Abstract
Objective. To identify predictors of bone remodelling in children and young adults with SLE.
Methods. Ninety subjects with SLE aged 8–22 years underwent yearly measurements of height, bone age, bone turnover markers, serum Type I IFNs, SLEDAI and BMD. Predictors of bone turnover were examined using serum osteocalcin as a marker of bone formation and both serum tartrate-resistant acid phosphatase (TRAP) and urine N-telopeptide (NTx) as markers of bone resorption.
Results. Subjects demonstrated short stature, high BMI and bone age delay. A spine BMD Z-score of less than −2.0 was seen in 16.1% of subject visits. Serum osteocalcin was negatively correlated with glucocorticoid dose (Spearman rank correlation coefficient R = −0.34, P < 0.0001) but was not associated with SLEDAI after adjustment for confounders. Serum TRAP was negatively associated with SLEDAI, even after controlling for confounders (P = 0.04). Similar results were obtained for urine NTx. There was a negative association between TRAP and serum IFN-β (P = 0.03).
Conclusions. In this population of children and young adults with moderate lupus disease activity, glucocorticoid dose was a negative predictor of bone formation, whereas lupus disease activity was not. Interestingly, lupus disease activity was a negative predictor of bone resorption, suggesting that lupus disease activity is not the primary factor contributing to the bone deficits of childhood-onset SLE. The potential protective role of IFN-β and the effects of SLE treatment on bone loss require further study.
Keywords: Bone turnover, Systemic lupus erythematosus, Children, Cytokines, Interferon
Introduction
Rheumatic diseases affect the skeletal development of children in a number of important ways. First, as in chronic kidney disease, IBD and sickle cell disease, children with chronic rheumatic illnesses are prone to delayed linear growth and altered skeletal maturation [1–6]. Secondly, rheumatic diseases are associated with low BMD in children due to complex processes including decreased physical activity and weight-bearing exercise, vitamin D deficiency from poor diet or decreased exposure to sunlight, use of medications such as glucocorticoids that promote bone loss and a pro-inflammatory cytokine milieu that affects bone turnover [7]. As a result, skeletal deficits in children with inflammatory disease include short stature, osteoporosis and increased fracture risk [8–10]. Understanding the impact of the rheumatic diseases on the growing skeleton may lead to improved prevention and treatment of these manifestations in children.
Childhood SLE is characterized by multi-organ inflammation and the presence of autoantibodies. Recent studies using dual-energy X-ray absorptiometry (DXA) have shown that ∼40% of children with SLE have osteopenia, as defined by a BMD Z-score between −1 and −2.5 and 20% have osteoporosis, as defined by a BMD Z-score less than −2.5 [11], making decreased bone density in SLE as common in children as in adults [12]. To date, studies of SLE and bone metabolism in children have identified longer disease duration and higher cumulative CS exposure as correlates of low BMD [11, 13–15]. While these measures relate to the cumulative effects of SLE and its treatment on the skeleton, it is not known how lupus activity affects bone metabolism during the period of disease flare. Furthermore, the contribution of disease activity to the observed reductions of BMD in childhood SLE is not fully understood.
Bone is a dynamic tissue continuously under the influence of the counter-balancing actions of bone-forming osteoblasts and bone-resorbing osteoclasts. The effects of SLE on bone may thus act via pathways of bone formation, bone degradation or both. In order to evaluate the relationship between bone turnover and inflammatory disease in children and young adults with SLE, we characterized height, skeletal maturation, bone turnover markers, SLE disease activity and bone density in an ethnically diverse cohort of young SLE patients. Our goal was to evaluate the effect of SLE disease activity on bone turnover in this population. Given the known elevation of Type I IFNs in periods of high SLE disease activity [16–18] and the role of IFN-β in regulation of bone metabolism [19–21], we also measured serum Type I IFN levels to determine what relationship, if any, existed between these cytokines and bone turnover markers.
Materials and methods
Study design and subjects
Subjects were enrolled in a prospective cohort study at the University of California, San Francisco (UCSF) Medical Center. Written informed consent was obtained according to the Declaration of Helsinki and local institutional review board requirements. The study was approved by the UCSF Committee on Human Research. To be eligible for the study, subjects must have been between the ages of 7 and 21 years and must have met ACR diagnostic criteria for SLE. Subjects were excluded if they: were taking or had previously taken a bisphosphonate or human growth hormone; had renal insufficiency (creatinine above normal for age); were undergoing haemodialysis for renal failure; or had hyperparathyroidism (PTH > 73 ng/l). Visits occurred between 1999 and 2006.
Clinical evaluation
Subjects underwent yearly assessment. A physical examination was performed at each visit by the principal investigator (E.vS.). Ethnicity, medical history, physical activity and medications were obtained by questionnaire. Medication doses were verified by medical record review. Oral and parenteral glucocorticoid formulations were converted to prednisone equivalents using standard conversion factors [22]. Current glucocorticoid dose was calculated in prednisone equivalents as mg/kg/day. Cumulative glucocorticoid exposure was calculated as mg/kg, where records were available (n = 53). Weight and height were measured in triplicate utilizing a digital scale to the nearest 0.1 kg and a wall-mounted stadiometer to the nearest 0.1 cm, respectively. SLE disease activity was calculated according to the SLEDAI [23].
