Abstract
Significant advances in our understanding of RA and its management have been made in the past decade, resulting in earlier intervention with biologic DMARDs, particularly in patients with evidence of aggressive, erosive disease. Here, one such biologic therapy, the T-cell co-stimulation modulator abatacept, is discussed, exploring clinical evidence published to date on its use in patients with very early arthritis/early RA who are MTX naïve, and in patients with established RA and an inadequate response to MTX or TNF antagonists. Data from relevant clinical trials are overviewed, discussing the clinical efficacy of abatacept in early disease, the clinical outcomes over long-term treatment in different patient populations and the effects of abatacept on structural damage. Findings from integrated safety analyses of abatacept clinical trial data, representing 10 366 patient-years of exposure are described, and clinically important safety events, including serious infections, malignancies and autoimmune events, are highlighted. It is concluded that abatacept represents an effective treatment option with an established safety profile across different patient populations, including patients with both early and erosive RA and those with established disease. Furthermore, efficacy data from studies in patients with early disease suggest that the risk–benefit profile of abatacept may be more favourable when introduced earlier in the treatment paradigm.
Keywords: Rheumatoid arthritis, Biological therapies, Clinical trials, Abatacept
Introduction
Significant advances in our understanding of RA and its management have demonstrated that early intervention, particularly in patients with evidence of aggressive, erosive disease, can prevent the irreversible structural damage characteristic of RA. The benefit observed is often optimized when combination treatment with both traditional and biologic DMARDs is administered [1–6]. Clinical practice guidelines recommend that the majority of patients should start treatment with a conventional DMARD, and in cases of treatment failure a biologic should be added; however, combination therapy should be considered early in DMARD-naïve patients if they present with poor prognostic factors, such as erosion, positivity for anti-CCP or RF and high disease activity [7]. Progress towards a framework for identifying patients with early disease who are at high risk of developing erosive and progressive RA—and thus would benefit from early DMARD intervention—has been made in the form of joint guidelines from the EULAR and the ACR [8], and guidelines from the stratégie thérapeutique de la polyarthrite (therapeutic strategies in RA) working group of the French Society of Rheumatology (study and follow-up of undifferentiated early arthritis). The latter specifically recommend very early use (≤6 months from diagnosis) of biologics in patients with poor prognostic factors [9]. However, one needs to take into account the benefit–risk profile of the therapeutic options available when considering this course of action [10].
Biologic DMARDs, including the TNF antagonists— infliximab, etanercept, adalimumab, golimumab and certolizumab—the B-cell depleter rituximab, the IL-6 receptor antagonist tocilizumab and the T-cell co-stimulation modulator abatacept, have demonstrated clinical efficacy and radiographic benefit in patients with moderate-to-severe RA who have demonstrated an inadequate response to at least one non-biologic DMARD [11–18]. Furthermore, efficacy benefits have been seen with some biologics in patients with severe, active and progressive early disease not previously treated with conventional DMARDs [19–23].
This review will focus on one of these biologic agents, abatacept, and the clinical experience to date examining intervention in various patient populations, including those with very early arthritis/early RA who are MTX naïve [23, 24], and in patients with established RA and an inadequate response to MTX [25, 26] or TNF antagonists [27].
Selective co-stimulation modulation
Abatacept is a selective co-stimulation modulator that inhibits T-cell activation by binding to CD80/86, and modulating its interaction with CD28 [28]—a co-stimulatory signal necessary for the full activation of T cells. Activated T cells are implicated in the pathogenesis of RA via amplification of the inflammatory cascade that leads to joint inflammation and destruction in RA [29, 30]. The effect of abatacept on the inflammatory cascade has been demonstrated by quantitative PCR studies and evaluation of synovial biopsies in patients with active RA who had previously failed TNF antagonist therapy. Findings from these studies demonstrate a reduction in expression of most inflammatory genes, and a small, largely non-significant reduction in cellular content following abatacept treatment; this suggests that abatacept reduces the inflammatory status of the synovium without disrupting cellular homoeostasis [31]. These observations are supported by clinical trial data, which have demonstrated a reduction in serum levels of inflammatory biomarkers to within ‘normal’ levels following abatacept treatment, implying that abatacept may help to normalize the levels of downstream inflammatory mediators. The unique mechanism of action of abatacept may offer significant therapeutic benefit to patients by specifically addressing the underlying RA pathophysiology [32].
Overview of abatacept clinical experience
Early disease
Abatacept study to gauge remission and joint damage progression in MTX-naïve patients with early erosive RA
The 2-year abatacept study to gauge remission and joint damage progression in MTX-naïve patients with early erosive RA (AGREE) study consisted of a 12-month double-blind (DB) period followed by a 12-month open-label period in MTX-naïve patients with early RA [23]. Patients had poor prognostic factors that are highly predictive of an aggressive disease course, including high CRP levels, radiographic evidence of erosions and seropositivity for RF or anti-CCP2. Patients were randomized 1 : 1 to receive abatacept (approved dose [33]) plus MTX (n = 256) or MTX alone (n = 253) [23]. All patients received open-label abatacept plus MTX from Year 1 onwards. The co-primary endpoints were 28-joint DAS (DAS-28)-defined remission and joint damage progression [Genant-modified [34] total score (TS)] at Year 1. At baseline, patients had short disease duration and high disease activity (Table 1).
Table 1.
