Abstract
A large body of literature advocates exercise as a successful intervention for increasing positive affect while also reducing negative affect and anxiety. Questions concerning the mechanisms driving these effects remain unanswered, particularly considering theorized attentional adaptations that may be elicited by acute exercise bouts. We investigated pre- and post-exercise attentional bias to examine possible attentional explanations that may account for these reported changes in affect. On separate visits to the laboratory, 30 high trait anxious participants completed 30 min of exercise on a cycle ergometer at 70% of their heart rate reserve, or completed a 30-min quiet rest protocol. During each intervention, pre-test and post-test modified dot-probe assessments of attentional bias were completed, as were a series of self-report anxiety and affect questionnaires. Attentional bias scores and reaction times were calculated. Post-exercise dot probe performance did not vary significantly as a function of the affective valence of presented stimuli. As hypothesized, however, positive affect and reaction time improved significantly following exercise compared with the pre- and post-rest conditions and the pre-exercise condition, suggesting that exercise facilitates a broadening of attentional scope. Implications of these findings and future directions are discussed within the context of traditional and contemporary theories of dispositional affect and state-specific emotional responses.
Keywords: Anxiety, attentional bias, aerobic, emotion
Introduction
Health care providers increasingly advocate exercise interventions independent of or in conjunction with psychological and pharmacological therapies for affective problems (Fox, 1999). Although exercise is not a panacea for mental or physical ailments, exercise prescription can reduce the burden of rising health care costs (Jones & Eaton, 1994) and can be viewed as a practical alternative or auxiliary treatment that may be more easily accessible than other treatment options. Most germane to the current study is the abundance of literature establishing the effectiveness of exercise as a treatment for maladaptive mood or emotional problems (Barbour & Blumenthal, 2005; Otto et al., 2007; Paluska & Schwenk, 2000; Penedo & Dahn, 2005).
Emotion-enhancing effects of exercise
Evidence suggests that physical activity enhances affect when performed at a “manageable level” for the individual engaged in the physical activity (Hansen, Stevens, & Coast, 2001; Salmon, 2001). Varying explanations have been proposed to account for these changes. Considerable evidence supports the role of physiological adaptations that drive such effects, as captured in such notions as the endorphin and monoamine hypotheses (Boecker et al., 2008; Cotman & Berchtold, 2002). Psychological accounts offer attractive and complementary approaches to understanding why there are improvements in positive affect and the amelioration of anxiety and negative affect following exercise. Conceptual notions such as self-efficacy theory and the distraction hypothesis have historically been adopted to explain these improvements (Craft, 2005). While self-efficacy has received the lion’s share of empirical investigation, attentional explanations remain viable, particularly when considering the hallmark symptoms of anxiety and affective problems.
The distraction hypothesis advocates that engaging in exercise results in a “time-out” that distracts one from ruminating on worrisome thoughts. Thus, reductions in state anxiety and negative affect reported by individuals after exercise are thought to result from breaking away from day-to-day routines and negative ideations that may perpetuate anxiety or additional maladaptive emotional states (Bahrke & Morgan, 1978; Morgan, 1985). Comparing exercise to alternative activities also serving as a distraction (i.e. relaxation, humour, and social contact) has been the primary method for investigating the merit of the distraction hypothesis (Szabo, Ainsworth, & Danks, 2005). The distraction hypothesis offers a reasonable account for the robust finding of reduced state anxiety and negative affect after exercise by asserting that exercise creates a diversion from unfavourable stimuli. It fails, however, to provide a rationale for why exercise induces positive affect. In addition, while evidence for an attentional effect has been inferred by designs comparing “other distracting activities” to exercise, the distraction hypothesis remains largely inadequate in explaining the mechanisms driving affective changes.
Affect and attentional bias
Although links among anxiety, affect, and attention have been presented in the context of the distraction hypothesis, researchers have not objectively assessed attention to establish how exercise influences these concepts. Whether (and how) attentional mechanisms account for reported changes in affect following exercise thus remains largely unknown. Merging mainstream methods from exercise psychology with a robust measure of affect-related attentional shifts provides an attractive approach to determining attentional mechanisms underlying emotional alterations following exercise.
A widely implemented means for investigating attentional biases is the now classic dot-probe paradigm (MacLeod, Mathews, & Tata, 1986) and its contemporary modifications. The typical dot-probe protocol consists of a valenced stimulus (unpleasant or pleasant) being simultaneously presented with a neutral stimulus. At stimuli offset, a dot replaces either the valenced or neutral cue, with the participant being required to respond to the location of the dot presentation as quickly and accurately as possible. To address criticisms of early versions of the dot-probe protocol, more recent studies implementing the task have relied on “modified” versions that use similar shaped characters (i.e. a “p” or “q”) to serve as the probes, rather than a single dot. Regardless of the probe type used, attention is inferred from response latency, with response times being quicker to probes replacing visually attended stimuli versus unattended ones. Considerable evidence indicates that individuals with increased emotional disturbance detect probes significantly faster (than moderate/low anxious controls) when the dot replaces a threat cue (see Bar Haim, Lamy, Pergamin, Bakermans-Kraneburg, & van Ijzendoorn, 2007; for reviews, see Mogg, Bradley, & Williams, 1995; Mogg, Mathews, & Eysenck, 1992), thus demonstrating an attentional bias towards threatening stimuli.
By utilizing tools like the dot-probe protocol and self-report measures, researchers have collected ample evidence indicating that individuals with high anxiety and those with affective disorders are biased towards threatening cues and are more likely to interpret stimuli as threatening (MacLeod & Mathews, 1988; Stopa & Clark, 2000; Williams, Watts, MacLeod, & Mathews, 1988, 1997; Yoon & Zinbarg, 2007; for exceptions, see Schmulke, 2005). Previously, researchers have shown that these attentional biases are common to several additional emotion-related disorders, and that they may precede and perpetuate maladaptive emotional responses (Mogg & Bradley, 2005; Mogg, Philippot, & Bradley, 2004). Importantly, researchers have yet to implement the dot-probe paradigm to assess how exercise may alter attentional biases responsible for perpetuating negative affect and anxiety, as is suggested by the distraction hypothesis.
Attentional biases have now been accommodated in theoretical approaches to understanding the influence of subclinical anxiety (and symptoms of clinical anxiety) levels on attentional control abilities and the resultant efficiency and effectiveness of performance. In particular, attentional control theory (Eysenck, Derakshan, Santos, & Calvo, 2007) offers a viable explanation as to why changes in anxiety (in this case, via aerobic activity) may lead to alterations in attentional patterns that manifest in performance changes. Critically, while attentional control theory has been used almost exclusively to explain the effects of anxiety on performance in numerous domains (i.e. cognitive tasks: Bonnot & Croizet, 2007; continuous motor tasks: Nieuwenhuys, Pijpers, Oudejans, & Bakker, 2008; Wilson, Vine, & Wood, 2009; reaction time tasks: Derakshan, Ansari, Hansard, Shoker, & Eysenck, 2009), we are implementing it here not to focus on performance, but to index and explain how attentional changes manifest in affective alterations.