Laboratory measures
The following laboratory assays were performed on untimed samples in the UCSF clinical laboratory: complete blood count, complement 3 (C3) level, complement 4 (C4) level, intact PTH level, 25-OH and 1,25-OH2 vitamin D levels [competitive protein binding radioassay (Esoterix, Calabasas Hills, CA, USA)], anti-dsDNA antibody titre and urinalysis with microscopic exam. Bone formation was assessed by immunoradiometric assay for osteocalcin (ng/ml; Quest Diagnostics, Madison, NJ, USA). Bone resorption was assessed by enzymatic measurement of serum tartrate-resistant acid phosphatase (U/l; TRAP; Quest Diagnostics) and urinary N-terminal telopeptide : creatinine ratio [nM bone collagen equivalents (BCE)/mM creatinine; N-telopeptide (NTx); Quest Diagnostics]. IFN-α2 was measured in serum samples utilizing a high-sensitivity bead assay (BioPlex, BioRad, Hercules, CA, USA). Single-cytokine ELISA was used to measure IFN-β (Fujirebio Inc., Tokyo, Japan). Assays were performed in duplicate and the results averaged.
Skeletal maturation and bone density assessment
In order to describe the bone health of this population, bone age and bone density were measured. Bone age assessments were made using non-dominant hand radiographs according to the method of Gruelich and Pyle [24]. Skeletal delay or advancement was calculated by subtracting bone age from chronological age. Incident fractures were based on clinical assessments with radiographic confirmation. Posterior–anterior lumbar spine (L1–L4) BMD (g/cm2) was determined by DXA using a Hologic 4500 scanner (Hologic, Inc., Bedford, MA, USA) in the fast array scan mode in the UCSF Department of Radiology. Standard phantoms were used for calibration. All scans were analysed using Hologic Delphi Manual Low-Density spine software. Spine BMD Z-scores for L1–L4 were calculated using chronological age, in comparison with age-, gender- and ethnicity specific reference curves [25]. Height-adjusted BMD Z-scores were estimated using simple arithmetic transformation of the non-adjusted BMD Z-scores, as in [6]. Longitudinal quality control scans were obtained each day when the subjects were scanned, with a minimum of three such scans per week, utilizing Hologic spine phantoms. An air scan was performed once a week to evaluate table top uniformity. The in vitro precision was 0.5%.
Statistical analysis
Descriptive methods were used to evaluate the clinical and laboratory measurements, which are reported as mean (s.d.) or percentiles. Univariate analyses utilized t-tests, one-way ANOVA or Spearman rank correlation coefficients (R), as indicated. General estimation equations were used to control for repeated measures in multivariate models. Explanatory variables were considered for inclusion in the multivariable models if P ≤ 0.10 in univariate analyses. Backward stepwise methods were utilized to determine final models. To control for coupling between bone formation and resorption in models predictive of osteocalcin or TRAP, we employed two strategies. First, in multivariate models predicting bone formation and resorption, we included the opposite marker in the model. For example, TRAP or NTx was added as an independent variable in models predicting osteocalcin, and osteocalcin was included as an independent variable in models predicting TRAP or NTx. The second strategy to control for the tight coupling between bone formation and resorption was to include ratios of formation and resorption markers as independent variables in the model (osteocalcin : NTx in models predicting TRAP or osteocalcin : TRAP in models predicting NTx) [26, 27]. For all final analyses, P ≤ 0.05 two-tailed tests were considered to be statistically significant. Statistical analyses were performed utilizing Stata 11 software (StataCorp LP, College Station, TX, USA).
Results
Clinical characteristics of the SLE cohort: an ethnically diverse group with moderately active lupus
The baseline cohort included 90 subjects (13 males, 77 females) ranging in age from 8.5 to 22.0 years [15.4 (3.3) years] of diverse ethnicity (31% Asian, 24% Caucasian, 17% Hispanic, 11% African-American, 17% mixed). Among the 90 subjects, 41 (46%) completed a second visit, 21 (23%) completed a third visit, 9 (10%) completed a fourth visit and 7 (8%) completed a fifth visit. Intervals between visits were 1.5 (0.7) years. In total, there were 168 subject visits. Clinical and demographic characteristics are presented in Table 1. Age at diagnosis of SLE was 12.2 (3.0) years (range 5–18 years) and the median disease duration at enrolment was 1.6 years (range 0.1–11.4 years). Tanner stages were distributed as follows: 8.6% at Stage 1; 7.4% at Stage 2; 4.9% at Stage 3; 21.6% at Stage 4; and 57.4% at Stage 5. Approximately half of the subjects’ parents (50.5%) reported physical activity that was much less or less than age-matched peers.
Table 1.
Demographic and clinical characteristics of the subjects (90 subjects, 168 visits)
| Parameter | Value |
|---|---|
| Age, mean (s.d.), yearsa | 15.4 (3.3) |
| Gender (female), % | 86 |
| Ethnicity (%) | |
| African-American | 11 |
| Asian | 31 |
| Caucasian | 24 |
| Hispanic | 17 |
| Mixed | 17 |
| SLE disease duration, median (range)a, years | 1.6 (0–11.4) |
| SLEDAI, median (range)b | 4 (0–29) |
| SLICC damage index (s.d.)b, units | 0.2 (0.5) |
| Current prednisone dose, median (range)b, mg/kg/day | 0.13 (0–1.9) |
| Use of CYC during lifetime, % | 26.8 |
| Current medications, % | |
| Prednisone | 85.7 |
| HCQ | 84.9 |
| AZA | 1.8 |
| MMF | 1.2 |
| Laboratory parametersb | |
| C3, median (range), mg/dl | 91 (21–196) |
| C4, median (range), mg/dl | 14 (0–46) |
| dsDNA antibody, median (range), IU/ml | 105 (0–6333) |
| ESR, median (range), mm/h | 17 (0–90) |
aAt baseline visit. bAveraged across all visits.
