Abstract
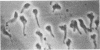
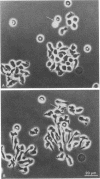
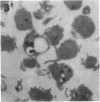
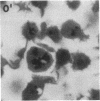
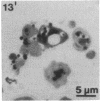
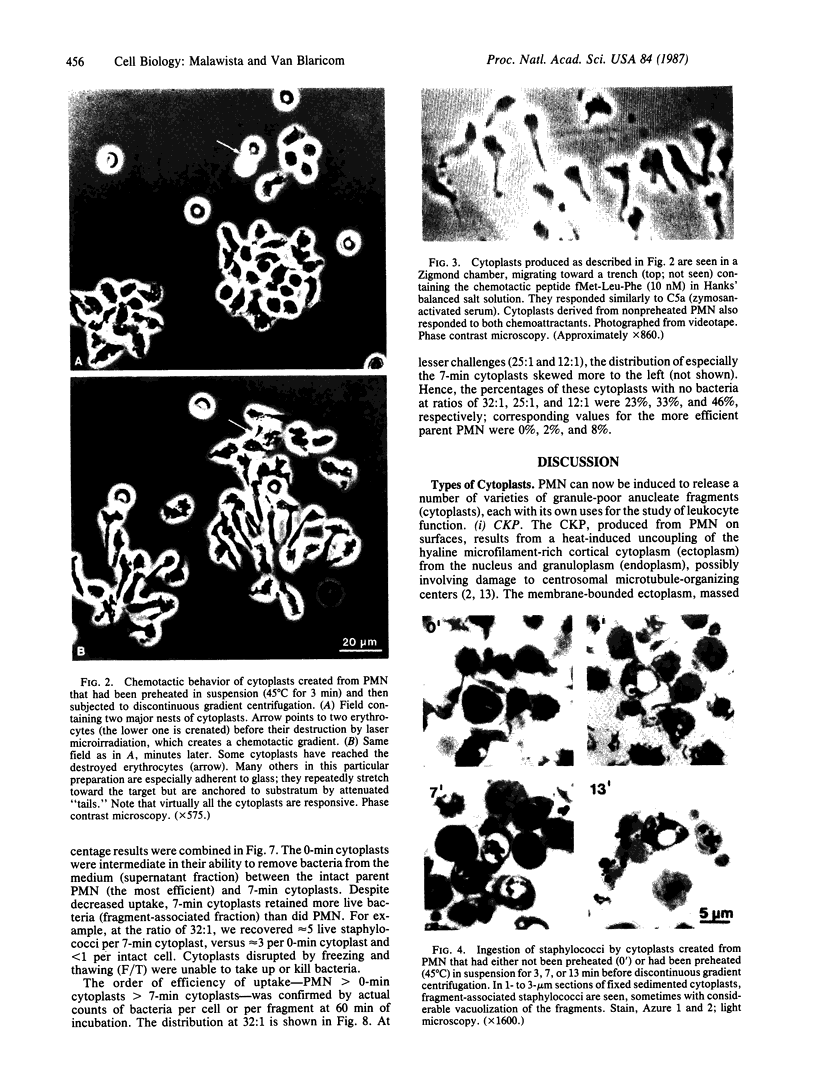
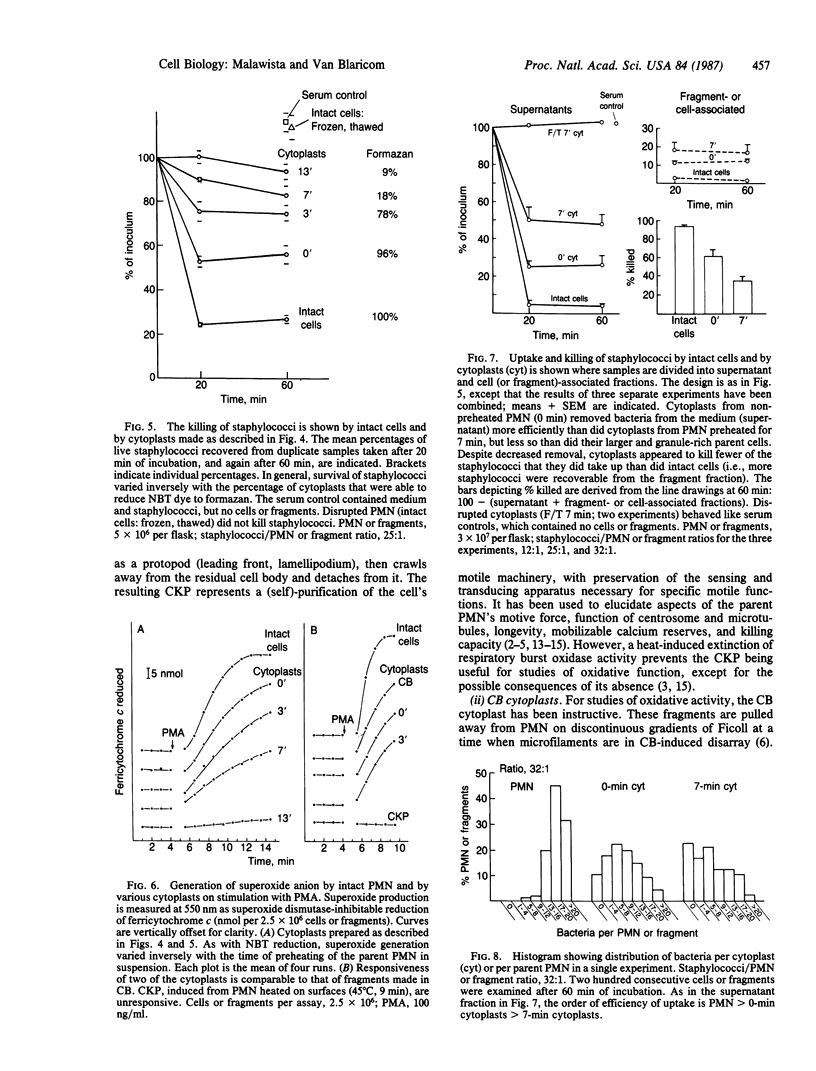
Anucleate fragments (cytoplasts) from polymorphonuclear leukocytes (PMN) are simplified systems that can be used to elucidate specific pathways by which cell function is altered. PMN cytoplasts in current use are defective either in activatable respiratory burst oxidase activity or in motile function. By centrifugation of PMN on discontinuous gradients of Ficoll without cytochalasin B, we have created granule-poor cytoplasts in which both these capacities are preserved. Specifically, they generate superoxide anion (O2-.) and reduce nitroblue tetrazolium dye on appropriate stimulation; they respond chemotactically to erythrocytes destroyed by laser microirradiation or to the specific chemoattractants fMet-Leu-Phe (10 nM) and C5a (zymosan-activated serum); and they ingest and kill staphylococci. We can improve the yield of these fragments progressively by preheating (45 degrees C) the cells in suspension for increasing periods of time, but those treatments are attended by a decreasing percentage of cytoplasts with activatable oxidase activity, and a progressive inability of the cytoplasts to ingest and to kill staphylococci. These easily made and multipotent cytoplasts readily lend themselves to studies of PMN physiology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis M. Editorial: Une forme particuliére de chimiotaxis: le nécrotaxis. Nouv Rev Fr Hematol. 1973 May-Jun;13(3):285–290. [PubMed] [Google Scholar]
- Dyett D. E., Malawista S. E., Naccache P. H., Sha'afi R. I. Stimulated cytokineplasts from human polymorphonuclear leukocytes mobilize calcium and polymerize actin. Cytoplasts made in cytochalasin B retain a defect in actin polymerization. J Clin Invest. 1986 Jan;77(1):34–37. doi: 10.1172/JCI112297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyett D. E., Malawista S. E., Van Blaricom G., Melnick D. A., Malech H. L. Functional integrity of cytokineplasts: specific chemotactic and capping responses. J Immunol. 1985 Sep;135(3):2090–2094. [PubMed] [Google Scholar]
- Gallin J. I., Metcalf J. A., Roos D., Seligmann B., Friedman M. M. Organelle-depleted human neutrophil cytoplasts used to study fmet-leu-phe receptor modulation and cell function. J Immunol. 1984 Jul;133(1):415–421. [PubMed] [Google Scholar]