Attentional control theory asserts that high anxious individuals possess reduced attentional control, specifically characterized by an inability to shift away from and inhibit threatening stimuli or worrisome thoughts. Such impairments of the inhibition and shifting functions of the central executive result in cognitive interference, which reduces the processing and temporary storage capacity of the working memory system and induces attentional bias (Eysenck et al., 2007; MacLeod & Rutherford, 1992; Mogg et al., 2000). Consequently, cognitive anxiety exhausts attentional resources that otherwise would be devoted to the task, thereby perpetuating a narrow attentional focus and often resulting in reduced performance efficiency (Derakshan et al., 2009; Janelle, Singer, & Williams, 1999). To further underscore the impact of anxiety on attention, Coombes and colleagues (Coombes, Higgins, Gamble, Cauraugh, & Janelle, 2009) recently found that regardless of emotional manipulation (pleasant, unpleasant or neutral), high trait anxious participants demonstrate less attentional control and perform significantly less efficiently than low trait anxious participants. Importantly, while attentional control theory makes specific predictions regarding attentional changes based on the amelioration of anxiety, it does not account for alterations in affect possibly driven by modulations in attentional scope. Complementary work, however, indicates a broadened attentional scope is associated with greater working memory capacity (Forster, Friedman, Ozelsel, & Denzler, 2006) and positive affect (Gable & Harmon-Jones, 2008; Gasper, 2004; Gasper & Clore, 2002; Rowe, Hirsh, & Anderson, 2007). Although studies showing this association typically assert that positive affect induces a broad attentional focus, a viable alternative assertion is that the relationship is cyclical; attentional broadening following exercise may permit a shift towards positive affective experience.
The tenets of attentional control theory provide a theoretical framework to explain the underlying attentional mechanisms responsible for post-exercise alterations in anxiety and affect. More specifically, it is reasonable to posit that aerobic exercise induces an attentional shift (via distraction) away from worrisome thoughts, thereby releasing attentional resources that can then be directed towards task-oriented stimuli (e.g. monitoring heart rate, motor demands of the exercise task). Previously, researchers have indicated that a broadened attentional focus is strongly associated with positive affect (Fredrickson, 2001; Gable & Harmon-Jones, 2008). Thus, shifting attention away from worrisome stimuli may serve not only to reduce anxiety and negative affect but also to enable an increase in positive affect. Furthermore, focusing on exercise-related thoughts such as feelings of mastery or accomplishment (Miller & Bartholomew, 2007), attributing physiological arousal (i.e. elevated heart and respiration rates) formerly associated with somatic anxiety to exercise activity (Clark, 1986; van Zijderveld et al., 1992), and interest in task demands and information (Fredrickson, 2001; Ryan & Deci, 2000) are predictive of positive affect and reductions in state anxiety.
Recent work has indicated that attentional bias protocols may be effective in assessing the efficacy of interventions aimed at extinguishing maladaptive emotional responses (Townshend & Duka, 2007). To date, however, the interplay of attentional bias and exercise has been neglected. A notable exception is the work of Berry (2006) who, using a Stroop task, found that exercisers showed an attentional bias for exercise-related words, while non-exercisers were biased towards words related to a sedentary lifestyle. While novel and important, Berry did not implement an exercise intervention. As such, no study, as far as we are aware, has examined the effects of exercise on affect in anxious individuals via the modified dot-probe protocol.
In summary, researchers agree that exercise improves affect but the mechanisms that underpin these effects remain unknown. Despite numerous explanations of why exercise acts to increase positive affect while reducing negative affect and state anxiety, the postulate that exercise ameliorates maladaptive allocation of attention that tends to characterize those with emotional disturbances remains viable yet unexplored. Thus, the purpose of this experiment was to determine whether a behavioural index of attentional bias known to characterize affective dysfunction would be altered by acute aerobic exercise.
To address this aim, we measured emotion-related attentional biases before and after participants engaged in a 30-min exercise bout and a 30-min quiet rest protocol. Before and immediately after each intervention, the modified dot-probe protocol and inventories measuring state anxiety and affective disposition were administered. Three hypotheses were tested. First, we predicted that improvements in positive affect, negative affect, and state anxiety would manifest following exercise. Second, it was anticipated that exercise would induce greater attentional capacity and an overall broadening of attentional scope, demonstrated by faster reaction times irrespective of probe location and emotional stimuli. Increased efficiency would be evidenced by significantly faster reaction time following exercise compared with pre- and post-rest conditions and the pre-exercise condition. In addition, this speeded response would be ubiquitous across emotional stimuli presented. Lastly, we hypothesized that an acute bout of exercise would result in a relative bias away from unpleasant stimuli (and towards pleasant stimuli) as indexed by a bias score, suggesting that the attentional bias towards negatively valenced cues often exhibited in anxious individuals is attenuated by exercise.
Methods
Participants
Altogether, 30 participants (19 females, 11 males; mean age 19.8 years, s =0.9) completed the experiment and received extra credit as compensation. Sample size was determined by an a priori analysis using the G*Power general power analysis program (Erdfelder, Faul, & Buchner, 1996). Alpha was set at 0.05 with a corresponding power of 0.80 to detect a medium effect size. A medium effect size was selected according to the convention established by Cohen (1988) for estimating effect size in the absence of existing data. Accordingly, a sample size of 30 was determined to be adequate. Participants were prescreened from a total sample of 726 individuals who were registered for various courses in the College of Health and Human Performance at the University of Florida and selected for inclusion contingent on their trait scores from the State–Trait Anxiety Inventory (Spielberger, 1983). Participants who scored higher than 40 on the State–Trait Anxiety Inventory were classified as high trait anxious (mean 53.6, s =4.20) (Bradley, Mogg, Falla, & Hamilton, 1998; MacLeod & Mathews, 1988; Mogg & Bradley, 2002) and contacted for inclusion. All procedures were approved by the university’s institutional review board and all participants provided written informed consent before taking part in the experiment.
Instrumentation and task
Self-report inventories were used to measure participants’ ability to engage in physical activity safely and as indices of personality and affect. The Physical Activity Readiness Questionnaire (Shephard, 1988), State–Trait Anxiety Inventory (Spielberger, 1983), and Positive and Negative Affect Schedule (Watson, Clarke, & Tellegen, 1988) were administered. Given the comorbidity between anxiety and depression, the revised Beck Depression Inventory (Beck & Steer, 1987) was also administered. However, no participants reported a high level of depression (30 or above), and consequently the sample size was unchanged for all statistical analyses.
Physical Activity Readiness Questionnaire
The Physical Activity Readiness Questionnaire was administered to determine whether participants could safely engage in physical activity. The questionnaire serves as a screening tool that asks “yes” or “no” questions pertaining to the participant’s medical history before his or her involvement in exercise. “Yes” responses serve as a warning to those prescribing exercise that the participant should be seen by a physician prior to exercising. For this study, no participants were denied inclusion, as responses indicated that all participants were able to safely complete the exercise protocol.
