Abstract
A novel class of pH sensitive complexation hydrogels composed of methacrylic acid and functionalized poly(ethylene glycol) tethers, referred to as P(MAA-g-EG) WGA, was investigated as an oral protein delivery system. The PEG tethers were functionalized with wheat germ agglutinin (WGA), a lectin that can bind to carbohydrates in the intestinal mucosa, to improve residence time of the carrier and absorption of the drug at the delivery site. The ability of P(MAA-g-EG) WGA to improve insulin absorption was observed in two different intestinal epithelial models. In Caco-2 cells, P(MAA-g-EG) WGA improved insulin permeability by 9-fold as compared to an insulin only solution. P(MAA-g-EG) WGA was also evaluated in a mucus-secreting culture that contained Caco-2 and HT29-MTX cells. Insulin permeability was increased by 5-fold in the presence of P(MAA-g-EG) WGA. Overall, it is clear that P(MAA-g-EG) WGA enhances insulin absorption and holds great promise as an oral insulin delivery system.
Keywords: Caco-2, HT29-MTX, mucus-secreting, oral protein delivery, wheat germ agglutinin, mucoadhesion
INTRODUCTION
Current estimates indicate that the cost to bring a drug to market is nearing $1 billion dollars.3 With this in mind, it is clear that in vitro tools that can screen and accurately predict the potential success of a drug candidate are necessary. Cell culture models to predict drug permeability are becoming a routine part of drug development within the pharmaceutical industry. While cell culture studies to predict drug absorption often take a minimum of 3 weeks, in vitro experiments have proven to be a more cost-effective method for initial permeability screening than in vivo studies.
The most well-established and accepted cell culture model for predicting drug absorption uses human colon adenocarcinoma cells (Caco-2).11,15 Previous research with this cell line has shown that studies are reproducible and correlate well with in vivo data.2 Caco-2 cell monolayers are similar to the small intestine epithelial layer in that they differentiate into columnar absorptive cells and form a polarized monolayer that includes tight junctions. In addition, Caco-2 cells display a brush border, excrete typical brush border enzymes, and express many carrier-mediated transport systems.5,11 However, Caco-2 cells only differentiate into absorptive enterocytes, whereas the intestinal epithelial layer consists of a variety of different cell types that include goblet cells (mucus-secreting), enteroendocrine cells, and M-cells. Also, due to the lack of goblet cells, there is no mucus layer that lines the cellular monolayer.
Hilgendorf et al. 16 noted that a Caco-2 cellular monolayer forms tight junctions which more closely represent the tightness of the junctions present in the colon as opposed to the looser junctions present in the small intestine. The disadvantage of this is that permeability will be decreased for compounds which are transported predominantly by the paracellular mechanism. It has been shown that the permeability of paracellular markers using Caco-2 cells can be 100 times lower than the permeability in human small intestine.34
For these reasons, researchers have investigated a variety of different cell lines to assess drug permeability. Some of the more common cell lines include MDCK (dog kidney epithelial cells), LLC-PK1 (pig kidney epithelial cells), and TC-7 (subclone of Caco-2).3,27 While all of these cell lines have similar properties and certain advantages and disadvantages over the Caco-2 cell line, they all lack a mucus producing cell.
The HT29 cell line is a human colon carcinoma cell line that contains both mucus and columnar absorptive cells.21 Researchers have developed various subclones of this cell line that differentiate into predominantly mucus secreting cells. Many of the HT29 subclones have been used in co-culture with Caco-2 cells and also by themselves to design a model that more accurately mimics the small intestinal epithelial layer.6,18,25,27,33,34
Our lab has successfully developed a class of environmentally sensitive complexation hydrogels containing methacrylic acid (MAA) and poly(ethylene glycol) (PEG) tethers (designated as P(MAA-g-EG)).7,26 More specifically, P(MAA-g-EG) is a pH responsive hydrogel that is capable of swelling and deswelling due to the formation of temporary physical crosslinks, or interpolymer complexes, between the PMAA pendant groups and the tethered PEG chains. These systems can therefore utilize the pH shift between the stomach and the small intestine (from ~ pH 2 to 7) as an environmental trigger to deliver protein to the targeted site of delivery, which is the small intestine. Furthermore, the PEG tethers were functionalized with wheat germ agglutinin (WGA), a lectin that can bind to carbohydrates in the intestinal mucosa, to improve residence time of the carrier and absorption of the drug at the delivery site. In this research, we seek to evaluate insulin transport in the presence of functionalized complexation hydrogels across a Caco-2 cell monolayer and a Caco-2/HT29-MTX monolayer to determine differences in permeability.