Baseline demographics and clinical characteristics
| Parameter | MTX-naïve patients |
Patients with inadequate response to MTX |
Patients with inadequate response to anti-TNF agents |
||||
|---|---|---|---|---|---|---|---|
| AGREE abatacept + MTX group (n = 256) | ADJUST abatacept group (n = 28) | Phase IIB abatacept 10 mg/kg group (n = 115) | AIM abatacept + MTX group (n = 433) | ATTEST abatacept + MTX group (n = 156) | ATTAIN abatacept + DMARD group (n = 258) | ARRIVE all patients (n = 1046) | |
| Demographics | |||||||
| Age, years | 50.1 (12.4) | 44.8 (10.9) | 55.8 (range: 17–83) | 51.5 (12.9) | 49.0 (12.5) | 53.4 (12.4) | 54.4 (12.4) |
| Women, % | 76.6 | 71.4 | 75 | 77.8 | 83.3 | 77.1 | 81.2 |
| Caucasian, % | 78.9 | 89.3 | 87 | 87.5 | 80.8 | 96.1 | 92.4 |
| Disease duration | 6.2 (7.5) months | 8.8 (4.2) months | 9.7 (9.8) years | 8.5 (7.3) years | 7.9 (8.5) years | 12.2 (8.5) years | 11.6 (9.5) years |
| MTX dose, mg/week | NA | NA | 15.0 (4.4) | 16.1 (3.6) | 16.5 (3.7) | 15.2 (5.3) | – |
| Clinical characteristics | |||||||
| Tender joints, n | 31.3 (14.8) | – | 30.8 (12.2) | 31.0 (13.2) | 31.6 (13.9) | 31.2 (13.0) | 17.8 (6.0) |
| Swollen joints, n | 22.9 (11.3) | – | 21.3 (8.4) | 21.4 (8.8) | 21.3 (8.6) | 22.3 (10.2) | 13.6 (5.5) |
| Pain, 100 mm VAS | – | – | 62.1 (21.4) | 63.3 (21.1) | – | 70.8 (19.8) | – |
| HAQ-DI score (0–3) | 1.7 (0.7) | 0.8 (0.6) | M-HAQ: 1.0 (0.5) | 1.7 (0.7) | 1.8 (0.6) | 1.8 (0.6) | 1.7 (0.6) |
| Patient global assessment, 100 mm VAS | – | – | 60.1 (20.7) | 62.7 (21.2) | – | 69.2 (19.7) | 72.9 (16.5) |
| Physician global assessment, 100 mm VAS | – | – | 62.1 (14.8) | 68.0 (16.0) | – | 68.8 (17.7) | – |
| DAS-28 | CRP: 6.3 (1.0) | CRP: 3.6 (1.1) | CRP: 5.5 (0.63) | – | ESR: 6.9 (1.0) | – | CRP: 6.2 (0.7) |
| CRP level, mg/dl | 3.1 (3.1) | 1.12 (1.43) | 2.9 (2.8) | 3.3 (3.1) | 3.1 (2.7) | 4.6 (4.0) | 2.1 (3.0) |
| RF, % | 96.1 | 85.7 | – | 81.8 | 87.2 | 73.3 | 61.3 |
| ES | 5.4 (6.1) | 3.2 (3.5) | NA | 21.7 (18.1) | NA | NA | NA |
| JSN score | 2.1 (4.2) | 0.1 (0.4) | NA | 22.8 (20.2) | NA | NA | NA |
| TS | 7.5 (9.7) | 3.3 (3.6) | NA | 44.5 (37.3) | NA | NA | NA |
| Anti-rheumatic medication at enrolment, n (%) | |||||||
| MTX | 0 | 0 | 99.1% | 433 (100) | 156 (100) | 195 (75.6) | 730 (69.8) |
| Other DMARDs | 7 (2.7) | 0 | 16.5% | 53 (12.2) | – | 72 (27.9) | – |
| Biologics | 0 | 0 | NA | 1 (0.2) | – | 7 (2.7) | – |
| CS | 131 (51.2) | 5 (17.9) | 60.0% | 312 (72.1) | 118 (75.6) | 181 (70.2) | 611 (58.4) |
| NSAIDs | 203 (79.3) | 22 (78.6) | – | 370 (85.5) | 133 (85.3) | 181 (70.2) | – |
| Other | – | – | – | 1 (0.2) | – | – | – |
At Year 1, significantly more patients treated with abatacept plus MTX achieved DAS-28 (CRP)-defined remission and ACR50 and ACR70 responses (Table 2), and the difference between treatment arms was significant by Month 2. Over 1 year, 27.3 vs 11.9% of abatacept plus MTX- vs MTX alone-treated patients (P < 0.001) achieved a major clinical response (ACR70 maintained for ≥6 consecutive months) [23]. Significant improvements were also seen in physical function at Year 1, for abatacept plus MTX- vs MTX alone-treated patients [23]. In addition, abatacept plus MTX demonstrated a higher likelihood of increasing or maintaining initial improvements in ACR responses and physical function over 1 year than MTX alone in patient-level post hoc analyses [35].
Table 2.
Clinical efficacy with abatacept across clinical trials
| Efficacy outcome | MTX-naïve patients |
Patients with inadequate response to MTX |
Patients with inadequate response to anti-TNF agents |
||
|---|---|---|---|---|---|
| AGREE, Year 1 [23] | Phase IIb, Year 1a [38] | AIM, Year 1 [41] | ATTEST, Month 6 [45] | ATTAIN, Month 6 [48] | |
| Abatacept vs placebo | |||||
| ACR20, % | NR | 62.6 vs 36.1 (P < 0.001) | 73.1 vs 39.7 (P < 0.001) | 66.7 vs 41.8 (P < 0.001) | 50.4 vs 19.5 (P < 0.001) |
| ACR50, % | 57.4 vs 42.3 (P < 0.001) | 41.7 vs 20.2 (P < 0.001) | 48.3 vs 18.2 (P < 0.001) | 40.4 vs 20.0 (P < 0.001) | 20.3 vs 3.8 (P < 0.001) |
| ACR70, % | 42.6 vs 27.3 (P < 0.001) | 20.9 vs 7.6 (P = 0.003) | 28.8 vs 6.1 (P < 0.001) | 20.5 vs 9.1 (P = 0.019) | 10.2 vs 1.5 (P = 0.003) |
| LDAS, % | 54.3 vs 36.8 (P < 0.001)b | 49.6 vs 21.9 (P < 0.05) | 42.5 vs 9.9 (P < 0.001) | 20.7 vs 10.8 | 17.1 vs 3.1 (P < 0.001) |
| Remission, % | 41.4 vs 23.3 (P < 0.001) | 34.8 vs 10.1 (P < 0.001) | 23.8 vs 1.9 (P < 0.001) | 11.3 vs 2.9 | 10.0 vs 0.8 (P < 0.001) |
| HAQ-DI response, % | 71.9 vs 62.1 (P = 0.024) | 49.6 vs 27.7 (P < 0.001) | 63.7 vs 39.3 (P < 0.001) | 61.5 vs 40.9 (P = 0.001) | 47.3 vs 23.3 (P < 0.001) |
Data are taken from [23, 39, 42, 46, 49]. aAbatacept data are for the 10 mg/kg group. bData on file; LDAS: DAS-28 ≤3.2; remission: DAS-28 < 2.6; HAQ response: change from baseline in HAQ-DI ≥0.3 U for all trials except for the Phase IIb trial, defined as HAQ-DI mean change from baseline ≥0.22 U; NR: not reported.
Improvements in disease activity and ACR responses were sustained or improved over the second year for patients remaining on abatacept plus MTX therapy, with 55.2% achieving remission at Year 2 [36]. After patients randomized to MTX alone were initiated on abatacept plus MTX at Year 1, improvements in these efficacy endpoints were seen, with 44.5% in remission at Year 2, increased from 26.9% at Year 1 [36].
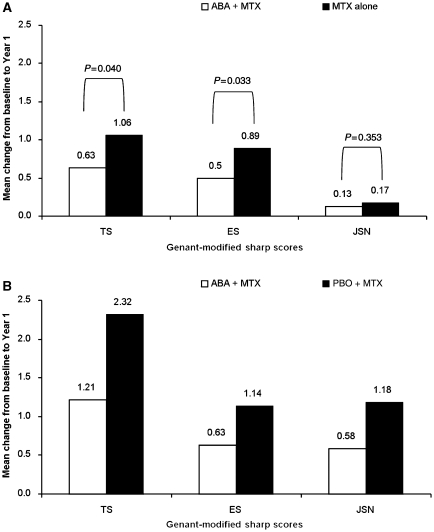
Changes from baseline to Year 1 in Genant-modified Sharp TS and erosion score (ES) were significantly lower for MTX-naïve patients randomized to abatacept plus MTX vs MTX alone (Fig. 1A) [23]. Furthermore, there was an increasing degree of inhibition of progression in Year 2 relative to Year 1 for patients originally randomized to abatacept [37]. For patients originally receiving MTX alone, structural damage progression was reduced over Year 2 relative to Year 1, following the addition of abatacept [37]. However, overall structural damage progression at Year 2 remained greater for these patients compared with patients who received abatacept from baseline [37].