State–Trait Anxiety Inventory
The trait and state versions of the State–Trait Anxiety Inventory assess global and transient levels of anxiety, respectively. While the inventory has been identified as a unideminsional tool for assessing anxiety, an individual’s aggregate State–Trait Anxiety Inventory score is arrived at by totalling scores on multiple questions assessing several different dimensions including apprehension, tension, nervousness, and worry. Each inventory comprises 20 questions scored on a 4-point Likert scale. Reliability scores range from 0.65 to 0.86 for the trait version of the State–Trait Anxiety Inventory, while reliability scores for the state version range from 0.16 to 0.62.
Positive and Negative Affect Schedule
The Positive and Negative Affect Schedule is a 20-question self-report measure quantifying positive and negative affect. The 10 positive affects are: interested, excited, strong, enthusiastic, proud, alert, inspired, determined, attentive, and active. The 10 negative affects are: distressed, upset, guilty, scared, hostile, irritable, ashamed, nervous, jittery, and afraid. The reliability of the positive affect and negative affect scales is 0.89 and 0.85, respectively.
Revised Beck Depression Inventory
The Beck Depression Inventory measures characteristic attitudes and symptoms associated with depression. The inventory consists of 21 items each rated on a 4-point scale with 0 being the lowest score and 3 being the highest score for each item presented. Total scores above 30 are indicative of severe depression. The measure is routinely implemented and found to be reliable (0.93) and consistent (0.91) (Beck, Steer, & Garbin, 1988).
Modified dot-probe task
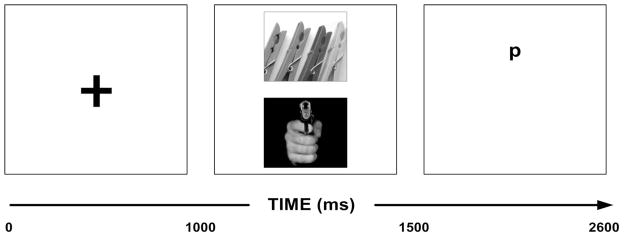
As shown in Figure 1, each trial began with a white fixation cross appearing in the centre of a black presentation screen for 1000 ms. Appearance of the fixation cross served as a reference point for the initiation of each trial. Following the offset of the fixation cross, two images became visible for 500 ms, one stacked vertically above the other. At image offset, the screen went blank aside from a white lower-case “p” or “q” presented at the centre location of the upper or lower image. The “p” or “q” functioned as the “probe” with participants being required to press the corresponding response key as quickly as possible following picture offset. The probe was visible for 1100 ms. If no response was made within 1100 ms, the trial timed out and an error was recorded. In total, the modified dot-probe task consisted of 48 experimental trials. Positions of the probe and emotional scenes were randomized and counterbalanced so that each appeared in both locations an equal number of times. Moreover, on each of the four test occasions a different presentation format and order were used. Accuracy of responses, reaction times, timed out trials, and slide pairings were recorded by a Lab-VIEW computer program (National Instruments 8.0, Austin, TX).
Figure 1.
Schematic representation of the modified dot-probe protocol. (1) A fixation cross appears. (2) Paired stimuli appear. (3) Following the offset of the stimuli pair, a probe appears in a spatial location occupied by one of the previous stimuli. Participants are asked to respond via a keyboard whether they saw a “p” or “q” as quickly and accurately as possible.
Emotional manipulation
The International Affective Picture System (CSEA-NIMH, 2005) is a standardized catalogue of visual stimuli used worldwide to manipulate emotion. The International Affective Picture System (IAPS) consists of affective pictures (unpleasant, pleasant, and neutral) that have been normed on the dimensions of stimulus valence, arousal, and dominance (Lang, Bradley, & Cuthbert, 2005) (Table I). Experimental trials were created by selecting two pictures from the International Affective Picture System, including scenes representing pleasant (i.e. sport/adventure, erotica, babies), neutral (i.e. household objects, neutral faces), and unpleasant (disgust, mutilation, attack) valence categories. Pictures were presented full screen on a 19-inch LCD monitor (1024 × 768 resolution; 75-Hz refresh rate, 32-bit colour). Each stimulus array included one valenced image (either pleasant or unpleasant) and one neutral picture. Participants completed 48 trials, viewing two pictures per trial. Accordingly, 24 pleasant, 24 unpleasant, and 48 neutral pictures were selected based on affective normative ratings.
Table I.
IAPS means, standard deviations, and slide identification numbers for pleasant, unpleasant, and neutral images.
| Pleasant | Unpleasant | Neutral | |
|---|---|---|---|
| Valence | 6.81 (0.53) | 1.88 (0.36) | 5.01 (0.40) |
| Arousal | 6.18 (0.61) | 6.36 (0.61) | 3.13 (0.63) |
| Slide number | 4643, 4645, 4647, 4649, 4650, 4651, 4652, 4653, 4656, 4658, 4659, 4660, 4664, 4666, 4669, 4670, 4672, 4676, 4677, 4680, 4681, 4683, 4687, 4689, 4690, 4694, 4695, 4800, 4810, 5600, 5611, 5623, 5626, 5628, 5629, 5700, 6521, 8080, 8170, 8200, 8205, 8400, 8420, 8300, 8180, 8185, 8193, 8179 | 3015, 6230, 9254, 9425, 9428, 3500, 3530, 3170, 3181, 3191, 3101, 3053, 3100, 3102, 3110, 3120, 3140, 9252, 9433, 9300, 3250, 3266, 3000, 3010, 3016, 3017, 3030, 3051, 3061, 3062, 3063, 3068, 3069, 3071, 3080, 3130, 3150, 3168, 3225, 9050, 9500, 3400, 9405, 9400, 9410, 9040, 9250 | 1945, 2102, 2104, 2200, 2206, 2210, 2214, 2215, 5030, 5120, 5130, 5500, 5510, 5520, 5530, 5531, 5532, 5533, 5534, 5535, 5740, 6150, 7000, 7002, 7004, 7006, 7009, 7010, 7020, 7025, 7030, 7031, 7034, 7035, 7036, 7037, 7038, 7039, 7040, 7041, 7042, 7043, 7046, 7050, 7052, 7053, 7054, 7055, 7056, 7057, 7058, 7059, 7060, 7080, 7090, 7095, 7096, 7100, 7110, 7130, 7140, 7150, 7160, 7161, 7170, 7175, 7179, 7180, 7182, 7183, 7184, 7185, 7186, 7187, 7188, 7190, 7192, 7205, 7207, 7211, 7217, 7233, 7234, 7235, 7236, 7237, 7242, 7247, 7248, 7249, 7500, 7547, 7705, 7950, 9210, 9700 |
Exercise protocol
The current study adhered to practical recommendations for engaging in exercise offered by the American College of Sports Medicine (2006). The exercise prescription was also similar to previous research in both intensity (Hansen et al., 2001; Russell et al., 2003) and duration (Hansen et al., 2001; Kilpatrick, Jarreau, Bartholomew, & Kraemer, 2004; Tate & Petruzzello, 1995).