MATERIALS AND METHODS
Hydrogel Synthesis and Functionalization
Hydrogel microparticles were prepared by UV-initiated free radical solution polymerization with a diameter of 90–150 μm as previously described.8 P(MAA-g-EG) was functionalized with biotinylated-WGA (B-WGA) through a biotin-avidin linkage. Prior to polymerization, PEG chains were functionalized with biotin by established protocols 4,14 to allow for the addition of B-WGA by use of an avidin linker.
Microparticles containing PEG-biotin tethers were added to PBS, pH 7.4. Avidin D (Vector Laboratories, Burlingame, CA) was added in a 1:1 molar ratio to biotin. The solution was stirred for 30 minutes, after which the particles were filtered and washed. Particles were then resuspended in PBS, pH 7.4, and B-WGA (Vector Laboratories) was added in a 1.5:1 molar ratio of B-WGA:avidin. After 1 hour of agitation, particles were washed and filtered. Particles were then lyophilized and stored at −20 °C until use. Functionalization was confirmed via HPLC (Waters 2695 Separations Module, Milford, MA).
General Cell Culture
Caco-2 cells were obtained from American Type Culture Collection (ATCC, Rockwell, MD) and HT29-MTX cells were a kind gift from Dr. Thecla Lesuffleur, INSERM, Paris, France. HT29-MTX cells are a sub-population of HT29 cells that were adapted to 10−6 M methotrexate (MTX).21,22 All cell types were cultured in Dulbecco’s modified Eagle medium (DMEM) (Mediatech, Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum (Cambrex, East Rutherford, NJ), 1% non-essential amino acids (Mediatech), 100 U/ml penicillin, and 100 μg/ml streptomycin (Mediatech).
Cultures were maintained in T-75 flasks (Corning, Corning, NY) at 37 °C and a humidified environment of 5% CO2 in air. The medium was changed every other day. Cells were routinely passaged at 80% confluency, which occurred between 6 and 7 days after seeding. A passage operation consisted of a 2 times wash with DPBS w/o Ca2+ and Mg2+ (Mediatech) and then the addition of 1 ml of a 0.5% Trypsin/0.2% EDTA solution (Sigma, St. Louis, MO). Cells were then incubated with the trypsin/EDTA solution for 5 minutes, after which time cells were detached from the flasks and could then be counted and reseeded.
Caco-2 cells were seeded at a density of 3.0 × 103 cells/cm2 and used between passages 60 to 80. HT29-MTX cells were seeded at a density of 2.0 × 104 cells/cm2 and used between passages 8 to 20.
Cytocompatibility
Cytocompatibility experiments were performed in a 96-well plate (Corning) using both Caco-2 cells and HT29-MTX cells. Caco-2 cells were seeded at a density of 1.4 × 104 cells/cm2 while HT29-MTX cells were seeded at a density of 2.8 × 104 cells/cm2. Cells were fed every other day and cytotoxicity studies were conducted when cells reached 90% confluence (6–7 days).
P(MAA-g-EG) and P(MAA-g-EG) WGA were prepared as described; for this study microparticles were sized between 90 and 150 μm. Growth medium was removed from each well and P(MAA-g-EG) microparticles and P(MAA-g-EG) WGA functionalized microparticles were added to wells at concentrations ranging between 0.5 mg/mL to 2.5 mg/mL in Hank’s balanced salt solution (HBSS) (Mediatech). Prior to addition, the pH of each suspension was adjusted to 7.4 with 0.1 N NaOH. Microparticles were then incubated with the cells for 2 hours at 37 °C and 5% CO2. The microparticle suspension was removed from each well and the wells were rinsed three times with HBSS.