Fig. 1.
Radiographic progression in early and established RA over 1 year of abatacept treatment. (A) Mean change from baseline in TS, ES and JSN at Year 1 of the AGREE trial for abatacept plus MTX- and MTX alone-treated patients [23]. Adapted from Westhovens et al. [23] copyright 2009, with permission from the BMJ Publishing Group Ltd. (B) Mean change from baseline in TS, ES and JSN at Year 1 of the AIM trial for abatacept- and placebo-treated patients [42]. ABA: abatacept; PBO: placebo. Adapted from Kremer et al. [42].
Abatacept study to determine the effectiveness in preventing the development of RA in patients with undifferentiated inflammatory arthritis and to evaluate safety and tolerability
The potential for early treatment with abatacept to delay the development or progression of RA in patients with very early disease was investigated in the Phase II, exploratory, 2-year ADJUST trial [abatacept study to determine the effectiveness in preventing the development of RA in patients with undifferentiated inflammatory arthritis (UA) (ADJUST) trial and to evaluate safety and tolerability]. Following 6 months of DB, randomized (1 : 1) treatment with either abatacept at the approved dose (n = 28) or placebo (n = 28), abatacept treatment was terminated. The proportion of patients who developed RA according to ACR 1987 criteria [38] or discontinued due to lack of efficacy at Year 1 was assessed.
Patients had a short disease duration (Table 1), and although patients did not have RA according to ACR 1987 criteria, more than half already had evidence of one or more erosion. As such it is likely that a significant proportion had early RA. When abatacept was stopped at Month 6, 22 and 17 patients treated with abatacept and placebo, respectively, remained in the trial (i.e. had not developed RA); by Year 2, 7 and 4 patients remained in the trial. Numerically more placebo than abatacept patients developed RA over 1 year (66.7 vs 46.2%), although CI overlapped. Radiographic assessments demonstrated an inhibitory effect on structural damage progression at Month 6, which was maintained for 6 months following therapy cessation, with similar trends observed for MRI-assessed osteitis, erosion and synovitis [24].
Established disease
MTX-inadequate responders Phase IIb trial
The Phase IIb trial in MTX-inadequate responders was a 12-month, randomized (1 : 1 : 1) DB study designed to evaluate the safety and efficacy of abatacept [2 mg/kg (n = 105) or 10 mg/kg (n = 115)] plus MTX compared with placebo plus MTX (n = 119) [39]. The primary endpoint was ACR20 response at Month 6. Patients completing the DB period were eligible to enter an open-label long-term extension (LTE), in which all patients received abatacept (approved dose). Results from the LTE have been published up to 5 years [25], with experience reported up to 7 years [40].
Patients had high baseline disease activity (Table 1). At Year 1, significantly greater improvements in RA signs and symptoms (Table 2) and clinically meaningful improvements in physical function were seen with abatacept 10 mg/kg vs placebo [39]. The 2 mg/kg dose was considered suboptimal and was not pursued in Phase III.
Over 12 months, serum levels of inflammatory biomarkers were significantly lower with abatacept 10 mg/kg vs placebo treatment, with numerical reductions in TNF-α and RF also reported [41]. In particular, sIL-2R, IL-6, soluble E-selectin and TNF-α were brought to within the range considered normal.
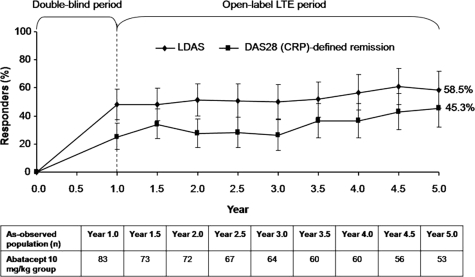
Of the patients who entered the LTE, 59 and 52% remained on treatment at Years 5 and 7, respectively, with 11.0% discontinuing due to lack of efficacy [25, 40]. Sustained efficacy improvements over 5 years were observed in patients remaining on treatment (Fig. 2) [25]. Furthermore, low disease activity state (LDAS) and ACR70 were reported in ∼70 and ∼50% of patients at Year 7, respectively [40]. Reductions in functional disability were also maintained over 5 and 7 years [25, 40].
Fig. 2.
Long-term clinical efficacy over 5 years of treatment with abatacept. The proportion of patients originally randomized to the 10 mg/kg abatacept group of the Phase IIb trial experiencing LDAS (DAS-28 CRP ≤3.2) and DAS-28-defined remission (DAS-28 CRP < 2.6) by visit day. Responses are based on the intent-to-treat population for patients with data available at the visit of interest (as-observed analysis). Broken line represents the DB period; data are presented with 95% CIs. Reproduced from Westhovens et al. [25] with permission from the Journal of Rheumatology.
Abatacept in inadequate responders to MTX
The Phase III AIM trial included a similar patient population of MTX-inadequate responders with established disease and high baseline disease activity (Table 1) [26, 42]; however, this trial also evaluated radiographic outcomes. The design of this trial has been reported extensively [18, 42]. Here, patients received either abatacept (approved dose; n = 433) or placebo (n = 219) on a background of MTX for 1 year, after which patients who continued into the LTE received open-label abatacept [26]. The co-primary endpoints were ACR20 response, clinically meaningful improvement in physical function and joint damage progression as assessed by Genant-modified ES.
Approximately three-quarters of patients who entered the LTE were still participating after 5 years, with 5.0% of discontinuations during the LTE due to lack of efficacy and 8.7% to AEs [26]. Yearly discontinuations were generally low (Years 2, 3, 4 and 5: 12.2, 6.3, 7.1 and 8.0%, respectively).
Through the 1-year DB period, improvements in clinical efficacy and physical function were significantly greater for abatacept vs placebo (Table 2; [42]). Post hoc analyses demonstrated statistically significant improvements from Months 6 to 12 in the proportions of abatacept-treated patients achieving ACR50 and ACR70 responses [42].
Throughout the open-label LTE, efficacy improvements were maintained for patients who remained on treatment [18, 26]. At Year 5, 33.7% of patients had achieved DAS-28-defined remission, with 83.6, 61.1 and 39.6% of patients achieving ACR20, ACR50 and ACR70 responses, respectively [26]. Approximately three-quarters of patients achieved clinically meaningful improvements in physical function [improvement of ≥0.3 in HAQ-disability index (HAQ-DI)] at Year 5 [26].
In post hoc, patient-level analyses from AIM, the majority of patients maintained or improved their treatment response or disease status from Months 3 to 12, suggesting that patients who have not responded by Month 3 may still achieve a clinically meaningful response over time [43]. The sustainability of patient-level responses was also evaluated for the LTE [44], revealing that the majority of patients who had achieved LDAS, remission or normalized physical function (i.e. HAQ-DI ≤ 0.5) by Year 1 sustained these outcomes through 5 years.