Biopsy-proven nephritis was the most common end-organ involvement of SLE, seen in 31 subjects (34%), with diffuse proliferative GN in 12 subjects (13%), mesangial GN in 5 subjects (6%), membranous GN in 4 subjects (4%), focal segmental GN in 1 subject (1%), membranoproliferative GN in 1 subject (1%) and mixed type in 8 subjects (9%). Complement protein C3 was reduced (<71 mg/dl) in 24.2% of subjects, C4 levels were reduced (<13 mg/dl) in 37.2% of subjects, dsDNA antibodies were detectable (>30 IU/ml) in 70.8% of the subjects and ESR was elevated (>15 mm/h in females or >10 mm/h in males) in 51.8% of the subjects (Table 1). Medications used during study visits included HCQ (84.9%), MMF (1.2%), AZA (1.8%) and prednisone (85.7%). Twenty-seven per cent of patients had used CYC at some point during their lifetime. Levels of serum 25-OH vitamin D were <30 ng/ml in 77.4% of patient visits and were <20 ng/ml in 37.1% of patient visits, making vitamin D deficiency common in the cohort.
Disease activity assessed by SLEDAI was variable [median (range) 4 (0–29)] at the study visits, with active disease present during 46% of visits as defined by SLEDAI ≥ 6 [28]. Glucocorticoid dose ranged from 0 to 1.9 mg/kg/day (median 0.13 mg/kg/day) prednisone equivalents at the study visits. Across all study visits, 14.3% of subjects were not taking any glucocorticoid at the time of the visit. Among subjects who used glucocorticoids, median cumulative CS dose was 112.1 mg/kg prednisone equivalents (range 8.5–2433.0 mg/kg). The cohort demonstrated increased BMI Z-score {median [interquartile range (IQR)] 0.93 (0.26–1.50)}. Weight Z-score was also increased [median (IQR) 0.45 (−0.33 to 1.31)].
Skeletal deficits: SLE subjects had reduced height, skeletal maturation and bone density
SLE subjects were short when compared with age-matched peers. The median height-for-age Z-score was −0.68 (IQR −1.70 to 0.39), with median change in height Z-score/year of −0.08 (IQR −0.15 to 0.04), indicating a progressive height delay compared with age-matched peers. The mean (s.d.) height Z-score was −0.71 (1.32) for ages 8–15 years, −0.77 (1.36) for ages 15–18 years and −0.50 (1.37) for ages ≥18 years, demonstrating more severe height deficits in younger compared with older age groups. The median growth velocity across all study visits was 0.6 cm/year for girls (IQR 0–2.1) and 1.5 cm/year for boys (IQR 0.8–3.6). The mean (s.d.) difference in growth velocity at each study visit from a 50th-percentile age- and sex-matched normal child was −0.66 (2.56) cm/year for ages 8–15 years, 0.45 (1.40) cm/year for ages 15–18 years and 0.18 (0.80) for ages ≥18 years, suggesting that retardation of growth velocity was most pronounced in younger age groups [29]. Bone age delay was seen at 52.3% of the visits (Table 2). Of those subjects with bone age delay, median delay was 1.2 years (range 0.1–4.2 years). Accelerated skeletal maturation (bone age greater than chronological age) was seen at 19.6% of the visits. Among those with advanced bone age, median advancement was 0.8 years (range 0.1–3.8 years).
Table 2.
Anthropomorphic and skeletal characteristics of the subjectsa
| Parameter | Value |
|---|---|
| Height | |
| Mean (s.d.), cm | 56.2 (11.6) |
| Z-score, median (IQR) | −0.68 (−1.70 to 0.39) |
| Annualized change in Z-score, median (IQR)b, cm/year | −0.08 (−0.15 to 0.04) |
| Weight | |
| Mean (s.d.), kg | 60.1 (17.6) |
| Z-score, median (IQR) | 0.45 (−0.33 to 1.31) |
| BMI | |
| Mean (s.d.), kg/m2 | 24.5 (5.5) |
| Z-score, median (IQR) | 0.93 (0.26 to 1.5) |
| Bone age | |
| Delayed, % | 52.3 |
| Advanced, % | 19.6 |
| Spine BMD Z-score, median (IQR) | −0.47 (−1.50 to 0.32) |
| Height-adjusted spine BMD Z-score, median (IQR) | −0.50 (−1.0 to −0.2) |
aAveraged across all visits; n = 168 visits for height, weight and BMI; n = 142 visits for bone age; n = 161 visits for BMD. bBetween each visit.
Lumbar spine BMD ranged from 0.414 to 1.214 g/cm2 [mean (s.d.) 0.846 (0.163)]. Spine BMD Z-scores were variable, ranging from −6.3 to 2.7. The median spine BMD Z-score was −0.47 (IQR −1.5 to 0.3). After adjusting for body size [6], the range of BMD Z-scores narrowed (−2.60 to 1.41), but the median BMD Z-score remained essentially unchanged at −0.50 (IQR −1.0 to −0.2). Median change in spine BMD Z-score/year was −0.08 (IQR −0.45 to 0.14), indicating a progressive, slight decline in BMD compared with age-matched peers. A BMD Z-score of less than −1.0 at the spine was seen in 34.8% of subject visits, while a BMD Z-score of less than −2.0 at the spine was seen in only 16.1% of subject visits. Fractures were reported in only two subjects; one was a traumatic metatarsal fracture, and the other occurred prior to diagnosis of SLE.