To determine cell viability, a cellular metabolic assay was used to measure NADPH production (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI). Results were compared to control wells that were not incubated with microparticles, but only with HBSS and the CellTiter 96® reagent.
Caco-2 Transwell® Culture
All transport and transepithelial electrical resistance (TEER) experiments were conducted using a Costar Transwell® plate (Corning) with a polycarbonate membrane, 0.4 μm pore size, and a cell growth area of either 4.7 cm2 (6 well) or 1 cm2 (12 well). Cells were seeded at a density of 6 × 104 cells/cm2 (6 well) or 1 × 105 cells/cm2 (12 well) after a passaging procedure and cultured for 21 to 24 days. Media were changed every other day and TEER values were measured with an EVOM volt-ohm meter and a chopstick electrode (World Precision Instruments, Sarasota, FL) to monitor development of tight junctions. It has been well documented that Caco-2 cells form an absorptive polarized monolayer, develop an apical brush border, and secrete enzymes after culture for 21 days.5,11 Caco-2 cell seeding densities and media volumes for the different plate types are listed in Appendix 1.7.
Caco-2/HT29-MTX Transwell® Culture
Caco-2 and HT29-MTX cells were maintained separately in T-75 flasks as previously described. After subculturing, cells were counted and mixed together in a 1:1 ratio before seeding on to the Transwell® plate at a density of 6 × 104 cells/cm2 (6 well) or 1 × 105 cells/cm2 (12 well). Previous research demonstrated that a 1:1 seeding ratio produced TEER values closest to those reported in vivo for human intestinal epithelia.16 As with the Caco-2 cells, media was changed every other day in the co-culture and TEER was used to monitor development of the tight junctions.
TEER Evaluation
TEER was used to evaluate the development of tight junctions in the Transwell® cultures. Measurements were taken every other day two hours after changing the media. In order to determine the resistance across the cellular monolayer, Rtrue tissue, it was important to subtract out the resistance due to the membrane and the media within the wells. A blank resistance measurement was taken in the presence of medium without cells, Rblank, and then subtracted from the experimental TEER value.
After Rtrue tissue was obtained, all TEER values were multiplied by the growth area, which provided a unit area resistance. This allowed for comparison of TEER values when cells were grown on different sized growth areas.
Experiments were also conducted to determine the effect of various microparticle concentrations on the TEER values in both the Caco-2 and Caco-2/HT29-MTX Transwell® cultures. Cells were cultured in a 12-well Transwell® plate for 21 to 24 days as previously described. The medium was then removed from the apical and basolateral chamber and replaced with pre-warmed HBSS after first washing both chambers one time with HBSS. Cells were allowed to equilibrate for one hour and TEER measurements were taken at 0, 0.5, and 1 hour.
During the TEER measurement, Transwell® plates were placed on a heating mat to maintain temperature at 37 °C. P(MAA-g-EG) microparticles sized between 90 and 150 μm were added to the apical chamber at four different concentrations after cell equilibration. Both samples 1 and 2 were added as dry microparticles to each well. Sample 1 contained 3.33 mg microparticles (3.33 mg/cm2) and sample 2 contained 2.12 mg of microparticles (2.12 mg/cm2), meaning the concentration of microparticles in the apical chamber was 6.67mg/ml and 4.24 mg/ml, respectively. The last two samples were suspended in HBSS and heated for 30 minutes prior to the addition to each well. Sample 3 was added at a concentration of 1 mg/ml (0.5 mg/cm2) and sample 4 was added at a concentration of 5 mg/ml (2.5 mg/cm2). Control wells contained HBSS without microparticles. TEER values were monitored at various time points over the course of 3 hours. After this time, microparticles were removed from the wells by washing with HBSS 3 times. Media was then added to both the apical and basolateral chambers and TEER values were monitored over the next 24 hours.
Insulin Transport
Experiments were performed to determine the amount of insulin transported across a cellular monolayer in both the presence and absence of microparticles. Cells were cultured in a 6-well Transwell® plate for 21 to 24 days as already described. Media were then removed from the apical and basolateral chambers and replaced with pre-warmed HBSS after first washing both chambers one time with HBSS. The HBSS in both the apical and basolateral chambers contained Ca2+ at a concentration of 1.26 mM. Cells were allowed to equilibrate for one hour and TEER measurements were taken at 0, 0.5, and 1 hour. For all insulin samples and TEER measurements, Transwell® plates were placed on a heating mat to maintain temperature at 37 °C.