At the end of the DB period, a significant inhibition of structural damage progression was seen with abatacept vs placebo, with ∼50% reduction in change from baseline in Genant-modified Sharp scores compared with placebo (Fig. 1B) [42]. Progressive reductions in changes from baseline were observed in ES, joint-space narrowing (JSN) score and TS over 5 years, for patients originally randomized to abatacept, with progression reduced by ∼50% in the second year relative to the first and continued reductions in yearly progression up to Year 5. A similar trend was also seen over 2 years in MTX-naïve patients, in the AGREE trial [37]. Once the patients originally randomized to placebo had switched to abatacept, annual mean changes progressively decreased in a similar trend; however, differences in structural damage were still seen between the groups at Year 5 [45]. Furthermore, approximately half of all patients treated with abatacept over the entire study period exhibited no structural damage progression (change in TS of ≤0) through 5 years.
Abatacept or infliximab versus placebo, a trial for tolerability, efficacy and safety in treating RA
A third trial in MTX-inadequate responders provided the opportunity to evaluate two biologics in a single study. The placebo- and active-controlled ATTEST (abatacept or infliximab versus placebo, a trial for tolerability, efficacy and safety in treating RA) study, although not powered to detect superiority, provided information on the relative efficacy and safety profiles of abatacept and infliximab vs placebo in the same population [46]. Patients with an inadequate response to MTX were randomized (3 : 3 : 2) to abatacept (approved dose, n = 156), infliximab (3 mg/week, n = 165) or placebo (n = 110), with background MTX. At Month 6, patients in the placebo group were switched to abatacept, and infliximab and abatacept groups continued to Year 1, with blinding maintained.
The primary endpoint of this trial, reduction in DAS-28 (ESR) at Month 6 for abatacept vs placebo, was met, with mean reductions of −2.53 vs −1.48 (P < 0.001), respectively. The proportion of patients achieving states of low disease activity and DAS-28 remission was also greater with abatacept (Table 2). Improvements in ACR20, ACR50 and ACR70 responses at Month 6 were significantly greater vs placebo for both abatacept and infliximab. The onset of ACR20 responses was generally more rapid for infliximab than abatacept, but responses were similar by Month 3. By Year 1, DAS-28 (ESR) reductions of −2.88 and −2.25 were seen for abatacept- and infliximab-treated patients, respectively, and ACR responses were maintained from Month 6 with abatacept but not with infliximab treatment (Fig. 3) [46].
Fig. 3.
Clinical efficacy over 1 year in the ATTEST trial. ACR responses achieved over Year 1 of the ATTEST trial. Data are presented for the intent-to-treat population with a last observation carried forward analysis. aInfliximab was administered on Days 1, 15, 43, 85 and then every 56 days thereafter; abatacept dosing occurred at each visit day. Reproduced from Schiff et al. [46] copyright 2008, with permission from the BMJ Publishing Group Ltd.
The ATTEST trial continued through 2 years; during the second year of treatment, patients receiving infliximab were switched to abatacept. Efficacy benefits observed with abatacept in Year 1 were maintained through 2 years, as demonstrated by assessments of signs and symptoms, physical function and disease activity [47]. In patients who switched from infliximab to abatacept at Year 1, efficacy benefits increased over the second year and were similar to the original abatacept group by Year 2 [47]. In addition, a considerable proportion of infliximab non-responders (i.e. ACR20 non-responders, or patients with high disease activity state) who switched to abatacept after 1 year achieved improved clinical responses with abatacept over the second year [48]. For patients who had achieved LDAS or remission following 1 year of infliximab, a high proportion were able to maintain these disease states over Year 2.
TNF antagonist-inadequate responders abatacept trial in treatment of anti-TNF inadequate responders
The efficacy of abatacept in patients with RA who have an inadequate response to TNF antagonists was examined in the Phase III ATTAIN (abatacept trial in treatment of anti-TNF inadequate responders) trial [49]. Patients had an inadequate response to ≥3 months of treatment with etanercept, infliximab or both. Patients were randomized (2 : 1) to receive abatacept (n = 258) or placebo (n = 133), plus one or more background DMARD, for the 6-month DB period; patients entering the LTE received open-label abatacept. The co-primary endpoints were ACR20 response and improvement in physical function. Patients had high baseline disease activity (Table 1). After 4 years of open-label therapy, approximately half of all patients who entered the LTE remained on treatment [50].
At the end of the 6-month DB period, improvements in clinical efficacy and physical function were significantly greater for abatacept vs placebo (Table 2). The proportion of patients achieving improvements in ACR20, ACR50 and ACR70 responses increased over 4.5 years of treatment for patients who remained on treatment, as did the proportions of patients achieving LDAS and remission (18.3 vs 37.1% and 11.1 vs 25.7% at Month 6 vs Year 4.5, respectively), and clinically meaningful improvements in physical function [50].
Abatacept researched in rheumatoid arthritis patients with an inadequate anti-TNF response to validate effectiveness
The second trial conducted in TNF-inadequate responders was a Phase IIIb/IV, 6-month, open-label study. The ARRIVE (abatacept researched in rheumatoid arthritis patients with an inadequate anti-TNF response to validate effectiveness) trial was the first to assess the safety of abatacept in patients who switched directly from TNF antagonist therapy without undergoing washout [51]. This approach may be more clinically relevant for day-to-day practice.
Patients in this trial had high levels of disease activity at baseline (Table 1); overall, the inclusion criteria resulted in a patient population that was more representative of clinical practice than often included in randomized controlled trials [51]: patients had an inadequate response to up to three TNF antagonists that they could have failed for efficacy, safety or tolerability reasons. Patients were eligible even if they had a positive purified protein-derivative test result. Abatacept could be administered as monotherapy (USA only), and patients were not limited to a particular background DMARD.
Similar, clinically meaningful, improvements were seen in disease activity, physical function and health-related quality of life, regardless of whether there was a washout period or not. Post hoc analyses revealed that numerically more patients who had previously failed one TNF antagonist achieved DAS-28-defined remission and LDAS than those who had failed two or more [51].
Safety summary
Safety assessments from the trials discussed above have demonstrated that the incidence of overall AEs and serious AEs (SAEs) was generally comparable for abatacept- and placebo-treated patients [23, 24, 39, 42, 46, 49], although in some trials the frequency of SAEs was reported to be higher with abatacept [42]. The safety of long-term abatacept treatment is reported to be consistent, with the incidence of overall AEs and SAEs remaining stable up to 7 years [40].
Events that are of significant interest to the treatment of RA with biologic DMARDs, including serious infections, malignancies and autoimmune events, were examined in an integrated safety analysis that pooled data from the Phase IIb, AIM, ATTAIN, ATTEST and ARRIVE studies overviewed here, a Phase IIb study of abatacept 2 mg/kg in combination with etanercept [52], the Phase III ASSURE (abatacept study of safety in use with other RA therapies) study of abatacept with or without a biologic or non-biologic DMARD [53], and a Phase II synovial biopsy study [31], through December 2007. This included 4150 patients who were exposed to abatacept, representing 10 365 patient-years of exposure, with an average exposure period of ∼2.5 years [54–57].
Serious infections
In the integrated safety analysis, the incidence of serious infections and related serious infections was generally low, although it was higher for abatacept- compared with placebo-treated patients over 1 year (serious infections: 3.47 vs 2.41 events/100 patient-years, respectively) [54].