- Keller H. U., Bessis M. Chemotaxis and phagocytosis in anucleated cytoplasmic fragments of human peripheral blood leucocytes. Nouv Rev Fr Hematol. 1975 Jul-Aug;15(4):439–446. [PubMed] [Google Scholar]
- Korchak H. M., Roos D., Giedd K. N., Wynkoop E. M., Vienne K., Rutherford L. E., Buyon J. P., Rich A. M., Weissmann G. Granulocytes without degranulation: neutrophil function in granule-depleted cytoplasts. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4968–4972. doi: 10.1073/pnas.80.16.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E., Bodel P. T. The dissociation by colchicine of phagocytosis from increased oxygen consumption in human leukocytes. J Clin Invest. 1967 May;46(5):786–796. doi: 10.1172/JCI105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E., De Boisfleury Chevance A. The cytokineplast: purified, stable, and functional motile machinery from human blood polymorphonuclear leukocytes. J Cell Biol. 1982 Dec;95(3):960–973. doi: 10.1083/jcb.95.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E. Microtubule function in human blood polymorphonuclear leukocytes: analysis through heat-induced lesions. Ann N Y Acad Sci. 1986;466:858–866. doi: 10.1111/j.1749-6632.1986.tb38472.x. [DOI] [PubMed] [Google Scholar]
- Malawista S. E. Simple screening test on clotted blood for chronic granulomatous disease of childhood. Lancet. 1978 Apr 29;1(8070):943–943. doi: 10.1016/s0140-6736(78)90723-7. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Van Blaricom G., Cretella S. B. Cytokineplasts from human blood polymorphonuclear leukocytes. Lack of oxidase activity and extended functional longevity. Inflammation. 1985 Mar;9(1):99–106. doi: 10.1007/BF00915416. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Van Blaricom G. Phagocytic capacity of cytokineplasts from human blood polymorphonuclear leukocytes. Blood Cells. 1986;12(1):167–177. [PubMed] [Google Scholar]
- Malawista S. E., de Boisfleury-Chevance A., Maunoury R., Bessis M. Heat as a probe of centrosomal function: a phase-contrast and immunofluorescent study of human blood monocytes. Blood Cells. 1983;9(3):443–453. [PubMed] [Google Scholar]
- Petrequin P. R., Todd R. F., 3rd, Smolen J. E., Boxer L. A. Expression of specific granule markers on the cell surface of neutrophil cytoplasts. Blood. 1986 Apr;67(4):1119–1125. [PubMed] [Google Scholar]
- Roos D., Voetman A. A., Meerhof L. J. Functional activity of enucleated human polymorphonuclear leukocytes. J Cell Biol. 1983 Aug;97(2):368–377. doi: 10.1083/jcb.97.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]