The American College of Sports Medicine (2006) advocates that heart rate reserve is an efficacious means for gauging the intensity in which one should exercise aerobically. Additional American College of Sports Medicine guidelines suggest exercising at 40/50–85% of heart rate reserve, for 20–30 min (not including warm up and cool down periods) to achieve the necessary benefits associated with aerobic activity. Based on these recommendations, participants in the present study engaged in 30 min of exercise on a cycle ergometer at 70% of their heart rate reserve. The exercise protocol commenced with a 5-min warm-up of light-intensity pedalling (20% of heart rate reserve) and concluded with a 5-min cool down at the same exercise intensity.
Heart rate
Heart rate was measured using a Polar Favor heart rate monitor (Polar CIC Inc., New York). Participants were fitted with a chest strap and watch providing them with continuous feedback to assist them in maintaining the desired work rate prescribed (70% of heart rate reserve). Heart rate monitors have been shown to be a valid and reliable measure for gauging exercise intensity (American College of Sports Medicine, 2006).
Procedure
Participants made two separate visits to the laboratory with the average elapsed time between visits being 1.7 days (s =1.0). Before arrival at the laboratory, participants were informed as to which of the protocols they would complete first: the exercise or quiet rest condition. Upon arrival at the laboratory, to ensure participants understood the protocol and were able to safely engage in physical activity, they were asked to complete a university-approved informed consent form, and the Physical Activity Readiness Questionnaire. Before each experimental condition, the state scale of the State–Trait Anxiety Inventory and the state version of the Positive and Negative Affect Schedule were completed, followed by a pretest modified dot-probe assessment. Participants completed 10 practice trials consisting of neutral images before the pre- and post-test modified dot-probe tasks.
Participants assigned to the quiet rest condition were asked to rest for 30 min. To occupy their time they were allowed to bring leisure reading material (e.g. magazines) while resting alone and quietly in a sound-attenuated room. To avoid unintentional manipulations of anxiety levels before the start of the quiet rest period, the experimenter told the participants that they could read or rest as long as they did not fall asleep or study materials that were related to their schoolwork. After 30 min had elapsed, participants were immediately administered the state form of the State–Trait Anxiety Inventory and the Positive and Negative Affect Schedule for the second time, and completed the modified dot-probe post-test within 5 min of the end of the rest intervention.
For the exercise condition, participants engaged in 30 min of exercise on a cycle ergometer at 70% of their heart rate reserve (American College of Sports Medicine, 2006). A heart rate monitor and feedback from the experimenter were used to assist participants in maintaining their desired work rate. Like the non-exercise protocol, participants completed pre-and post-test State–Trait Anxiety Inventory and Positive and Negative Affect Schedule assessments and the modified dot-probe task before and after exercising within the same time frame as previously mentioned. The Beck Depression Inventory was administered following the completion of the participants’ second session to gather information regarding levels of depression. Upon completion of both experimental protocols and all questionnaires, participants were debriefed and thanked for their involvement. The total time for each of the required two sessions was approximately 1 h.
Data reduction and analysis
Participants’ reaction time to probes (“p” or “q”) replacing valenced stimuli were the primary dependent measures of interest. Reaction time was calculated as the time between the offset of the presented pictures (or onset of the probe) and the time it took participants to press the corresponding response key. In congruence with previous dot-probe studies, reaction times were averaged within pleasant and unpleasant conditions, and within match and mismatch trials (Dewitte, Koster, De Houwer, & Buysse, 2007; MacLeod et al., 1986). Match trials referred to trials where the “p” or “q” replaced the valenced image (pleasant or unpleasant) rather than the neutral image. Mismatch trials occurred when the probe replaced neutral as opposed to valenced images. Reaction time was analysed in a four-way repeated-measures analysis of variance (ANOVA): 2 (Intervention: rest, exercise) × 2 (Time: pre, post) × 3 (Valence: pleasant, unpleasant, neutral) × 2 (Location: match, mismatch).
As is typical with modified dot-probe studies, bias scores were created to determine whether attentional allocation had shifted as a result of intervention type. Bias scores were calculated by subtracting matched (valenced) scores from mismatched scores/trials. Based on this method of calculation (Bradley, Mogg, & Millar, 2000), higher (positive) bias scores indicate greater vigilance to the location of the valenced cues, while negative scores indicate avoidance of the valenced cues. The summary statistics were averaged and reduced similar to the reaction time data. Attentional bias scores were analysed in a three-way repeated-measures ANOVA: 2 (Intervention: rest, exercise) × 2 (Time: pre, post) × 2 (Valence: pleasant, unpleasant). Self-report scores were analysed in a two-way repeated-measures ANOVA: 2 (Intervention: rest, exercise) × 2 (Time: pre, post).
For all analyses, if the sphericity assumption was violated, Greenhouse-Geisser degrees of freedom corrections were applied. Follow-up analyses were conducted using simple effects tests and Tukey’s HSD procedure for significant interactions and main effects, respectively. For all analyses, the probability value was set at P < 0.05.
Results
Self-report data
Positive affect
Figure 2 shows positive affect scores across Intervention and Time. A significant main effect of Intervention on positive affect (F1,29 = 7.26, P < 0.05, ) was qualified by a significant Intervention × Time interaction (F1,29 = 42.03, P < 0.05, ). Follow-up analyses on the significant interaction showed that positive affect was significantly greater after exercise relative to all other conditions. A significant main effect of Time was not observed (F1,29 = 3.41, P > 0.05, ).
Figure 2.
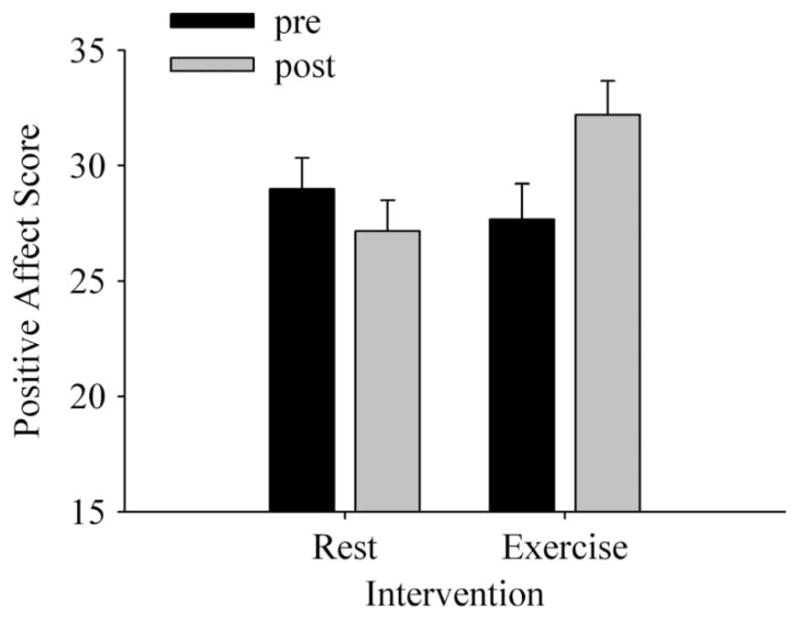
Positive Affect (Positive and Negative Affect Schedule) among high anxious participants before and after rest and exercise.