The incidence rates of serious infection and hospitalized infection remained stable for the DB vs cumulative periods, with 3.47 vs 2.98 serious infections, and 3.05 vs 2.73 hospitalized infections/100 patient-years, respectively [54]. The risk for serious infections did not appear to increase over time, as evidenced by incidence rates at annual intervals (Table 3) [54]. The most common hospitalized infections were pneumonia, bronchitis, cellulitis and urinary tract infection [55]. There were few opportunistic infections observed, including Mycobacterium tuberculosis (TB; 0.06 events/100 patient-years), aspergillosis (0.02), blastomycosis (0.01) and systemic candida (0.01) [55].
Table 3.
Incidence of serious infections and autoimmune events in the integrated safety summarya by annual intervals [54, 57]
| Events/100 patient-years (95% CI) |
||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | |
| Total exposure, patient-years | ∼3500 | ∼2400 | ∼1900 | ∼1500 | ∼700 | ∼180 |
| All serious infections | 3.68 (3.07, 4.37) | 2.77 (2.14, 3.53) | 2.41 (1.75, 3.23) | 2.61 (1.84, 3.60) | 2.16 (1.21, 3.57) | 3.05 (0.99, 7.13) |
| Hospitalized infectionsb | 3.31 (2.73, 3.97) | 2.55 (1.94, 3.28) | 2.34 (1.70, 3.16) | 2.46 (1.72, 3.42) | 1.87 (1.00, 3.20) | 3.02 (0.98, 7.06) |
| Autoimmune events | 1.64 (1.25, 2.13) | 2.02 (1.49, 2.68) | 1.61 (1.09, 2.30) | 1.25 (0.74, 1.97) | 0.99 (0.40, 2.04) | 0 (0, 1.99) |
aData are for all those patients who received at least one dose of abatacept during the cumulative study period, for the eight core abatacept trials. bHospitalized infection is a subset of serious infection. Adapted from Smitten et al. [54] with permission from BMJ Publishing Group Ltd, and Smitten et al. [57] with permission from the author.
Standardized incidence rates (SIRs) were calculated by comparing the observed number of hospitalized infections in the abatacept cumulative experience with that expected from external cohorts of RA patients treated with non-biologic DMARDs [55]. Hospitalized infections were not increased for abatacept-treated patients compared with established RA patients [55].
Malignancies
As reported in the integrated safety analyses, the incidence of malignancies [excluding non-melanoma skin cancer (NMSC)] during DB treatment was reported to be 0.59 vs 0.63 events/100 patient-years for abatacept- vs placebo-treated patients [56]. Incidence was generally low and did not increase with increasing exposure (0.59 and 0.71/100 patient-years in the DB and cumulative periods) [56]. Incidence of lung cancer and lymphoma, in particular, did not increase between the DB and cumulative periods, with 0.24 and 0.16 lung cancers, and 0.06 and 0.07 lymphomas/100 patient-years, respectively [56].
The incidence of malignancy in the abatacept clinical trial programme, as assessed in the integrated safety analyses, was compared with the incidence in five, observational non-biologic DMARD-treated RA patient cohorts—the British Columbia RA Cohort, the National Data Bank for Rheumatic Diseases, the UK General Practice Research Database, the UK Norfolk Arthritis Registry and the Sweden Early Rheumatoid Arthritis Register Cohort [56]. The SIRs calculated suggested that the overall risk of malignancy (excluding NMSC) was not significantly increased in abatacept- compared with non-biologic DMARD-treated patients; SIRs ranged from 0.40 to 1.06 for the cohorts. The risk of lung cancer did not appear to be increased with abatacept (SIRs ranged from 0.65 to 1.84), and there appeared to be a comparable risk for lymphoma (SIRs ranged from 0.60 to 1.23) [56].
Autoimmune events
During the integrated DB periods, autoimmune events were reported in 28 (1.4%) abatacept- and 8 (0.8%) placebo-treated patients; most events were mild or moderate in intensity [57]. Incidence of autoimmune disorders was generally low and did not increase with increasing exposure to abatacept (1.43 and 1.59/100 patient-years in the DB and cumulative periods, respectively) [57]. When incidence was assessed at annual intervals, the rate remained stable over time [57]. Psoriasis, the most frequently reported autoimmune event, did not increase between the DB and cumulative periods, with rates of 0.53 and 0.56 events/100 patient-years, respectively.
Abatacept has not been reported to lead to increased formation of ANA and anti-dsDNA antibodies, compared with placebo [33]. The integrated safety analyses support this suggestion, reporting a lower proportion of abatacept- vs placebo-treated patients seroconverting to positive anti-ANA status and positive anti-dsDNA status over 6 and 12 months [58].
Safety in ARRIVE
Comparable safety was seen in the ARRIVE trial (n = 1046) for both direct-switch and washout patients, with no increase in the overall frequency of AEs seen between groups either in the 6-month study period or monthly after initiation of abatacept therapy [51]. In addition, no cases of TB were reported, and no opportunistic infections occurred. Safety was also comparable regardless of the number of prior TNF antagonists received [59].
Safety in ATTEST
The ATTEST trial examined the relative safety profiles of two agents with differing mechanisms of action under the same study conditions. Over 1 year of DB treatment, SAEs (9.6 vs 18.2%), serious infections (1.9 vs 8.5%), acute infusional events (7.1 vs 24.8%) and discontinuations due to AEs (3.2 vs 7.3%) were less frequent in abatacept- vs infliximab-treated patients [46]. Infections and infestations were reported in 59.6 and 68.5%, and serious infections in 1.9 and 8.5%, respectively. The most frequently reported serious infection was pneumonia (1.3 and 1.8%, respectively). Five serious opportunistic infections were reported with infliximab treatment (herpetic encephalitis, pseudomonas lung infection, peritoneal TB, Pneumocystis jiroveci pneumonia and pulmonary TB); no opportunistic infections were reported with abatacept. Autoimmune events were uncommon in both groups (1.3 vs 0.6% for abatacept vs infliximab groups) [46].
Discussion and conclusions
The studies summarized here, encompassing up to 7 years of treatment, demonstrate that abatacept provides clinically meaningful and sustained benefits across multiple efficacy measures (signs and symptoms, structural damage and physical function), without dose adjustment, for patients with early, erosive disease who are MTX naïve, and patients with established, moderate-to-severe disease and an inadequate response to MTX/DMARDs, or to TNF antagonists.
As with other biologics [60], abatacept demonstrates statistical significance in achieving clinical efficacy outcomes compared with placebo, over short-term, DB treatment at the group level. Furthermore, the clinical efficacy improvements seen with abatacept have been observed at the individual patient level, with post hoc analyses suggesting that patients responding to treatment had a high probability of maintaining or further improving responses over time. Sustained/improved long-term effects were demonstrated with abatacept for signs and symptoms, physical function and structural damage, with data up to 7 years available in MTX-inadequate responders. Given that this type of sustained as opposed to intermittent treatment response could have an impact on long-term reduction of radiographic progression and improvement in physical function, these data are of particular interest, and evaluating this is strongly advised by guidelines from EULAR and ACR [61].