Bovine insulin (Sigma-Aldrich) solutions were prepared at a concentration of 0.2 mg/ml with HBSS. Bovine serum albumin (BSA) (Sigma-Aldrich) was also added to the solution at a final concentration of 0.5 mg/ml to inhibit insulin adsorption to the Transwell® plates. Insulin solutions were heated at 37 °C for 15 minutes prior to addition to the equilibrated cells. For samples containing microparticles, either P(MAA-g-EG) or P(MAA-g-EG) WGA, sized between 90 and 150 μm, was added to the insulin solution at a concentration of 1 mg/ml immediately before adding the sample to the apical chamber. Control wells contained only the insulin/BSA/HBSS solution and no microparticles. After sample addition, 0.1 ml insulin samples were taken from the apical chamber at 0 and 3 hours and from the basolateral chamber at 0, 0.5, 1, 2, and 3 hours. Samples were replaced with a pre-warmed HBSS solution containing 0.5 mg/ml BSA. TEER values were also monitored over the course of the experiment. After 3 hours, the contents of the apical chamber was removed and washed 2 times with HBSS. Media was then added to both the apical and basolateral chambers and TEER values were monitored over the next 24 hours.
Insulin concentration was determined by a bovine insulin ELISA kit (Alpco Diagnostics, Windham, NH). The maximum sensitivity of the ELISA kit was 6 ng/ml, so apical samples were diluted 25,000x and basolateral samples were diluted between 0 and 10x.
The apparent permeability coefficient, Papp, was calculated from the following equation:
where dQ/dt represents the steady-state flux of insulin across the monolayer, A is the surface area of the membrane, and Co is the initial insulin concentration in the apical chamber. The flux across the monolayer was calculated from the slope of insulin transported to the basolateral chamber versus time.
RESULTS AND DISCUSSION
Cytocompatibility
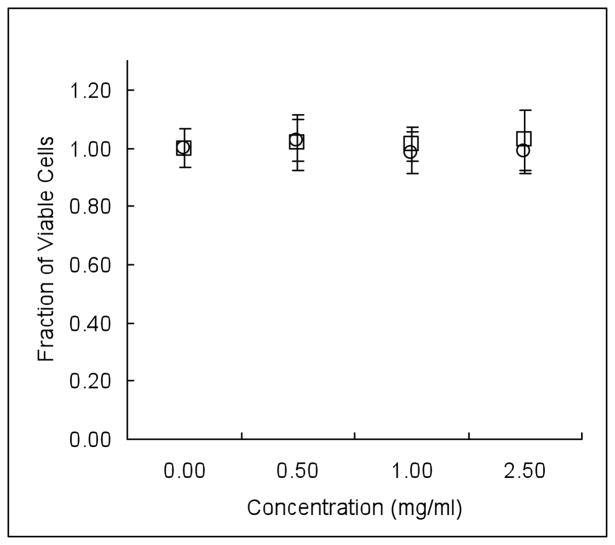
There was no significant decrease in cell viability when incubating the various concentrations of P(MAA-g-EG) and P(MAA-g-EG) WGA with Caco-2 cells as shown in, Figure 1. The addition of a biologically functional molecule, such as WGA in this case, could potentially lead to some cytocompatibility issues. In this experiment, functionalization of P(MAA-g-EG) with WGA did not alter the metabolic activity of the cells.
Figure 1.