Negative affect
Main effects of Time (F1,29 = 16.30, P < 0.05, ) and Intervention (F1,29 = 8.44, P < 0.05, ) were found for negative affect scores. Follow-up analyses indicated that negative affect scores were significantly lower for the post-test compared with the pre-test condition, and lower for the exercise intervention compared with the rest intervention. No significant interaction between Intervention and Time was observed (F1,29 = 0.04, P > 0.05, ).
State anxiety
Anxiety scores varied significantly as a function of Time (F1,29 = 5.01, P < 0.05, ) and Intervention (F1,29 = 5.50, P < 0.05, ), with follow-up analyses revealing that post-test levels of anxiety were significantly lower than pre-test levels, and finally that state anxiety scores for exercise were lower than for the rest intervention. The Intervention × Time interaction on state anxiety scores was not significant (F1,29 = 0.03, P > 0.05, ).
Reaction time data
Analysis of reaction time data indicated that reaction time was significantly faster following exercise (F1,29 = 12.33, P < 0.05, ). Follow-up tests confirmed visual inspection of the data (Figure 3). Specifically, reaction time following exercise was significantly reduced compared with the pre-rest, post-rest, and pre-exercise conditions. Main effects for Intervention, Time, Valence, and Location also reached significance (Intervention: F1,29 = 10.55, P < 0.05, ; Time: F1,29 = 39.43, P < 0.05, ; Valence: F1,29 = 5.77, P < 0.05, ; Location: F1,29 = 11.89, P < 0.05, ). Follow-up analyses revealed that reaction time was significantly faster following exercise compared with the rest intervention, faster for the post-test compared with the pre-test condition, and faster to probes that replaced unpleasant versus pleasant or neutral stimuli. Finally, with respect to location, reaction time was faster for match conditions in which probes replaced valenced stimuli, as opposed to pairings in which probes replaced a neutral stimulus.
Figure 3.
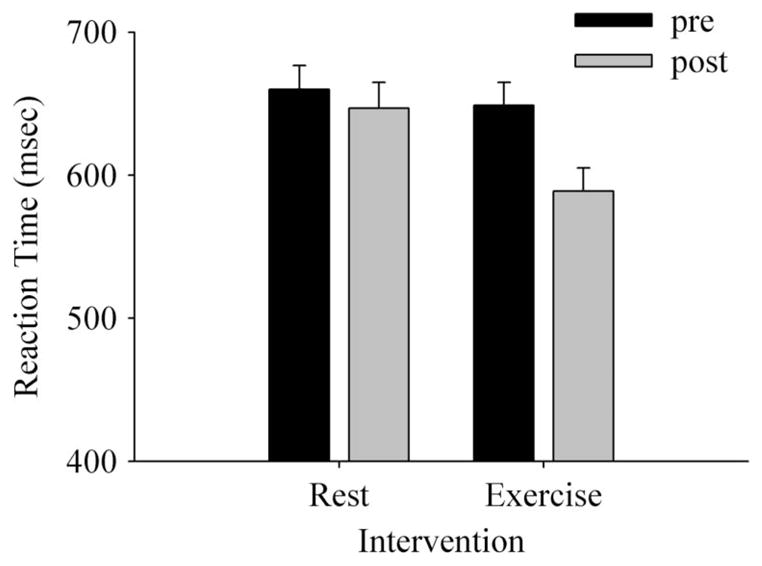
Reaction times among high anxious participants on the modified dot-probe task following an exercise and rest intervention.
Attentional bias scores
Analysis of attentional bias scores showed a significant Intervention × Valence interaction (F1,29 = 4.14, P < 0.05, ), but follow-up analyses were not significant. Visual inspection of Figure 4, however, suggests that exercise attenuates maladaptive attentional bias as evidenced by the greatest bias for pleasant stimuli and smallest bias towards unpleasant stimuli being exhibited after exercise.
Figure 4.
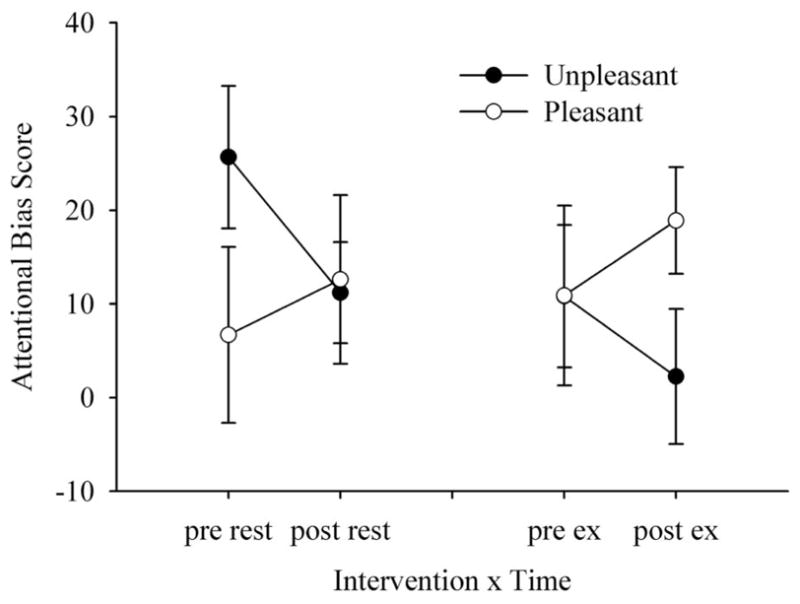
Attentional bias scores for modified dot-probe task. Higher (positive) bias scores indicate greater vigilance to the location of the valenced cues, while lower scores indicate avoidance of the valenced cues.
Discussion
To measure the effects of aerobic exercise on affect, we investigated pre- and post-exercise levels of attentional allocation that are believed to generate and perpetuate anxiety using a modified dot-probe task. High trait anxious participants completed exercise and quiet rest protocols on separate visits. We hypothesized exercise would induce improvements in positive affect, negative affect, and state anxiety. We also predicted exercise would broaden attention, illustrated by faster reaction times irrespective of probe location and emotional stimuli. Furthermore, we anticipated that exercise would attenuate the attentional bias towards negatively valenced cues that is typically characteristic of high anxious individuals.