Similarly to data for other biologics, the long-term efficacy data for abatacept discussed here are based on as-observed analyses, with no imputation rule for missing data. In addition, some results are from post hoc assessments, and data such as these should be interpreted with caution. As-observed analyses are more vulnerable to the discontinuation of patients, and may result in a perceived increase in efficacy (as the proportion of responders is calculated only from those patients still on treatment) compared with the more conservative intent-to-treat analysis. However, the trials discussed here report relatively high patient retention with long-term treatment. Furthermore, following only those patients who actually remain on therapy may be more relevant over the long term, given that the extrapolation of data over many years from the start of a study is generally not recommended. The data discussed here have been interpreted with these concerns in mind.
Beneficial effects on radiographic progression have been seen with abatacept plus MTX vs placebo plus MTX, in patients with both early and long-standing RA. Reductions in the annual rate of structural damage progression observed in both patient populations suggest that abatacept has an increasing disease-modifying effect on structural damage over time in the majority of patients who respond. For patients with early RA treated with MTX alone over 1 year, structural damage progression is reduced following the addition of abatacept; however, overall structural damage progression at Year 2 (after 1 year of abatacept treatment) remains greater for these patients compared with patients who receive abatacept from baseline. These findings suggest that delaying biologic therapy in this population has a significant and lasting impact on irreversible structural damage, and support the earlier initiation of abatacept. In addition, findings in patients with early erosive disease suggest that earlier addition of abatacept to MTX provides clinically meaningful benefits over delayed initiation in the prevention of irreversible structural damage; when structural damage progression was assessed in patients with very early disease who were treated with abatacept for 6 months, the inhibitory effect was maintained for at least 6 months following treatment cessation. These data suggest that it may be possible to alter the progression of RA when abatacept is administered at a very early stage in disease.
Clinical responses and radiographic benefits with abatacept appear to be greater in MTX-naïve patients compared with patients who have failed MTX or other DMARDs. In addition, patients who previously failed MTX treatment demonstrate higher clinical responses than patients who have failed TNF antagonists. Although abatacept provides considerable efficacy benefits irrespective of the number of previous TNF antagonists received, there is a trend towards greater treatment responses in patients who have failed fewer agents, demonstrating that the efficacy of abatacept can be optimized when patients are switched through fewer prior TNF antagonists.
Taken together, these results suggest that introducing abatacept earlier in the treatment paradigm may lead to more favourable results—a trend that has also been seen with other biologic agents [19–22]. This shift towards earlier, aggressive treatment in suitable patients will be further facilitated by the recently published joint EULAR and ACR guidelines for early RA [8].
Abatacept has demonstrated similar efficacy in patients with early RA relative to other approved biologics [62]. A meta-analysis in MTX-naïve patients with early disease was conducted to assess clinical remission and radiographic non-progression after 1 year of treatment with abatacept, adalimumab, etanercept or infliximab, plus MTX. Each of the biologics demonstrated favourable results for inducing clinical remission and radiographic non-progression compared with MTX monotherapy at Year 1. Despite some limitations, these results provided a broad view of the comparability of the efficacy of these biologics [62]. Similar findings were reported in a Cochrane Review meta-analysis of randomized, DB trials of biologic DMARDs for RA treatment (abatacept, adalimumab, anakinra, etanercept, infliximab and rituximab), which concluded that the different biologic agents showed similar efficacy in patients with established RA, with the exception of anakinra [63]. The observations made in the Cochrane Review meta-analysis are supported by the findings from the ATTEST study, which provided a unique opportunity to evaluate two biologic agents in a single study. Abatacept and infliximab demonstrated comparable efficacy over 6 months of treatment in MTX-inadequate responders. Furthermore, patients who were switched from infliximab to abatacept treatment at Year 1 maintained or improved their responses over Year 2, suggesting that patients who achieve a good response to infliximab but need to switch therapies, due to safety concerns, for example, could expect to maintain or improve their response with abatacept.
Long-term integrated safety data from up to eight abatacept trials, representing >10 000 patient-years of exposure, confirm that, overall, abatacept has a favourable safety profile that is consistent with observations from the short-term experience in all RA populations studied, with no new clinically important safety issues identified with long-term compared with short-term findings. This is supported by a recent Cochrane Review that reported the safety profile of abatacept to be acceptable [60].
The increased risk of serious infections associated with TNF antagonists has been well documented [64–68]. Serious infections, as reported in the integrated safety analyses discussed here, are more frequent in abatacept- compared with placebo-treated patients over 1 year [54, 58]. However, the incidence rate of serious infections with abatacept is at the lower end of the range observed in RA patients treated with other biologics [69–71], and an independent meta-analysis by Salliot et al. [72] reported that this risk was not significantly increased with abatacept treatment compared with placebo. Importantly, the incidence rate of serious infections is reported to remain stable with increasing exposure to abatacept [54], consistent with trends seen with anti-TNF agents.
Patients with RA may be at higher risk for lung cancer and lymphoma than the general population [73, 74]; it is, therefore, important to assess the incidence of malignancies in patients treated with biologic agents. The risk for malignancies, including lung cancer and lymphoma, reported for abatacept in the integrated safety analyses was generally low and comparable to that of the general DMARD-treated RA population.
The Cochrane Review meta-analysis assessed safety across the biologic DMARDs based on withdrawals from clinical trials due to AEs [63]. Based on this criterion, there was a trend towards a favourable safety profile of abatacept vs placebo, relative to adalimumab or infliximab [63]. In general, this is consistent with data from the ATTEST trial in patients with an inadequate response to MTX, which reported a higher frequency of SAEs and serious infections (including opportunistic infections) with infliximab compared with abatacept.
In summary, abatacept represents an effective treatment option with an established safety profile in DMARD-naïve patients with early disease, and in patients with RA who have experienced an inadequate response to either non-biologic or biologic DMARDs. Moving forwards, it will be of interest to evaluate efficacy and safety outcomes with abatacept treatment in clinical practice, as opposed to the clinical trial settings discussed here. Further evaluation of the efficacy and safety of abatacept in early and very early disease will also be of high clinical importance, along with investigation into the factors associated with response to abatacept therapy.

Acknowledgements
The author would like to thank Eve Guichard, Medicus International, for editorial assistance, which was funded by Bristol-Myers Squibb.
Funding: Funding for editorial support of the review was provided by Bristol-Myers Squibb. Funding to pay the Open Access publication charges for this article was provided by Bristol-Myers Squibb.
Disclosure statement: M.S. is a consultant for and has received grant support from Bristol-Myers Squibb.