Caco-2 cells were seeded at a density of 1.4 × 104 cells/cm2 and cultured in a 96-well plate until 90% confluence. P(MAA-g-EG) (○) and P(MAA-g-EG) WGA (□) microparticles were suspended in HBSS at various concentrations and incubated with the cells for 2 hours. Microparticles were removed via a wash step and metabolic activity was determined by a colorimetric assay. No significant toxicity was observed at the concentrations tested. n = 3–6 ± SD
These results are in agreement with previous research that demonstrated P(MAA-g-EG) microparticles using a 1:1 molar feed ratio of MAA:EG are not toxic to Caco-2 cells at concentrations less than 10 mg/ml.10,13,17,32 However, it should be noted that increasing the molar feed ratio of MAA:EG or AA:EG to 4:1 can have toxic effects on Caco-2 cells.13 It is hypothesized that the addition of more MAA leads to an increase in ionized carboxyl groups that can bind Ca2+, which could potentially disrupt cell function. Also, the ionization of the MAA would cause local acidic microenvironments, which could potentially decrease cell viability. In addition, Torres-Lugo et al. 32 showed that at a microparticle concentration of 10 mg/ml cell viability was reduced regardless of monomer composition.
It wasn’t anticipated that cytotoxicity results would be different for the Caco-2 and HT29-MTX cells, but it was important to conduct cytotoxicity experiments with both cell lines that we would later be using for drug transport studies. Figure 2 shows that P(MAA-g-EG) and P(MAA-g-EG) WGA did not decrease cell viability at the tested concentrations when incubated for 2 hours with HT29-MTX cells. We needed to ensure that incubating the microparticles with the cells did not decrease viability in either cell line because this could lead to false results in the drug transport experiments.
Figure 2.
HT29-MTX cells were seeded at a density of 2.8 × 104 cells/cm2 and cultured in a 96-well plate until 90% confluence. P(MAA-g-EG) (○) and P(MAA-g-EG) WGA (□) microparticles were suspended in HBSS at various concentrations and incubated with the cells for 2 hours. Microparticles were removed via a wash step and metabolic activity was determined by a colorimetric assay. No significant toxicity was observed at the concentrations tested. n = 6 ± SD
TEER Evaluation
TEER was used as a tool to evaluate the development of tight junctions within the cellular monolayers. The first step was to monitor the difference in TEER values between the Caco-2 cells and the co-cultured cells over the course of 21 days, Figure 3. Besides the addition of a mucus layer to the cellular model, the co-culture has the potential to more accurately mimic the resistance values present in human small intestinal epithelia, which has been measured to be between 50 and 100 Ω*cm2.3,15,28 After 21 days, the Caco-2 monolayer had a resistance of 401 ± 35 Ω*cm2, whereas the Caco-2/HT29-MTX monolayer reduced the resistance to 157 ± 36 Ω*cm2.
Figure 3.
Caco-2 cells were seeded on a 12-well Transwell® plate at a density of 1.0 × 105 cells/cm2. Caco-2/HT29-MTX cells were seeded on a 12-well Transwell® plate in a 1:1 ratio at a total cell density of 1.0 × 105 cells/cm2. TEER values were monitored for 21 days of both the Caco-2 (●) and Caco-2/HT29-MTX (○) cultures. TEER values in the co-cultured system were closer to reported values of human intestinal epithelia, which range between 50 and 100 Ω*cm2. n=12 ± SD.
Values are similar to previously published results for TEER after 21 days for both the Caco-2 and co-cultured monolayer.5,16 It must be noted that culture conditions, such as medium, passage number, seeding density, and membrane type can greatly influence TEER values.3 When examining drugs whose predominant mechanism of transport is paracellular, it is especially important that cellular model TEER values correlate well with in vivo TEER values to accurately predict in vivo drug absorption. While it is important for cellular model TEER values to be closer to in vivo TEER values, it should be noted that cellular models (even mucus secreting models) generally underestimate permeability of paracellular compounds in comparison to perfused rat small intestine studies.17,29,33
The next TEER study involved examining the effect of various microparticle concentrations over the course of 24 hours on both the Caco-2 and Caco-2/HT29-MTX monolayers. All four microparticle concentrations used reduced TEER values in the Caco-2 monolayer during the three hour experiment as compared to control, Figure 4. A concentration dependent reduction in TEER was observed and a maximum reduction of approximately 45% (as compared to initial TEER value) after three hours was seen in the wells containing 3.33 mg of initially dry microparticles, which corresponded to the highest concentration of microparticles. This result is in agreement with previous research, which has shown a reduction in TEER in the presence of P(MAA-g-EG) microparticles.9,12,17,19,23
Figure 4.