As hypothesized, positive affect increased significantly after exercise compared with the other test conditions. These findings replicate previous research regarding improvements in affect, and bolster claims advocating the use of exercise as an augmentation strategy in individuals with affective problems (Bartholomew, Morrison, & Ciccolo, 2005; Pilu et al., 2007; Trivedi, Greer, Grannemann, Chambliss, & Jordan, 2006). However, levels of state anxiety and negative affect were only marginally reduced following exercise. As pointed out by Sharpe and Gilbert (1998), there can be methodological problems when administering affective questionnaires multiple times to the same participants as possible testing effects may result. While testing effects may occur from repeated exposure to questionnaires, the opposite may also occur. From a methodological standpoint, reliability scores for the Positive and Negative Affect Schedule and State–Trait Anxiety Inventory are strong, but it is possible that the number of times the tests were administered to each participant within a relatively narrow time span led to a testing fatigue that resulted in a smaller than expected magnitude in difference scores measuring negative affect and anxiety. Indeed, “short-form” questionnaires and visual analog scales are often created with this notion in mind. While requisite, it can be argued that studies investigating exercise and affect have relied too heavily on self-report measures. Therefore, studies incorporating additional measures, whether they be behavioural (e.g. reaction time) or physiological (e.g. cortisol levels) in nature, may help support claims of emotional improvement.
Contrary to our prediction that improvements in negative affect and state anxiety would manifest following exercise, we did not observe significant reductions in negative affect and state anxiety. Although many studies have demonstrated significant changes in state anxiety and negative affect immediately following activity (Bahrke & Morgan, 1978; Bartholomew et al., 2005; Tate & Petruzzello, 1995), other research suggests that exercise may initially induce anxiety and negative affect, but shortly following the conclusion of activity a rebound effect occurs that can last as long as 2–5 h, where positive affect increases and anxiety is attenuated (Morgan, 1985), and therefore the magnitude of change may have been (significantly) higher at a later post-exercise time point. While negative affect and state anxiety changes were not significantly altered, the expected pattern was evident, with scores for negative affect and state anxiety being lowest after exercising. Considering the collective self-report affect data, it is reasonable to infer that the alterations in dot-probe performance were driven largely by increased positive affect following exercise as opposed to diminished changes in negative affect or anxiety.
Consistent with our hypothesis that exercise would broaden attention, as illustrated by faster reaction times, our results indicate reaction times were fastest following exercise. These findings are similar to those of Hogervorst and colleagues (Hogervorst, Riedel, Jeukendrup, & Jolles, 1996), who found that performance both on a simple reaction time test and the Stroop test were improved when individuals cycled at 75% of their maximal work capacity. Our reaction time results also concur with work documenting the facilitative effect of aerobic exercise on cognitive and/or motor tasks (for reviews, see Davranche, Burle, Audiffren, & Hasbroucq, 2006; McMorris & Graydon, 2000; Tomporowski, 2003). While consistent with previous findings, the current data are unique in assessing the impact of exercise on the modified dot-probe task, which directly measures emotion-motivated attentional allocation.
The significant reduction in reaction time following exercise (Figure 3) can be logically accounted for via changes in attention. Specifically, results indicate that attention was broadly facilitated by exercise, allowing participants to respond quicker to probes, regardless of where they were presented (match or mismatch condition) and irrespective of the valence category of the stimulus that preceded the probe (unpleasant or pleasant condition).
Conceptual tenets of attentional control theory can explain the general slowing of reaction time noted in our sample prior to exercise, and why exercise alleviates this slowing. Dispositional anxiety theoretically accounts for slower reaction times during both pretest sessions as no intervention had yet been introduced to ameliorate anxiety levels or significantly increase positive affect. According to attentional control theory, attentional resources that are required for the efficient execution of the task are exhausted by anxiety. Moreover, high trait anxiety is characterized by deficiencies in the inhibition and shifting functions of the central executive, leading to greater susceptibility to distraction and more automatic, biased processing of threat stimuli. When coupled, attentional control theory predicts that these two factors will result in decrements in the rate in which information is processed. Such reduced attentional control will be evidenced via slower reaction times (i.e. reduced performance efficiency) rather than performance errors (i.e. performance accuracy).
The pattern described above was evident in our data since participants’ performance effectiveness, as indexed by the number of correct responses to the modified dot-probe task, was not impacted by anxiety (e.g. 95% of trials were included in the analysis), but the speed in which they responded was significantly slower. Moreover, once the exercise intervention was introduced, the efficiency of response time improved significantly. While attentional control theory specifically predicts that a reduction in anxiety accounts for improvements in processing efficiency (i.e. improved reaction time), our findings suggest that moderate decreases in state anxiety along with significant increases in positive affect will enhance performance efficiency. These trends are consistent with our hypothesis that reductions in anxiety will free attentional resources, allowing for participants to broaden their attentional scope. Broadened attentional scope, in this case induced by exercise, accounts for subsequent improvements in processing efficiency and is consistent with previous research that has associated a broadening of attention with increases in positive affect (Derryberry and Tucker, 1994; Forster et al., 2006; Friedman & Forster, 2005).
While our predictions of a general facilitation of reaction time following exercise were upheld, the magnitude of the attentional bias noted in the pre-rest, post-rest, and pre-exercise conditions towards unpleasant stimuli was marginal. Thus, the hypothesis that an acute bout of aerobic exercise would result in a relative bias away from unpleasant stimuli (and towards pleasant stimuli) was not supported statistically. A review of the attentional bias literature would suggest that a significant bias towards negative stimuli, as evidenced by shorter response latencies to probes replacing unpleasant stimuli, should manifest in participants who report high trait anxiety before receiving an intervention (cf. Bar Haim et al., 2007). High anxious participants in the current study exhibited a predicted bias towards unpleasant stimuli (and away from pleasant stimuli) in the pre-rest condition (as seen in Figure 4), but bias scores for unpleasant and pleasant stimuli were similar for the pre-exercise condition. While the dot-probe task is generally robust in identifying attentional biases, the task has occasioned poor retest reliability over a short time period (within one week) with non-clinical samples (e.g. Schmulke, 2005). The multiple testing occasions in the current investigation may have ameliorated or masked actual pretest differences.
Another potential reason for the lack of pretest difference is that although participants reported a high mean trait anxiety score of 53.6, they cannot be classified as clinically anxious without a structured, clinical interview; therefore, they may only share symptomology with clinically anxious individuals. Despite this apparent anomaly, visual inspection of Figure 4 suggests attentional bias scores followed the predicted trend for the post-exercise condition, offering preliminary support that the attentional bias often exhibited in high anxious individuals may be attenuated by exercise. Specifically, participants were least vigilant to unpleasant cues and most vigilant to pleasant cues following exercise when compared with the pre-rest, post-rest, and pre-exercise conditions. Such promising trends warrant further study and provide compelling support for utilizing the dot-probe protocol to index affect-related attentional shifts induced by exercise.
Despite support for two of our three hypotheses, we acknowledge inherent limitations of the dot-probe protocol generally and in our investigation specifically. One such limitation is that the protocol only provides a brief “snapshot” of attention, with data explaining how attention was allocated only at the specific time period in which the probe was presented. The modified dot-probe task is therefore insufficient for delineating the entire time course of attentional biases present during task performance.