References
- 1.Landewe RB, Boers M, Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46:347–56. doi: 10.1002/art.10083. [DOI] [PubMed] [Google Scholar]
- 2.O’Dell JR, Leff R, Paulsen G, et al. Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: results of a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1164–70. doi: 10.1002/art.10228. [DOI] [PubMed] [Google Scholar]
- 3.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2008;58:S126–35. doi: 10.1002/art.23364. [DOI] [PubMed] [Google Scholar]
- 4.St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–43. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 5.Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–81. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 6.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 7.Aletaha D. ACR Concurrent Abstract Sessions: Rheumatoid Arthritis Therapy: Treatment Strategies. In: ACR/ARHP Annual Scientific Meeting. Philadelphia, PA, 2009. [Google Scholar]
- 8.Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 9.Le Loet X, Berthelot JM, Cantagrel A, et al. Clinical practice decision tree for the choice of the first disease modifying antirheumatic drug for very early rheumatoid arthritis: a 2004 proposal of the French Society of Rheumatology. Ann Rheum Dis. 2006;65:45–50. doi: 10.1136/ard.2005.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiely PD, Brown AK, Edwards CJ, et al. Contemporary treatment principles for early rheumatoid arthritis: a consensus statement. Rheumatology. 2009;48:765–72. doi: 10.1093/rheumatology/kep073. [DOI] [PubMed] [Google Scholar]
- 11.Maini RN, Breedveld FC, Kalden JR, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051–65. doi: 10.1002/art.20159. [DOI] [PubMed] [Google Scholar]
- 12.van der Heijde D, Klareskog L, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–74. doi: 10.1002/art.21655. [DOI] [PubMed] [Google Scholar]
- 13.Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 14.Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009;68:789–96. doi: 10.1136/ard.2008.099010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keystone E, Heijde D, Mason D, Jr, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58:3319–29. doi: 10.1002/art.23964. [DOI] [PubMed] [Google Scholar]
- 16.Korhonen R, Moilanen E. Anti-CD20 antibody rituximab in the treatment of rheumatoid arthritis. Basic Clin Pharmacol Toxicol. 2009;106:13–21. doi: 10.1111/j.1742-7843.2009.00452.x. [DOI] [PubMed] [Google Scholar]
- 17.Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–80. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 18.Kremer JM, Genant HK, Moreland LW, et al. Results of a two-year followup study of patients with rheumatoid arthritis who received a combination of abatacept and methotrexate. Arthritis Rheum. 2008;58:953–63. doi: 10.1002/art.23397. [DOI] [PubMed] [Google Scholar]
- 19.Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375–82. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 20.van der Kooij SM, le Cessie S, Goekoop-Ruiterman YP, et al. Clinical and radiological efficacy of initial vs delayed treatment with infliximab plus methotrexate in patients with early rheumatoid arthritis. Ann Rheum Dis. 2009;68:1153–8. doi: 10.1136/ard.2008.093294. [DOI] [PubMed] [Google Scholar]
- 21.Emery P, Genovese MC, van Vollenhoven R, Sharp JT, Patra K, Sasso EH. Less radiographic progression with adalimumab plus methotrexate versus methotrexate monotherapy across the spectrum of clinical response in early rheumatoid arthritis. J Rheumatol. 2009;36:1429–41. doi: 10.3899/jrheum.081018. [DOI] [PubMed] [Google Scholar]
- 22.Fleischmann RM, Emery P, Moreland LW, et al. Golimumab, a new human anti-TNF-a monclonal antibody, administered subcutaneously every 4 weeks in methotrexate-naive patients with active rheumatoid arthritis: a randomized, double-blind, placebo-controlled, GO-BEFORE study. Abstract 983. Arthritis Rheum. 2008;58:S530. [Google Scholar]
- 23.Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870–7. doi: 10.1136/ard.2008.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emery P, Durez P, Dougados M, et al. The impact of T-cell co-stimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept. Ann Rheum Dis. 2010;69:510–6. doi: 10.1136/ard.2009.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westhovens R, Kremer JM, Moreland LW, et al. Safety and efficacy of the selective costimulation modulator abatacept in patients with rheumatoid arthritis receiving background methotrexate: a 5-year extended phase IIB study. J Rheumatol. 2009;36:736–42. doi: 10.3899/jrheum.080813. [DOI] [PubMed] [Google Scholar]
- 26.Kremer JM, Russell A, Emery P, et al. Abatacept demonstrates consistent safety and sustained improvements in efficacy through 5 years of treatment in biologic-naïve patients with RA. Abstract FRI0263. Ann Rheum Dis. 2009;68:444. [Google Scholar]
- 27.Genovese MC, Schiff M, Luggen M, et al. Efficacy and safety of the selective co-stimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti-tumour necrosis factor therapy. Ann Rheum Dis. 2008;67:547–54. doi: 10.1136/ard.2007.074773. [DOI] [PubMed] [Google Scholar]
- 28.Yamada A, Salama AD, Sayegh MH. The role of novel T cell costimulatory pathways in autoimmunity and transplantation. J Am Soc Nephrol. 2002;13:559–75. doi: 10.1681/ASN.V132559. [DOI] [PubMed] [Google Scholar]
- 29.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 30.Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003;2:473–88. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 31.Buch MH, Boyle DL, Rosengren S, et al. Mode of action of abatacept in rheumatoid arthritis patients having failed tumour necrosis factor blockade: a histological, gene expression and dynamic magnetic resonance imaging pilot study. Ann Rheum Dis. 2009;68:1220–7. doi: 10.1136/ard.2008.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choy EH. Selective modulation of T-cell co-stimulation: a novel mode of action for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2009;27:510–8. [PubMed] [Google Scholar]
- 33.EMEA, London. Abatacept: summary of product characterisitics. 2010 [Google Scholar]
- 34.Genant HK, Jiang Y, Peterfy C, Lu Y, Redei J, Countryman PJ. Assessment of rheumatoid arthritis using a modified scoring method on digitized and original radiographs. Arthritis Rheum. 1998;41:1583–90. doi: 10.1002/1529-0131(199809)41:9<1583::AID-ART8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 35.Yazici Y, Moniz Reed D, Covucci A, Becker JC, Westhovens R. Patients with early RA treated with abatacept plus MTX have a higher likelihood of increasing or maintaining initial improvements in signs and symptoms and physical function over time than those treated with MTX alone. Abstract 1688. Arthritis Rheum. 2009;60:S631–2. [Google Scholar]
- 36.Westhovens R, Robles M, Nayiager S, et al. Disease remission is achieved within two years in over half of methotrexate naive patients with early erosive rheumatoid arthritis (RA) treated with abatacept plus MTX: results from the AGREE trial. Abstract 638. Arthritis Rheum. 2009;60:S239. [Google Scholar]
- 37.Bathon J, Genant H, Nayiager S, et al. Reduced radiographic progression in patients with early rheumatoid arthritis (RA) treated with abatacept + methotrexate compared to methotrexate alone: 24 month outcomes. Abstract 639. Arthritis Rheum. 2009;60:S239–40. [Google Scholar]
- 38.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 39.Kremer JM, Dougados M, Emery P, et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve-month results of a phase iib, double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:2263–71. doi: 10.1002/art.21201. [DOI] [PubMed] [Google Scholar]