Clinical correlates of bone formation: osteocalcin is associated with glucocorticoid exposure but not with SLE disease activity
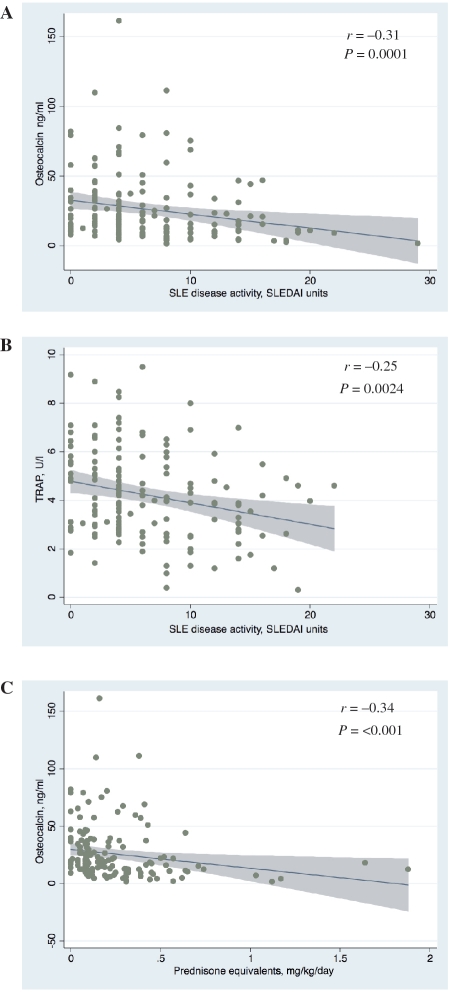
Serum osteocalcin was measured to assess bone formation. The median osteocalcin level was 18.2 ng/ml (range 1.6–161.5 ng/ml). Univariate analysis examining predictors of osteocalcin revealed significant positive correlations between osteocalcin and male gender, 25-OH vitamin D and 1,25-OH2 vitamin D, as well as significant negative correlations between osteocalcin and age, weight, current prednisone dose and SLEDAI (Table 3 and Fig. 1). BMD Z-score was not a significant predictor of serum osteocalcin, regardless of adjustment for body size. Thus, adjustment of BMD for body size did not alter its relationship to osteocalcin. As predicted, bone resorption markers were significantly associated with osteocalcin. To explore the negative association between bone formation and prednisone dose, we utilized a multivariate model controlling for confounders of osteocalcin. In this model, prednisone dose remained a significant negative predictor of osteocalcin (P < 0.001; β-coefficient −29.7 ng/ml/1 mg/kg/day increase in prednisone dose; 95% CI −43.2, −16.3; adjusted R2 = 0.37) after controlling for SLEDAI, age, weight, gender, 25-OH and 1,25-OH2 vitamin D and TRAP. Similarly, prednisone dose remained a significant negative predictor of osteocalcin (P = 0.01; β-coefficient −14.1 ng/ml/1 mg/kg/day increase in prednisone dose; 95% CI −25.0, −3.2; adjusted R2 = 0.64) after controlling for SLEDAI, age, weight, gender, 25-OH and 1,25-OH2 vitamin D and NTx instead of TRAP.
Table 3.
Association between clinical parameters and bone formation (osteocalcin) and bone resorption (TRAP, NTx)
| Osteocalcin |
TRAP |
NTx |
||||
|---|---|---|---|---|---|---|
| Parameter | R | P-value | R | P-value | R | P-value |
| Age, years | −0.37 | <0.0001 | −0.42 | <0.0001 | −0.66 | <0.0001 |
| Gender | – | 0.01 | – | 0.44 | – | 0.058 |
| Ethnicity | – | 0.52 | – | 0.31 | – | 0.90 |
| Height, cm | −0.07 | 0.37 | −0.18 | 0.02 | −0.26 | 0.002 |
| Weight, kg | −0.23 | 0.004 | −0.27 | 0.0006 | −0.32 | 0.0001 |
| SLEDAI, units | −0.29 | 0.0002 | −0.23 | 0.004 | −0.09 | 0.26 |
| Prednisone, mg/kg/day | −0.34 | <0.0001 | −0.04 | 0.67 | −0.04 | 0.64 |
| 25-OH-vitamin D, ng/ml | 0.15 | 0.07 | 0.15 | 0.07 | −0.05 | 0.58 |
| 1,25-OH2-vitamin D, ng/ml | 0.21 | 0.009 | 0.06 | 0.50 | 0.20 | 0.015 |
| PTH, ng/l | 0.02 | 0.79 | 0.003 | 0.97 | −0.01 | 0.87 |
| Osteocalcin, ng/ml | – | – | 0.36 | <0.0001 | 0.67 | <0.0001 |
| TRAP, U/l | 0.36 | <0.0001 | – | – | 0.41 | <0.0001 |
| NTxa | 0.03 | <0.0001 | 0.41 | <0.0001 | – | – |
R-values are reported for continuous variables. anM BCE/mM creatinine; n = 166 for osteocalcin; n = 157 for TRAP; n = 153 for NTx. P > 0.10.
Fig. 1.
Relationship between SLE disease activity, measured as SLEDAI or CS exposure and markers of bone turnover. Scatter plots are overlaid with fitted regression lines and 95% CIs. (A) Bone formation. Serum osteocalcin vs SLEDAI (n = 157). (B) Bone resorption. Serum TRAP vs SLEDAI (n = 148). (C) Serum osteocalcin vs prednisone equivalents.