Understanding how affective disposition and the motor system interact warrants further investigation. Motor deficits brought on by varying levels of anxiety and related negative affect can be disabling, as deficiencies in movement quality may lead to subsequent movement errors, compounding symptoms such as fatigue or restlessness that may in turn have dire functional consequences. The clinical implications of slowed reaction time are also worthy of note as they may perpetuate classic symptoms of anxiety and related emotional problems. In particular, fatigue, irritability, confusion, and motor tension are established diagnostic criteria for generalized anxiety disorder (American Psychiatric Association, 1994). While we acknowledge that the participants in the present experiment were not screened and diagnosed as “clinically anxious”, their high State–Trait Anxiety Inventory scores increase the likelihood of shared symptomology, and therefore have implications for related clinical populations.
In conclusion, our results confirm that attentional allocation and reaction time in highly anxious individuals are altered by aerobic exercise. Participants self-reported a significant increase in positive affect, mirroring previous work suggesting that exercise improves psychological well-being. Together with the increase in positive affect, data gathered from a modified dot-probe protocol provided objective behavioural evidence to support the notion that attentional breadth is facilitated following exercise. Such findings corroborate the notion that exercise-induced non-discriminate broadening of attention is accompanied by enhancements in positive affect. This evidence supports the idea that exercise interventions can be tailored to address the attentional mechanisms that underlie affective problems.
Table II.
Mean self-report scores and standard deviations for exercise history and affective measures recorded during screening (only Physical Activity Readiness Questionnaire and Beck Depression Inventory) and before and after each intervention (only State–Trait Anxiety Inventory and Positive and Negative Affect Schedule).
| Total | Pre-rest | Pre-exercise | Post-rest | Post-exercise | |
|---|---|---|---|---|---|
| PARQ | 47.6 (20.18) | – | – | – | – |
| BDI | 7.7 (4.96) | – | – | – | – |
| State Anxiety | – | 38.1 (8.85) | 35.3 (8.73) | 36.03 (8.66) | 33.57 (7.80) |
| Positive Affect | – | 29.0 (7.31) | 27.67 (8.5) | 27.17 (7.42) | 32.2 (8.13) |
| Negative Affect | – | 15.77 (5.07) | 14.1 (5.15) | 14.0 (5.32) | 12.13 (3.65) |
Note: Dashes indicate data were not collected at a time point. PARQ =Physical Activity Readiness Questionnaire, BDI =Beck Depression Inventory.
References
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 7. Baltimore, MD: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bahrke M, Morgan W. Anxiety reduction following exercise and meditation. Cognitive Therapy and Research. 1978;2:323–333. [Google Scholar]
- Barbour KA, Blumenthal JA. Exercise training and depression in older adults. Neurobiology of Aging. 2005;26:119–123. doi: 10.1016/j.neurobiolaging.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Bar Haim Y, Lamy D, Pergamin L, Bakermans-Kraneburg MJ, van Ijzendoorn MH. Threat-related attentional bias in anxious and non-anxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bartholomew JB, Morrison D, Ciccolo JT. Effects of exercise on mood and well being in patients with major depressive disorder. Medicine and Science in Sports and Exercise. 2005;37:2032–2037. doi: 10.1249/01.mss.0000178101.78322.dd. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Beck AT, Steer RM, Garbin M. Psychometric properties of the Beck Depression Inventory: 25 years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Berry T. Who’s even interested in the exercise message? Attentional bias for exercise and sedentary-lifestyle related words. Journal of Sport and Exercise Psychology. 2006;28:4–17. [Google Scholar]
- Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, et al. The runner’s high: Opiodergic mechanisms in the human brain. Cerebral Cortex. 2008;18:2523–2531. doi: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- Bonnot V, Croizet J. Stereotype internalization and women’s math performance: The role of interference in working memory. Journal of Experimental Social Psychology. 2007;43:857–866. [Google Scholar]
- Bradley BP, Mogg K, Fall SJ, Hamilton LR. Attentional bias for threatening facial expressions in anxiety: Manipulation of stimulus duration. Cognition and Emotion. 1998;12:737–753. [Google Scholar]
- Bradley BP, Mogg K, Millar NH. Covert and overt orienting of attention to emotional faces in anxiety. Cognition and Emotion. 2000;14:789–808. [Google Scholar]
- Clark DM. A cognitive approach to panic. Behavior Research and Therapy. 1986;24:461–470. doi: 10.1016/0005-7967(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Coombes SA, Higgins T, Gamble KM, Cauraugh JH, Janelle CM. Attentional control theory: Anxiety, emotion, and motor planning. Journal of Anxiety Disorders. 2009;23:1072–1079. doi: 10.1016/j.janxdis.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neuroscience. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Craft LL. Exercise and clinical depression: Examining two psychological mechanisms. Psychology of Sport and Exercise. 2005;6:151–171. [Google Scholar]
- Davranche K, Burle B, Audiffren M, Hasbroucq T. Physical exercise facilitates motor processes in simple reaction time performance: An electromyographic analysis. Neuroscience Letters. 2006;396:54–56. doi: 10.1016/j.neulet.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Ansari TL, Hansard M, Shoker L, Eysenck MW. Anxiety, inhibition, efficiency, and effectiveness. Journal of Experimental Psychology. 2009;56:48–55. doi: 10.1027/1618-3169.56.1.48. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Tucker DM. Motivating the focus of attention. In: Niedenthal PM, Kitayama S, editors. Heart’s eye: Emotional influences in perception and attention. New York: Academic Press; 1994. pp. 167–196. [Google Scholar]
- Dewitte M, Koster EHW, De Houwer JD, Buysse A. Attentive processing of threat and adult attachment: A dot-probe study. Behaviour Research and Therapy. 2007;45:1307–1317. doi: 10.1016/j.brat.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behavior Research Methods, Instruments, and Computers. 1996;28:1–11. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Forster J, Friedman RS, Ozelsel A, Denzler M. Enactment of approach and avoidance behavior influences the scope of perceptual and conceptual attention. Journal of Experimental Social Psychology. 2006;42:133–146. [Google Scholar]
- Fox KR. The influence of physical activity on mental well-being. Public Heath Nutrition. 1999;2:411–418. doi: 10.1017/s1368980099000567. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. The role of positive emotions in positive psychology. American Psychologist. 2001;56:218–226. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RS, Forster J. Effects of motivational cues on perceptual asymmetry: Implications for creativity and analytical problem solving. Journal of Personality and Social Psychology. 2005;88:263–275. doi: 10.1037/0022-3514.88.2.263. [DOI] [PubMed] [Google Scholar]
- Gable PA, Harmon-Jones E. Approach-motivated positive affect reduced breadth of attention. Psychological Science. 2008;19:476–482. doi: 10.1111/j.1467-9280.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- Gasper K. Do you see what I see? Affect and visual information processing. Cognition and Emotion. 2004;18:405–421. [Google Scholar]