- 40.Westhovens R. Consistent safety and sustained improvement in disease activity and treatment response over 7 years of abatacept treatment in biologic-naïve patients with RA. In: Proceedings of the EULAR, 2009 10–13th June, Copenhagen, Poster SAT0108. [Google Scholar]
- 41.Weisman MH, Durez P, Hallegua D, et al. Reduction of inflammatory biomarker response by abatacept in treatment of rheumatoid arthritis. J Rheumatol. 2006;33:2162–6. [PubMed] [Google Scholar]
- 42.Kremer JM, Genant HK, Moreland LW, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144:865–76. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 43.Dougados M, Kremer J, Le Bars M, et al. Abatacept improves disease activity status over time in patients with rheumatoid arthritis and an inadequate response to methotrexate. Abstract SAT0099. Ann Rheum Dis. 2009;68:573–4. [Google Scholar]
- 44.Westhovens R, Dougados M, Hall S, et al. Disease remission, radiographic non-progression and normalization of function achieved at Year 1 are sustained long-term in a majority of patients: 5-year outcomes with abatacept in biologic-naïve patients. Abstract 1657. Arthritis Rheum. 2009;60:S618. [Google Scholar]
- 45.Genant H. Abatacept increases the proportion of patients who remain free from structural damage progression through 5 years in methotrexate inadequate responders with RA. In: Proceedings of the EULAR, 2009 10–13th June, Copenhagen, Poster FRI0253. [Google Scholar]
- 46.Schiff M, Keiserman M, Codding C, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiff M. Two-year efficacy and safety in abatacept-treated patients with RA who received continuous therapy or switched from infliximab to abatacept: the ATTEST trial. In: Proceedings of the EULAR, 2009 10–13th June, Copenhagen, Poster SAT0103. [Google Scholar]
- 48.Schiff M, Keiserman M, Moniz Reed D, et al. An increasing proportion of patients achieve a low disease activity state or remission when switched from infliximab to abatacept regardless of initial infliximab treatment response: results from the ATTEST trial. In: ACR, 2009 10–16th October, Philadelphia, Poster 1659. [Google Scholar]
- 49.Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 50.Genovese MC, Schiff M, Luggen M, et al. Abatacept demonstrates consistent safety and sustained improvements in efficacy through 4 years of open-label treatment in patients with an inadequate response to anti-TNF therapy. Abstract 1689. Arthritis Rheum. 2009;60:S632. [Google Scholar]
- 51.Schiff M, Pritchard C, Huffstutter JE, et al. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: the ARRIVE trial. Ann Rheum Dis. 2009;68:1708–14. doi: 10.1136/ard.2008.099218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinblatt M, Schiff M, Goldman A, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann Rheum Dis. 2007;66:228–34. doi: 10.1136/ard.2006.055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: a one-year randomized, placebo-controlled study. Arthritis Rheum. 2006;54:2807–16. doi: 10.1002/art.22070. [DOI] [PubMed] [Google Scholar]
- 54.Smitten AL, Covucci A, Simon TA. Descriptive analysis of serious infections, hospitalized infections and malignancies over time in the abatacept rheumatoid arthritis clinical development program: a safety update with >10,000 person–years of exposure. In: EULAR, 2008, Paris, Poster FRI0162. [Google Scholar]
- 55.Smitten AL, Simon TA, Qi K, et al. Hospitalized infections in the abatacept RA clinical development program: an epidemiological assessment with >10,000 person–years of exposure. In: ACR Annual Scientific Meeting, 2008, San Francisco, CA, Poster 1674. [Google Scholar]
- 56.Smitten AL, Simon TA, Qi K, et al. Malignancies in the abatacept RA clinical development program: an updated epidemiological assessment with >10,000 person–years of exposure. In: ACR Annual Scientific Meeting, 2008, San Francisco, CA, Poster 1675. [Google Scholar]
- 57.Smitten AL, Qi K, Simon TA, Becker JC. Autoimmune adverse events in the abatacept RA clinical development program: a safety analysis with >10,000 person–years of exposure. In: ACR Annual Scientific Meeting, 2008, San Francisco, CA, Poster 1673. [Google Scholar]
- 58.Sibilia J, Westhovens R. Safety of T-cell co-stimulation modulation with abatacept in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:S46–56. [PubMed] [Google Scholar]
- 59.Schiff M, Le Bars M, Gaillez C, Wu G, Poncet C, Genovese MC. Efficacy and safety of abatacept in patients with RA and an inadequate response to anti-TNF therapy by number of prior anti-TNF therapies used. Abstract SAT0102. Ann Rheum Dis. 2009;68:574–5. [Google Scholar]
- 60.Maxwell L, Singh A. Abatacept for rheumatoid arthritis. Cochrane Database of Syst Rev 2009;(4):CD007277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis. 2008;67:1360–4. doi: 10.1136/ard.2008.091454. [DOI] [PubMed] [Google Scholar]
- 62.Kuriya B, Arkema V, Bykerk VP, Keystone E. Efficacy of initial methotrexate monotherapy versus combination therapy with a biologic agent in early rheumatoid arthritis: a meta-analysis of clinical and radiographic remission. Abstract 2010. Arthritis Rheum. 2009;60:S751–2. doi: 10.1136/ard.2009.118307. [DOI] [PubMed] [Google Scholar]
- 63.Singh A, Christensen R, Wells GA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. doi: 10.1002/14651858.CD007848.pub2. Cochrane Database of Syst Rev 2009;4:CD007848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furst DE. Serum immunoglobulins and risk of infection: how low can you go? Semin Arthritis Rheum. 2009;39:18–29. doi: 10.1016/j.semarthrit.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Listing J, Strangfeld A, Kary S, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52:3403–12. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 66.Kroesen S, Widmer AF, Tyndall A, Hasler P. Serious bacterial infections in patients with rheumatoid arthritis under anti-TNF-alpha therapy. Rheumatology. 2003;42:617–21. doi: 10.1093/rheumatology/keg263. [DOI] [PubMed] [Google Scholar]
- 67.Salliot C, Gossec L, Ruyssen-Witrand A, et al. Infections during tumour necrosis factor-alpha blocker therapy for rheumatic diseases in daily practice: a systematic retrospective study of 709 patients. Rheumatology. 2007;46:327–34. doi: 10.1093/rheumatology/kel236. [DOI] [PubMed] [Google Scholar]
- 68.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 69.Klareskog L, Gaubitz M, Rodriguez-Valverde V, Malaise M, Dougados M, Wajdula J. A long-term, open-label trial of the safety and efficacy of etanercept (Enbrel) in patients with rheumatoid arthritis not treated with other disease-modifying antirheumatic drugs. Ann Rheum Dis. 2006;65:1578–84. doi: 10.1136/ard.2005.038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schiff MH, Burmester GR, Kent JD, et al. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:889–94. doi: 10.1136/ard.2005.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Askling J, Dixon W. The safety of anti-tumour necrosis factor therapy in rheumatoid arthritis. Curr Opin Rheumatol. 2008;20:138–44. doi: 10.1097/BOR.0b013e3282f4b392. [DOI] [PubMed] [Google Scholar]
- 72.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68:25–32. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smedby KE, Baecklund E, Askling J. Malignant lymphomas in autoimmunity and inflammation: a review of risks, risk factors, and lymphoma characteristics. Cancer Epidemiol Biomarkers Prev. 2006;15:2069–77. doi: 10.1158/1055-9965.EPI-06-0300. [DOI] [PubMed] [Google Scholar]
- 74.Smitten AL, Simon TA, Hochberg MC, Suissa S. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10:R45. doi: 10.1186/ar2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kremer JM, Westhovens R, Leon M. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–15. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]