In addition to examining the relationship between osteocalcin and prednisone dose, we explored the finding that SLE disease activity is negatively associated with osteocalcin using multivariable modelling. In this case, however, SLEDAI did not remain a significant predictor of osteocalcin after controlling for weight, gender, prednisone dose, age, 25-OH and 1,25-OH2 vitamin D and TRAP (P = 0.31; β-coefficient −0.40; 95% CI −1.18, 0.37; adjusted R2 = 0.37; Table 4). Likewise, SLEDAI did not remain a significant predictor of osteocalcin after controlling for weight, gender, prednisone dose, age, 25-OH and 1,25-OH2 vitamin D and NTx instead of TRAP (P = 0.44; β-coefficient −0.23; 95% CI −0.82, 0.36; adjusted R2 = 0.64). Inclusion of either formation : resorption ratio to the model in place of TRAP or NTx still did not reveal a significant association between osteocalcin and SLEDAI. Inclusion of BMI instead of weight and inclusion of Tanner stage or bone age instead of chronological age did not significantly alter the model for osteocalcin.
Table 4.
Multivariable model examining predictors of osteocalcin and TRAP
| Parameter | β-coefficient (95% CI) | P-value |
|---|---|---|
| Multivariable model for osteocalcina | ||
| SLEDAI, units | −0.40 (−1.18, 0.37) | 0.31 |
| Age, year | −2.59 (−3.95, −1.22) | <0.001* |
| Weight, kg | −0.25 (−0.47, −0.02) | 0.03* |
| Prednisone, mg/kg/day | −29.7 (−43.2, −16.3) | <0.001* |
| 25-OH vitamin D, ng/ml | −0.21 (−0.58, 0.17) | 0.19 |
| 1,25-OH2 vitamin D, ng/ml | 0.06 (−0.14, 0.26) | 0.54 |
| TRAP, U/l | 3.16 (0.97, 5.4) | 0.005* |
| Male gender | 15.5 (5.77, 25.1) | 0.002* |
| Multivariable model TRAPb | ||
| SLEDAI, units | −0.06 (−0.11, −0.002) | 0.04* |
| Age, year | −0.12 (−0.22, −0.02) | 0.02* |
| Height, cm | −0.02 (−0.05, 0.01) | 0.28 |
| Weight, kg | 0.003 (−0.02, 0.02) | 0.78 |
| 25-OH vitamin D, ng/ml | 0.02 (−0.008, 0.05) | 0.16 |
| Osteocalcin, ng/ml | 0.02 (0.0001, 0.03) | 0.049* |
| NTxc | 0.002 (−0.001, 0.005) | 0.23 |
aMultivariable model examining predictors of osteocalcin shows that SLEDAI is not significantly associated with bone formation after controlling for confounders. Model-adjusted R2 = 0.37 (n = 130 observations, 77 subjects). bMultivariable model examining predictors of TRAP shows that SLEDAI is negatively associated with bone resorption after controlling for confounders. Model-adjusted R2 = 0.29 (n = 132 observations, 81 subjects). cBCE/mM creatinine. *P < 0.05.
Clinical correlates of bone resorption: TRAP and NTx are negatively associated with SLE disease activity
Predictors of bone resorption markers (TRAP or NTx) were next assessed. TRAP and NTx measure different stages of the bone resorption process: TRAP is an early marker of osteoclast differentiation, whereas NTx measures osteoclast function [30, 31]. The median TRAP level was 3.9 U/l (range 0.31–9.5 U/l), and the median NTx level was 78 nM BCE/mM creatinine (range 1–1114 nM BCE/mM). Univariate analysis revealed that age, height, weight and SLEDAI were significant predictors of TRAP and that age, height, weight, gender and 1,25-OH2 vitamin D were significant predictors of NTx (Table 3 and Fig. 1). BMD Z-score was not significantly correlated with either TRAP or NTx, regardless of adjustment for body size. Thus, adjustment of BMD for body size did not alter its relationship to bone resorption markers. As predicted, there was a significant association between both TRAP and NTx and osteocalcin, reflecting coupling between bone formation and resorption. Prednisone dose was not associated with either bone resorption marker. Multivariate models were used to further explore whether SLE disease activity was a significant predictor of bone resorption. SLEDAI remained a negative predictor of TRAP after controlling for age and osteocalcin (P = 0.04; β-coefficient −0.06; 95% CI −0.11, −0.002; adjusted R2 = 0.29; Table 4). Inclusion of height, weight and/or 25-OH vitamin D, variables associated with TRAP, in the univariate analysis or inclusion of either formation : resorption ratio in place of osteocalcin did not improve the fit of the model. SLEDAI was not a significant predictor of NTx after controlling for age, gender, height, weight, 1,25-OH2 vitamin D and osteocalcin (P = 0.976; β-coefficient −0.05; 95% CI −3.20, 3.10; adjusted R2 = 0.64). Inclusion of the formation : resorption ratio in place of osteocalcin, however, suggested a significant association between SLEDAI and NTx (P = 0.046; β-coefficient −4.25; 95% CI −8.43, −0.07; adjusted R2 = 0.35 using osteocalcin : TRAP ratio). Inclusion of BMI instead of height and weight and inclusion of Tanner stage or bone age instead of chronological age did not significantly change these models for TRAP and NTx. Together, these data suggest that SLE disease activity is negatively correlated with bone resorption in a manner that is independent of bone formation and other confounders.