- Gasper K, Clore G. Attending to the big picture: Mood and global versus local processing of visual information. Psychological Science. 2002;13:34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]
- Hansen CJ, Stevens LC, Coast JR. Exercise duration and mood state: How much is enough to feel better? Health Psychology. 2001;20:267–275. doi: 10.1037//0278-6133.20.4.267. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Riedel W, Jeukendrup A, Jolles J. Cognitive performance after strenuous physical exercise. Perceptual and Motor Skills. 1996;83:479–488. doi: 10.2466/pms.1996.83.2.479. [DOI] [PubMed] [Google Scholar]
- Janelle CM, Singer RN, Williams AM. External distraction and attentional narrowing: Visual search evidence. Journal of Sport and Exercise Psychology. 1999;21:70–91. [Google Scholar]
- Jones TF, Eaton CB. Cost benefit analysis of walking to prevent coronary heart disease. Archives of Family Medicine. 1994;3:703–710. doi: 10.1001/archfami.3.8.703. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M, Jarreau D, Bartholomew J, Kraemer R. Comparing exercise bouts of differing intensities and durations on post-exercise mood. Medicine and Science in Sports and Exercise. 2004;36:S286–S287. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report #A-6. Gainesville, FL: University of Florida; 2005. International Affective Picture System (IAPS): Instruction manual and affective ratings. [Google Scholar]
- MacLeod C, Mathews A. Anxiety and the allocation of attention to threat. Quarterly Journal of Experimental Psychology A. 1988;40:653–670. doi: 10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford EM. Anxiety and the selective processing of emotional information: Mediating roles of awareness, trait and state variables, and personal relevance of stimulus materials. Journal of Behavioral Research Therapy. 1992;30:479–491. doi: 10.1016/0005-7967(92)90032-c. [DOI] [PubMed] [Google Scholar]
- McMorris T, Graydon J. The effect of incremental exercise on cognitive performance. International Journal of Sport Psychology. 2000;31:66–81. [Google Scholar]
- Miller BM, Bartholomew JB. Feelings of mastery and post-exercise psychological states in different exercise conditions. Medicine and Science in Sports and Exercise. 2007;39(5):S47. [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behaviour Research and Therapy. 2002;40:1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognitive Therapy and Research. 2005;29:29–45. [Google Scholar]
- Mogg K, Bradley BP, Dixon C, Fisher S, Twelftree H, McWilliams A. Trait anxiety, defensiveness and selective processing of threat: A investigation using two measures of attentional bias. Journal of Personality and Individual Differences. 2000;28:1063–1077. [Google Scholar]
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: The role of awareness. British Journal of Clinical Psychology. 1995;34:17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Eysenck M. Attentional bias in clinical anxiety states. Cognition and Emotion. 1992;6:149–159. [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Morgan WP. Affective beneficence of vigorous physical activity. Medicine and Science in Sports and Exercise. 1985;17:94–100. [PubMed] [Google Scholar]
- Nieuwenhuys A, Pijpers JR, Oudejans RRD, Bakker FC. The influence of anxiety on visual attention in climbing. Journal of Sport and Exercise Psychology. 2008;30:171–185. doi: 10.1123/jsep.30.2.171. [DOI] [PubMed] [Google Scholar]
- Otto MW, Church TS, Craft LL, Greer TL, Smits JAJ, Madhukar HT. Exercise for mood and anxiety disorders. The Primary Care Companion to the Journal of Clinical Psychiatry. 2007;9:287–294. doi: 10.4088/pcc.v09n0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluska SA, Schwenk TL. Physical activity and mental health: Current concepts. Sports Medicine. 2000;29:167–180. doi: 10.2165/00007256-200029030-00003. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry. 2005;18:189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Pilu A, Sorba M, Hardoy MC, Floris AL, Mannu F, Seruis ML, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clinical Practice and Epidemiology in Mental Health. 2007;3(8):1–4. doi: 10.1186/1745-0179-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe G, Hirsh JB, Anderson AK. Positive affect increases the breadth of attentional selection. Proceedings of the National Academy of Sciences USA. 2007;104:383–388. doi: 10.1073/pnas.0605198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W, Pritschet B, Frost B, Emmett J, Pelley TJ, Black J, et al. A comparison of post-exercise mood enhancement across common exercise distraction activities. Journal of Sport Behavior. 2003;26:368–383. [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist. 2000;55:68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clinical Psychology Review. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Schmulke SC. Unreliability of the dot probe task. European Journal of Personality. 2005;19:595–605. [Google Scholar]
- Sharpe JP, Gilbert DG. Effects of repeated administration of the Beck Depression Inventory and other measures of negative mood states. Personality and Individual Differences. 1998;24:457–463. [Google Scholar]
- Shephard RJ. PAR-Q, Canadian Home Fitness Test and exercise screening alternatives. Sports Medicine. 1988;5:185–195. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stopa L, Clark DM. Social phobia and interpretation of social events. Behaviour Research and Therapy. 2000;38:273–283. doi: 10.1016/s0005-7967(99)00043-1. [DOI] [PubMed] [Google Scholar]
- Szabo A, Ainsworth SE, Danks PK. Experimental comparison of the psychological benefits of aerobic exercise, humor, and music. International Journal of Humor Research. 2005;18:235–246. [Google Scholar]
- Tate AK, Petruzzello SJ. Varying the intensity of acute exercise: Implications for changes in affect. Journal of Sports Medicine and Physical Fitness. 1995;35:295–302. [PubMed] [Google Scholar]
- Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychologica. 2003;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Avoidance of alcohol-related stimuli in alcohol-dependent inpatients. Alcoholism: Clinical and Experimental Research. 2007;31:1349–1357. doi: 10.1111/j.1530-0277.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Grannemann BD, Chambliss HO, Jordan AN. Exercise as an augmentation strategy for treatment of major depression. Journal of Psychiatry Practice. 2006;12:205–213. doi: 10.1097/00131746-200607000-00002. [DOI] [PubMed] [Google Scholar]
- van Zijderveld GA, van Doornen LJP, Orlebeke JF, Snieder H, Van Faassen I, Tilders FJH. The psychophysiological effects of adrenaline infusions as a function of trait anxiety and aerobic fitness. Anxiety Research. 1992;4:257–274. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS Scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. Chichester: Wiley; 1988. [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. 2. Chichester: Wiley; 1997. [Google Scholar]
- Wilson MR, Vine SJ, Wood G. The influence of anxiety on visual attentional control in basketball free-throw shooting. Journal of Sport and Exercise Psychology. 2009;31:152–168. doi: 10.1123/jsep.31.2.152. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Zinbarg RE. Threat is in the eye of the beholder: Social anxiety and the interpretation of ambiguous facial expressions. Behavior Research and Therapy. 2007;45:839–847. doi: 10.1016/j.brat.2006.05.004. [DOI] [PubMed] [Google Scholar]