Caco-2 cells were seeded on a permeable Transwell® plate and cultured for 21 days. P(MAA-g-EG) microparticles were incubated with the cells for 3 hours and TEER values were monitored. Microparticles were added dry at concentrations of 6.67 mg/ml (□) and 4.24 mg/ml (◇), which corresponds to 3.33 mg/cm2 and 2.12 mg/cm2, respectively. Microparticles were pre-suspended in HBSS and added at concentrations of 5 mg/ml (△) and 1 mg/ml (○), which corresponds to 2.5 mg/cm2 and 0.5 mg/cm2, respectively. The control well contained only cells with HBSS (✕). n=3 ± SD
After three hours, microparticles were removed by a wash step and TEER was monitored over the next 24 hours, Figure 5. The 1 mg/ml concentration was the only sample to have TEER values to return to control after 24 hours, which indicates a reversible opening of the tight junctions. Difficulty in completely removing the microparticles from the cells may have resulted in some of the TEER values of the higher microparticle concentrations not returning to control or it is possible that the higher concentrations did cause permanent damage to the tight junctions.
Figure 5.
Caco-2 cells were seeded on a permeable Transwell® plate and cultured for 21 days. P(MAA-g-EG) microparticles were incubated with the cells for 3 hours and TEER values were monitored. Microparticles were removed via a wash step after 3 hours, cell medium was added, and TEER values were monitored for 24 hours. Microparticles were added dry at concentrations of 6.67 mg/ml (□) and 4.24 mg/ml (◇), which corresponds to 3.33 mg/cm2 and 2.12 mg/cm2, respectively. Microparticles were pre-suspended in HBSS and added at concentrations of 5 mg/ml (△) and 1 mg/ml (○), which corresponds to 2.5 mg/cm2 and 0.5 mg/cm2, respectively. The control well contained only cells with HBSS (✕). n=3 ± SD
Recent work by Sipahigil et al. 30 used histopathology and TEM to analyze cell damage and tight junction disruption after oral administration of P(MAA-g-EG) to rats. After administration of 100 mg of P(MAA-g-EG), there was minimal tight junction disruption at 2 and 10 hours, and after 20 hours there was no indication of cell damage. In contrast, administering 385 mg of P(MAA-g-EG) showed an increase in the tight junction disruption, which led to epithelial cell damage. While it is difficult to correlate this study with the comparably small surface area and static cell culture set-up, it is important to note that too high a concentration of microparticles can lead to disruptions in the integrity of the intestinal epithelial layer. Overall, the presence of the microparticles must not create a chronic opening of the tight junctions, which would allow for the free passage of bacteria and toxins into the bloodstream.
The TEER results from the microparticle experiment with the co-cultured cells are presented in Figure 6 and Figure 7. In contrast to the results with the Caco-2 cells, the microparticle concentrations used in the co-culture experiment did not produce a significant drop in resistance across the monolayer as compared to the control well (containing only HBSS) as shown in Figure 8. Even when comparing the percent change in TEER from the initial value for each sample, the 1 mg/ml sample produced the greatest change with only a 12% reduction in the TEER value. These samples did not produce a concentration dependent reduction in TEER as compared to the Caco-2 cells.
Figure 6.
Caco-2/HT29-MTX cells were seeded on a permeable Transwell® plate and cultured for 21 days. P(MAA-g-EG) microparticles were incubated with the cells for 3 hours and TEER values were monitored. Microparticles were added dry at concentrations of 6.67 mg/ml (□) and 4.24 mg/ml (◇), which corresponds to 3.33 mg/cm2 and 2.12 mg/cm2, respectively. Microparticles were pre-suspended in HBSS and added at concentrations of 5 mg/ml (△) and 1 mg/ml (○), which corresponds to 2.5 mg/cm2 and 0.5 mg/cm2, respectively. The control well contained only cells with HBSS (✕). n=3 ± SD
Figure 7.