Type I IFNs: TRAP is negatively associated with serum IFN-β
We hypothesized that the negative association between SLE disease activity and bone resorption may be mediated by Type I IFNs (IFN-α and IFN-β), as these cytokines have been shown to inhibit osteoclast differentiation. To test this hypothesis, we measured Type I IFN levels in serum in our cohort. Consistent with the observation that the majority of patients in the cohort had SLEDAI values <6, the distribution of values for both IFN-α and IFN-β was left-skewed, with the majority of patients having low cytokine levels. The median IFN-α level was 57.7 pg/ml (range 0.01–497.64 pg/ml), and the median IFN-β level was 2.49 IU/ml (range 2.49–202.25 IU/ml). To test the hypothesis that the effect of SLE disease activity on bone resorption was Type I IFN mediated, we used a multivariate model examining the predictors of bone resorption. In this model, IFN-β was negatively associated with TRAP (P = 0.03; β-coefficient −0.02 U/l/1 IU/ml increase in IFN-β; 95% CI −0.03, −0.002; adjusted R2 = 0.12) after controlling for age, prednisone dose and osteocalcin : NTx ratio. Similarly, there was a trend towards a negative association between IFN-β and TRAP (P = 0.06; β-coefficient −0.011 U/l/1 IU/ml increase in IFN-β; 95% CI −0.022, 0; adjusted R2 = 0.27) after controlling for age, prednisone and osteocalcin. A similar association was not observed between IFN-β and NTx or between IFN-α and either TRAP or NTx.
Discussion
Although there have been several studies characterizing the effect of SLE on skeletal parameters in children and young adults, this is the first report to specifically examine the relationship between SLE disease activity and bone turnover in this population. Such a distinction is important because the effects of SLE treatment and SLE disease activity itself on the developing skeleton may be distinct. For example, while we found evidence of decreased bone formation as a result of SLE treatment (as measured by glucocorticoid dose), we also found an independent association between SLE disease activity and reduced bone resorption in this population. Although it may initially seem counterintuitive to observe serum biomarker evidence of reduced bone resorption among individuals with more active SLE, especially in light of multiple reports indicating decreased BMD in this population [11, 32], such a result may explain why patients with SLE have less bone loss than expected compared with other rheumatic diseases such as RA [33]. For example, in a comparison of young adults with SLE on high-dose steroids with those with RA on low-dose steroids, patients with SLE had higher bone mass than did RA patients, despite the higher steroid doses in these SLE patients [33]. In keeping with this observation, subjects in our cohort had decreased BMD, though in most cases this was not severe. It is notable that, in contrast to RA, the arthritis of SLE does not produce bony erosions, perhaps reflective of a greater propensity for bone preservation in SLE than in RA. As our study shows that the predominant effect of SLE disease activity on bone metabolism is a decrease in bone resorption, we propose that the low BMD seen in SLE may result primarily from disease treatment (e.g. with CSs) rather than from the underlying disease itself. This idea is supported by reports showing that CS dose is a greater contributor than disease duration to low BMD in childhood SLE [15] as well as in adult SLE [34, 35]. In addition, our data demonstrate a robust negative association between CS dose and serum osteocalcin, suggesting that CSs have a powerful inhibitory effect on bone formation.
A significant association between bone turnover markers and BMD was not found in this study, likely reflecting the fact that BMD is a long-term measure of overall bone mineralization, whereas serum bone turnover markers in children are more dynamic measures. Supporting this idea are studies showing a variable association between baseline levels of bone turnover markers and longitudinal changes in BMD in children [36, 37].
The mechanism by which SLE disease activity leads to decreased bone resorption is currently not known. Our data suggest a role for the Type I IFNs, specifically IFN-β, in the effect of SLE on bone turnover in children. The Type I IFNs are elevated in active SLE, leading to altered gene expression in peripheral mononuclear cells [16–18]. Children receiving exogenous Type I IFNs for treatment of hepatitis B have increased BMD, pointing to a bone-protective effect of these cytokines [38]. Of the Type I IFNs, IFN-β has a particularly important function in regulating bone metabolism. Mice deficient in IFN-β have severe osteopenia resulting from enhanced osteoclastogenesis, resulting from IFN-β’s direct inhibition of osteoclastogenesis via inhibition of c-fos [20]. The potential therapeutic benefit of IFN-β has been demonstrated in an animal model of endotoxin-induced inflammatory arthritis, where daily administration of IFN-β inhibited bone resorption [21]. A recent study in humans demonstrated that IFN-β is 100-fold more potent than IFN-α in inhibiting osteoclast development [19]. Our studies did not find a significant association between IFN-α and bone turnover markers in SLE. This may be due to the rapid breakdown of IFN-α in serum, which has made measurement of this molecule in serum samples problematic [39]. Whether IFN-β or other Type I IFNs are essential mediators of decreased bone resorption in childhood SLE remains speculative and should be further explored in future studies.
While our study uniquely examines bone remodelling in a moderately sized and ethnically diverse cohort of children and young adults, several limitations must be considered. First, our study was restricted to the outpatient population, leading to a bias towards less active SLE. Secondly, as our bone turnover and cytokine samples were collected at outpatient clinic visits, we did not control for diurnal variation. Thirdly, the use of bone turnover markers in children is always complicated by growth-related changes in bone formation and resorption [40, 41]. For this reason, we included age in our multivariate models. Lastly, our measurements of TRAP were not specific to the 5b isoform of this enzyme, which is viewed to be more specific to osteoclasts [42]. Thus, measured TRAP levels in our study may reflect the production of this enzyme by other cells of the myeloid lineage such as macrophages and dendritic cells in addition to that produced by osteoclasts [43]. Despite this limitation, it is reassuring that our observations for TRAP were consistent when applied to urine NTx.
In summary, our study demonstrates an association between increased SLE disease activity and reduced bone resorption in children and young adults. Our results suggest that enhanced bone resorption due to SLE activity itself is not the primary factor driving bone loss in childhood-onset SLE; rather, another factor, such as treatment effects, likely drives this process. If so, minimizing CS exposure may be more important than prevention of disease flares in protecting the skeleton from adverse effects of childhood-onset SLE. Our findings also suggest that bone loss in SLE may differ from that seen in RA and other rheumatic diseases, pointing to the need for a disease-specific approach when examining the nature of bone loss among the rheumatic diseases. Improved understanding of the pathways involved in bone metabolism in childhood-onset inflammatory diseases will ultimately inform the development of improved prevention and treatment strategies for the adverse skeletal outcomes in SLE and related illnesses.
