrations of AA and tocopherol in lung lining fluid have been reported to be low in patients with mild asthma.33 Several approaches have been used to replenish the decreased antioxidants in patients with asthma. However, oral therapies using antioxidants have not been very successful in improving symptoms of asthma.34 Here we show that intrapulmonary administration of antioxidants increases antioxidant potential in the airway lining fluid. In future studies, it will be important to determine whether delivery of antioxidants locally into the airways induces a longlasting increase in airway antioxidant potential and, if so, whether it is more effective than systemic administration.
In the current study, we show that when antioxidants are administered 4 or 24 hours after RWE challenge in mice, they fail to inhibit allergic inflammation. An outstanding question was whether antioxidants block allergic airway inflammation nonspecifically because of their biochemical action in the airways. However, this possibility was ruled out because antioxidants failed to prevent allergic airway inflammation induced by nonoxidant allergen OVA, indicating that antioxidants specifically inhibit inflammation by oxidant allergens only. These observations are somewhat surprising because they suggest that either ROS produced by neutrophils cannot be blocked by antioxidants or these ROSs are not relevant for allergic inflammation. Antioxidants blocked second RWE challenge induced augmentation in allergic airway inflammation during late and resolution phases. These findings indicate that when antioxidants are administered in concert with RWE, antioxidants are able to exert its effects even after robust inflammation is already present. Regardless of what is the correct explanation, these observations suggest that if antioxidants are used as therapy in allergic inflammation, their role will be in prevention and not reversal of ongoing allergic airway inflammation.
In summary, our data indicate that ROS generated by NADPH oxidase in RWE (signal 1) continues to play a pro-inflammatory role during repeated pollen exposure. We propose that increasing antioxidant potential in the airways may be a novel therapeutic strategy to attenuate pollen-induced allergic airway inflammation.
Acknowledgments
Supported by grants from the National Institutes of Health (# 1R01 HL071163, S.S.; CA84461, I.B.), National Institute of Allery and Infectious Diseases (NIAID) Program Project Grant (#1P01 AI062885; S.S., PI on Project 4; I.B., PI on Project 5), National Heart, Lung, and Blood Institute Proteomics Initiative (#N01-HV28184) to S.S., and National Institute of Environmental Health and Sciences Center grant (#ES06676) to I.B.
We thank Drs B. K. Sur and S. Chitranshi, Kanpur, India, for their thought-provoking ideas on the role of antioxidants in asthma. We also thank Saurabh Bajpai of the Indian Institute of Technology, Kanpur, India, for his technical assistance with data analyses.
Abbreviations used
- AA
Ascorbic acid
- BAL
Bronchoalveolar lavage
- H2DCF-DA
2′–7′-Dihydro-dichlorofluorescein diacetate
- 4-HNE
4-Hydroxynonenal
- ID50
Inhibitory dose 50
- NAC
N-acetyl cysteine
- OVA
Ovalbumin
- PAS
Periodic acid-Schiff
- ROS
Reactive oxygen species
- RWE
Ragweed pollen extract
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
REFERENCES
- 1.Baulig A, Garlatti M, Bonvallot V, Marchand A, Barouki R, Marano F, et al. Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L671–L679. doi: 10.1152/ajplung.00419.2002. [DOI] [PubMed] [Google Scholar]
- 2.Hiltermann TJ, Peters EA, Alberts B, Kwikkers K, Borggreven PA, Hiemstra PS, et al. Ozone-induced airway hyperresponsiveness in patients with asthma: role of neutrophil-derived serine proteinases. Free Radic Biol Med. 1998;24:952–958. doi: 10.1016/s0891-5849(97)00381-x. [DOI] [PubMed] [Google Scholar]
- 3.Becker S, Soukup JM, Gallagher JE. Differential particulate air pollution induced oxidant stress in human granulocytes, monocytes and alveolar macrophages. Toxicol In Vitro. 2002;16:209–218. doi: 10.1016/s0887-2333(02)00015-2. [DOI] [PubMed] [Google Scholar]
- 4.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 5.Kagan V, Serbinova E, Packer L. Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem Biophys Res Commun. 1990;169:851–857. doi: 10.1016/0006-291x(90)91971-t. [DOI] [PubMed] [Google Scholar]
- 6.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sur S, Kita H, Gleich GJ, Chenier TC, Hunt LW. Eosinophil recruitment is associated with IL-5, but not with RANTES, twenty-four hours after allergen challenge. J Allergy Clin Immunol. 1996;97:1272–1278. doi: 10.1016/s0091-6749(96)70195-1. [DOI] [PubMed] [Google Scholar]
- 8.Sur S, Wild JS, Choudhury BK, Sur N, Alam R, Klinman DM. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J Immunol. 1999;162:6284–6293. [PubMed] [Google Scholar]