Caco-2/HT29-MTX cells were seeded on a permeable Transwell® plate and cultured for 21 days. P(MAA-g-EG) microparticles were incubated with the cells for 3 hours and TEER values were monitored. Microparticles were removed via a wash step after 3 hours, cell medium was added, and TEER values were monitored for 24 hours. Microparticles were added dry at concentrations of 6.67 mg/ml (□) and 4.24 mg/ml (◇), which corresponds to 3.33 mg/cm2 and 2.12 mg/cm2, respectively. Microparticles were pre-suspended in HBSS and added at concentrations of 5 mg/ml (△) and 1 mg/ml (○), which corresponds to 2.5 mg/cm2 and 0.5 mg/cm2, respectively. The control well contained only cells with HBSS (✕). n=3 ± SD
Figure 8.
Caco-2 and Caco-2/HT29-MTX cells were seeded on a permeable Transwell® plate and cultured for 21 days. P(MAA-g-EG) microparticles were added dry at a concentration of 4.24 mg/ml and incubated with Caco-2 cells (○) and Caco-2/HT29-MTX cells (□) for 3 hours. TEER values were monitored over the duration of the experiment. The control well contained only Caco-2 cells (●) or Caco-2/HT29-MTX cells (■) with HBSS. A drop in TEER values as compared to control and initial values was only seen in the Caco-2 monolayer and not in the Caco-2/HT29-MTX co-culture. n=3 ± SD
There are several possible explanations for why a significant reduction in TEER was not seen. The obvious conclusion is that the presence of the mucus layer inhibited the microparticles from exhibiting as great of an effect on the cellular monolayer. Previous research by Madsen and Peppas 24 demonstrated that the binding of extracellular Ca2+ by P(MAA-g-EG) modulated a decrease in TEER, which was linked to an opening of the tight junctions. It has been well documented that a decrease in extracellular Ca2+ from the basolateral chamber leads to an opening of the tight junctions, whereas a decrease of extracellular Ca2+ in the apical chamber has little effect on the integrity of the tight junctions.20
Ichikawa et al. 17 hypothesized that the presence of P(MAA-g-EG) microparticles in the apical chamber is able to modulate Ca2+ concentration in the basolateral chamber, thus leading to an opening of the tight junctions. It is thought that the Ca2+ chelation properties of P(MAA-g-EG) increase the Ca2+ flux from the basolateral-to-apical direction. The increased distance of the microparticles from the cellular monolayer due to the presence of the mucus layer might have decreased the permeation enhancement effect as previously seen with the microparticles. A second possible explanation is that the co-culture already has a reduced resistance as compared to the Caco-2 cells, so the percentage drop in resistance in the co-cultured system would possibly be less than in the Caco-2 system.
In contrast to the Caco-2 monolayer, after removal of the microparticles from the co-cultured monolayer all TEER values returned to above control TEER values and the values also surpassed the initial TEER values for each well. Hence, in the presence of the mucus layer, there was no permanent damage to the tight junctions due to the microparticles. As seen from the in vivo work of Sipahigil et al.,30 there is still an effect of the microparticles on the tight junctions in the presence of a mucus layer and a high enough concentration of microparticles can be damaging to the epithelial layer. Measurement of TEER values in the two different cellular intestinal models may not be the most suitable method for determining tight junction response to microparticles in vivo.
Insulin Transport
Insulin transport studies were conducted to examine the difference in transport in the presence of microparticles and also to compare transport between the two different cellular models. The first study looked at insulin transport across a Caco-2 monolayer with an insulin solution only, an insulin solution and P(MAA-g-EG), and an insulin solution and P(MAA-g-EG) WGA. Microparticles were used at a concentration of 1 mg/ml and were not loaded with insulin prior to the experiment. Unloaded microparticles were used to eliminate differences in the rate of insulin release from the non-functionalized and functionalized microparticles.
Both microparticle samples caused a significant drop in TEER values as compared to the insulin solution only, which then resulted in an increase in the amount of insulin transported across the monolayer as seen in Figure 9. For all samples the insulin permeability, Papp was calculated. The insulin permeability increased by approximately 9-fold in wells containing P(MAA-g-EG) (14.21 ± 2.80 × 10−9 cm/s) and P(MAA-g-EG) WGA (15.15 ± 1.33 × 10−9 cm/s) as compared to the permeability of an insulin solution (1.61 ± 0.38 × 10−9 cm/s) as shown in Table 1. The significant increase in the Papp value (p<0.01) can be explained by the ability of the P(MAA-g-EG) and P(MAA-g-EG) WGA microparticles to open cell tight junctions by binding Ca2+, as previously discussed, which was demonstrated by a reduction in TEER.