Acknowledgements
We thank Dr Nancy E. Lane for her insightful review of the article.
Funding: This publication was supported by National Institutes of Health/National Center for Research Resources UCSF-Clinical and Translational Science Institute Grant Number UL1 RR024131 and NIH/NCRR grant number 16319. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. J.C.B.-L. was supported by NIH Academic Rheumatology and Clinical Immunology Training Grant Number AR007304. M.C.N. was supported by the Department of Veterans’ Affairs.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Cassone R, Falcone A, Rossi F, et al. Unilateral destructive wrist synovitis in juvenile idiopathic arthritis. Clin Exp Rheumatol. 2004;22:637–42. [PubMed] [Google Scholar]
- 2.Heuschkel R, Salvestrini C, Beattie RM, Hildebrand H, Walters T, Griffiths A. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008;14 doi: 10.1002/ibd.20378. [DOI] [PubMed] [Google Scholar]
- 3.Leonard MB. Glucocorticoid-induced osteoporosis in children: impact of the underlying disease. Pediatrics. 2007;119(Suppl. 2):S166–74. doi: 10.1542/peds.2006-2023J. [DOI] [PubMed] [Google Scholar]
- 4.Nissel R, Lindberg A, Mehls O, Haffner D. Factors predicting the near-final height in growth hormone-treated children and adolescents with chronic kidney disease. J Clin Endocrinol Metab. 2008;93 doi: 10.1210/jc.2007-2302. [DOI] [PubMed] [Google Scholar]
- 5.White PH. Growth abnormalities in children with juvenile rheumatoid arthritis. Clin Orthop Relat Res. 1990;259:46–50. [PubMed] [Google Scholar]
- 6.Zemel BS, Kawchak DA, Ohene-Frempong K, Schall JI, Stallings VA. Effects of delayed pubertal development, nutritional status, and disease severity on longitudinal patterns of growth failure in children with sickle cell disease. Pediatr Res. 2007;61:607–13. doi: 10.1203/pdr.0b013e318045bdca. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan A, Sylvester FA. Chronic pediatric inflammatory diseases: effects on bone. Rev Endocr Metab Disord. 2008;9:107–22. doi: 10.1007/s11154-007-9070-0. [DOI] [PubMed] [Google Scholar]
- 8.Lilleby V. Bone status in juvenile systemic lupus erythematosus. Lupus. 2007;16:580–6. doi: 10.1177/0961203307079040. [DOI] [PubMed] [Google Scholar]
- 9.Roth J, Bechtold S, Borte G, Dressler F, Girschick HJ, Borte M. Osteoporosis in juvenile idiopathic arthritis–a practical approach to diagnosis and therapy. Eur J Pediatr. 2007;166:775–84. doi: 10.1007/s00431-007-0484-1. [DOI] [PubMed] [Google Scholar]
- 10.Bacon MC, White PH, Raiten DJ, et al. Nutritional status and growth in juvenile rheumatoid arthritis. Semin Arthritis Rheum. 1990;20:97–106. doi: 10.1016/0049-0172(90)90022-8. [DOI] [PubMed] [Google Scholar]
- 11.Compeyrot-Lacassagnes TP, Atenafu E, Doria AS, Stephens D, Gilday D, Silverman ED. Prevalence and etiology of low bone mineral density in juvenile systemic lupus erythematosus. Arthritis Rheum. 2007;56:1966–73. doi: 10.1002/art.22691. [DOI] [PubMed] [Google Scholar]
- 12.Bultink IE, Lems WF, Kostense PJ, Dijkmans BA, Voskuyl AE. Prevalence of and risk factors for low bone mineral density and vertebral fractures in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:2044–50. doi: 10.1002/art.21110. [DOI] [PubMed] [Google Scholar]
- 13.Alsufyani KA, Ortiz-Alvarez O, Cabral DA, et al. Bone mineral density in children and adolescents with systemic lupus erythematosus, juvenile dermatomyositis, and systemic vasculitis: relationship to disease duration, cumulative corticosteroid dose, calcium intake, and exercise. J Rheumatol. 2005;32:729–33. [PubMed] [Google Scholar]
- 14.Lilleby V, Lien G, Frey FK, Haugen M, Flato B, Forre O. Frequency of osteopenia in children and young adults with childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2005;52:2051–9. doi: 10.1002/art.21115. [DOI] [PubMed] [Google Scholar]
- 15.Trapani S, Civinini R, Ermini M, Paci E, Falcini F. Osteoporosis in juvenile systemic lupus erythematosus: a longitudinal study on the effect of steroids on bone mineral density. Rheumatol Int. 1998;18:45–9. doi: 10.1007/s002960050056. [DOI] [PubMed] [Google Scholar]
- 16.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:26100–15. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erhythematosus blood. J Exp Med. 2003;197:711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronnblom L, Alm GV. Systemic lupus erythematosus and the type I interferon system. Arthritis Res Ther. 2003;5:68–75. doi: 10.1186/ar625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coelho LF, Magno de Freitas AG, Mennechet FJ, Blangy A, Uze G. Interferon-alpha and -beta differentially regulate osteoclastogenesis: role of differential induction of chemokine CXCL11 expression. Proc Natl Acad Sci USA. 2005;102:11917–22. doi: 10.1073/pnas.0502188102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takayanagi H, Kim S, Matsuo K, et al. RANKL maintains bone homeostasis through c-fos-dependent induction of interferon-beta. Nature. 2002;416:744–9. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 21.Takayanagi H, Sato K, Takaoka A, Taniguchi T. Interplay between interferon and other cytokine systems in bone metabolism. Immunol Rev. 2005;208:181–93. doi: 10.1111/j.0105-2896.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 22.Punthakee Z, Legault L, Polychronakos C. Prednisolone in the treatment of adrenal insufficiency: a re-evaluation of relative potency. J Pediatr. 2003;143:402–5. doi: 10.1067/S0022-3476(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 23.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. a disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 24.Anderson M. Use of the greulich-pyle “atlas of skeletal development of the hand and wrist” in a clinical context. Am J Phys Anthropol. 1971;35:347–52. doi: 10.1002/ajpa.1330350309. [DOI] [PubMed] [Google Scholar]
- 25.Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–99. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 26.Cahue S, Sharma L, Dunlop D, et al. The ratio of type II collagen breakdown to synthesis and its relationship with the progression of knee osteoarthritis. Osteoarthritis Cartilage. 2007;15:819–23. doi: 10.1016/j.joca.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzuca SA, Poole AR, Brandt KD, Katz BP, Lane KA, Lobanok T. Associations between joint space narrowing and molecular markers of collagen and proteoglycan turnover in patients with knee osteoarthritis. J Rheumatol. 2006;33:1147–51. [PubMed] [Google Scholar]
- 28.Abrahamowicz M, Fortin PR, du Berger R, Nayak V, Neville C, Liang MH. The relationship between disease activity and expert physician’s decision to start major treatment in active systemic lupus erythematosus: a decision aid for development of entry criteria for clinical trials. J Rheumatol. 1998;25:277–84. [PubMed] [Google Scholar]
- 29.CDC. http://www.cdc.gov/GROWTHcharts/ (June 2010, date last accessed)
- 30.Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res. 2000;15:1477–88. doi: 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
- 31.Rosen HN, Dresner-Pollak R, Moses AC, et al. Specificity of urinary excretion of cross-linked n-telopeptides of type I collagen as a marker of bone turnover. Calcif Tissue Int. 1994;54:26–9. doi: 10.1007/BF00316285. [DOI] [PubMed] [Google Scholar]
- 32.Kalla AA, Fataar AB, Jessop SJ, Bewerunge L. Loss of trabecular bone mineral density in systemic lupus erythematosus. Arthritis Rheum. 1993;36:1726–34. doi: 10.1002/art.1780361212. [DOI] [PubMed] [Google Scholar]
- 33.Kalla AA, Meyers OL, Kotze TJ, Laubscher R. Corticosteroid therapy and bone mass – comparison of rheumatoid arthritis and systemic lupus erythematosus. S Afr Med J. 1994;84:404–9. [PubMed] [Google Scholar]
- 34.Kipen Y, Briganti E, Strauss B, Will R, Littlejohn G, Morand E. Three year followup of bone mineral density change in premenopausal women with systemic lupus erythematosus. J Rheumatol. 1999;26:310–7. [PubMed] [Google Scholar]
- 35.Sinigaglia L, Varenna M, Binelli L, et al. Determinants of bone mass in systemic lupus erythematosus: a cross sectional study on premenopausal women. J Rheumatol. 1999;26:1280–4. [PubMed] [Google Scholar]
- 36.Swolin-Eide D, Magnusson P, Hansson S. Bone mass, biochemical markers and growth in children with chronic kidney disease: a 1-year prospective study. Acta Paediatr. 2007;96:720–5. doi: 10.1111/j.1651-2227.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 37.Jurimae J, Pomerants T, Tillmann V, Jurimae T. Bone metabolism markers and ghrelin in boys at different stages of sexual maturity. Acta Paediatr. 2009;98:892–6. doi: 10.1111/j.1651-2227.2008.01193.x. [DOI] [PubMed] [Google Scholar]
- 38.Gur A, Dikici B, Nas K, Bosnak M, Haspolat K, Sarac AJ. Bone mineral density and cytokine levels during interferon therapy in children with chronic hepatitis B: does interferon therapy prevent from osteoporosis? BMC Gastroenterol. 2005;5:30. doi: 10.1186/1471-230X-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radwanski E, Perentesis G, Jacobs S, et al. Pharmacokinetics of interferon alpha-2b in healthy human volunteers. J Clin Pharmacol. 1987;27:432–5. doi: 10.1002/j.1552-4604.1987.tb03044.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen CJ, Chao TY, Janckila AJ, Cheng SN, Ku CH, Chu DM. Evaluation of the activity of tartrate-resistance acid phosphatase isoform 5b in normal chinese children – a novel marker for bone growth. J Pediatr Endocrinol Metab. 2005;18:55–62. doi: 10.1515/jpem.2005.18.1.55. [DOI] [PubMed] [Google Scholar]
- 41.Cole DE, Carpenter TO, Gundberg CM. Serum osteocalcin concentrations in children with metabolic bone disease. J Pediatr. 1985;106:770–6. doi: 10.1016/s0022-3476(85)80351-6. [DOI] [PubMed] [Google Scholar]
- 42.Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Vaananen HK. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res. 2000;15:1337–45. doi: 10.1359/jbmr.2000.15.7.1337. [DOI] [PubMed] [Google Scholar]
- 43.Lamp EC, Drexler HG. Biology of tartrate-resistant acid phosphatase. Leuk Lymphoma. 2000;39:477–84. doi: 10.3109/10428190009113378. [DOI] [PubMed] [Google Scholar]