- 9.Sur S, Gleich GJ, Offord KP, Swanson MC, Ohnishi T, Martin LB, et al. Allergen challenge in asthma: association of eosinophils and lymphocytes with interleukin-5. Allergy. 1995;50:891–898. doi: 10.1111/j.1398-9995.1995.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 10.Sur S, Lam J, Bouchard P, Sigounas A, Holbert D, Metzger WJ. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. J Immunol. 1996;157:4173–4180. [PubMed] [Google Scholar]
- 11.Stafford S, Li H, Forsythe PA, Ryan M, Bravo R, Alam R. Monocyte chemotactic protein-3 (MCP-3)/fibroblast-induced cytokine (FIC) in eosinophilic inflammation of the airways and the inhibitory effects of an anti-MCP-3/FIC antibody. J Immunol. 1997;158:4953–4960. [PubMed] [Google Scholar]
- 12.Michalec L, Choudhury BK, Postlethwait E, Wild JS, Alam R, Lett-Brown M, et al. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J Immunol. 2002;168:846–852. doi: 10.4049/jimmunol.168.2.846. [DOI] [PubMed] [Google Scholar]
- 13.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, et al. Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc Natl Acad Sci U S A. 2001;98:5175–5180. doi: 10.1073/pnas.081510898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol. 1999;162:6233–6237. [PubMed] [Google Scholar]
- 16.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider T, van Velzen D, Moqbel R, Issekutz AC. Kinetics and quantitation of eosinophil and neutrophil recruitment to allergic lung inflammation in a brown Norway rat model. Am J Respir Cell Mol Biol. 1997;17:702–712. doi: 10.1165/ajrcmb.17.6.2849. [DOI] [PubMed] [Google Scholar]
- 18.MacPherson JC, Comhair SA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, et al. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166:5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 19.Bogdan C, Rollinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 20.Kloek J, Van Ark I, De Clerck F, Bloksma N, Nijkamp FP, Folkerts G. Modulation of airway hyperresponsiveness by thiols in a murine in vivo model of allergic asthma. Inflamm Res. 2003;52:126–131. doi: 10.1007/s000110300025. [DOI] [PubMed] [Google Scholar]
- 21.Bacsi A, Dharajiya N, Choudhury BK, Sur S, Boldogh I. Effect of pollen-mediated oxidative stress on immediate hypersensitivity reactions and late-phase inflammation in allergic conjunctivitis. J Allergy Clin Immunol. 2005;116:836–843. doi: 10.1016/j.jaci.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pourazar J, Mudway IS, Samet JM, Helleday R, Blomberg A, Wilson SJ, et al. Diesel exhaust activates redox-sensitive transcription factors and kinases in human airways. Am J Physiol Lung Cell Mol Physiol. 2005;289:L724–L730. doi: 10.1152/ajplung.00055.2005. [DOI] [PubMed] [Google Scholar]
- 23.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 24.Komiya T, Tanigawa Y, Hirohashi S. Cloning and identification of the gene gob-5, which is expressed in intestinal goblet cells in mice. Biochem Biophys Res Commun. 1999;255:347–351. doi: 10.1006/bbrc.1999.0168. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino M, Morita S, Iwashita H, Sagiya Y, Nagi T, Nakanishi A, et al. Increased expression of the human Ca2+-activated Cl− channel 1 (CaCC1) gene in the asthmatic airway. Am J Respir Crit Care Med. 2002;165:1132–1136. doi: 10.1164/ajrccm.165.8.2107068. [DOI] [PubMed] [Google Scholar]
- 26.Gruber AD, Elble RC, Ji HL, Schreur KD, Fuller CM, Pauli BU. Genomic cloning, molecular characterization, and functional analysis of human CLCA1, the first human member of the family of Ca2+-activated Cl− channel proteins. Genomics. 1998;54:200–214. doi: 10.1006/geno.1998.5562. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 28.Vachier I, Damon M, Le Doucen C, de Paulet AC, Chanez P, Michel FB, et al. Increased oxygen species generation in blood monocytes of asthmatic patients. Am Rev Respir Dis. 1992;146:1161–1166. doi: 10.1164/ajrccm/146.5_Pt_1.1161. [DOI] [PubMed] [Google Scholar]
- 29.Emelyanov A, Fedoseev G, Abulimity A, Rudinski K, Fedoulov A, Karabanov A, et al. Elevated concentrations of exhaled hydrogen peroxide in asthmatic patients. Chest. 2001;120:1136–1139. doi: 10.1378/chest.120.4.1136. [DOI] [PubMed] [Google Scholar]
- 30.Jarjour NN, Calhoun WJ. Enhanced production of oxygen radicals in asthma. J Lab Clin Med. 1994;123:131–136. [PubMed] [Google Scholar]
- 31.Vural H, Uzun K. Serum and red blood cell antioxidant status in patients with bronchial asthma. Can Respir J. 2000;7:476–480. doi: 10.1155/2000/907478. [DOI] [PubMed] [Google Scholar]
- 32.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154:1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 33.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999;354:482–483. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 34.Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M, et al. Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo-controlled trial. Clin Exp Allergy. 2003;33:1355–1359. doi: 10.1046/j.1365-2222.2003.01777.x. [DOI] [PubMed] [Google Scholar]