Figure 9.
Caco-2 cells were seeded on a permeable Transwell® plate and cultured for 21 days. Cells were incubated with 1 mg/ml P(MAA-g-EG) + 0.2 mg/ml insulin (△), 1 mg/ml P(MAA-g-EG) WGA + 0.2 mg/ml insulin (○), and a 0.2 mg/ml insulin solution (□) for 3 hours. (Top) TEER values are represented as a percent of initial values as measured during an insulin transport experiment. (Bottom) Insulin concentration in the basolateral chamber was determined by ELISA. The presence of microparticles significantly enhanced insulin transport.
Table 1.
Permeability Values for Insulin Transport in Caco-2 and Caco-2/HT29-MTX Monolayers
| Culture Type | Formulation | Papp × 109 (cm/s)* |
|---|---|---|
| Caco - 2 | Insulin soln | 1.61 ± 0.38 |
| P(MAA-g-EG) + insulin soln | 14.21 ± 2.80 | |
| P(MAA-g-EG) WGA+ insulin soln | 15.15 ± 1.33 | |
| Caco-2/HT29-MTX | Insulin soln | 2.98 ± 0.27 |
| P(MAA-g-EG) + insulin soln | 15.01 ± 0.65 | |
| P(MAA-g-EG) WGA+ insulin soln | 15.20 ± 1.43 |
Permeability values were calculated for the different formulations in the Caco-2 and Caco-2/HT29-MTX cultures using the previously described equation. The flux across the monolayer was calculated from the slope of insulin transported to the basolateral chamber versus time.
Previous research in our lab has demonstrated a 1.5 to 6 fold increase in the apparent permeability of insulin in the presence of microparticles, which is lower than the 9-fold increase seen in this work.13,17,19,23 All of those studies contained 1.26 mM Ca2+ in the apical and basolateral chambers. In the absence of Ca2+ in the apical chamber and 0.20 mM Ca2+ in the basolateral chamber, Ichikawa et al.17 even reported a 21 fold permeation enhancement effect with P(MAA-g-EG). The increase in transport could be directly correlated to a decrease in TEER values. In wells containing microparticles and equimolar amounts of Ca2+, TEER only decreased by 19% whereas wells containing microparticles and minimal amounts of Ca2+ had a 47% reduction in TEER from initial values. This work helped to demonstrate the ability of the microparticles to chelate Ca2+ and further strengthened the argument that the predominant mechanism of insulin transport is paracellular.
Work by Torres-Lugo et al.,31 which involved the transport of salmon calcitonin instead of insulin, also claimed that the main mechanism of transport was by the paracellular route. In these studies, transport of FITC-dextran, a paracellular marker, was compared to transport of calcitonin at two different temperatures (37 °C and 5 °C), the apical-to-basolateral direction, and the basolateral-to-apical direction. Differences in transport at either temperature or between either direction would potentially indicate an active transport mechanism, but this was not observed. The presence of P(MAA-g-EG) increased transport of FITC-dextran and calcitonin at both temperatures and in both directions, and did not appear to affect the mechanism of transport.
Recently, Kavimandan et al.19 investigated a transcellular mechanism of transport by conjugating insulin to transferrin. From his work, it was shown that P(MAA-g-EG) microparticles increased insulin transport 6 fold. When insulin was conjugated to transferrin, apparent permeability of the conjugate increased by 16 fold as compared to insulin. The largest increase in permeability was seen with the insulin-transferrin conjugate in the presence of the microparticles, where apparent permeability increased by 24-fold. This research demonstrated the potential of using both paracellular and transcellular mechanisms of transport.
Actual Papp values from this work (14.21 × 10−9 cm/s) are slightly higher than previously published values using P(MAA-g-EG) microparticles and insulin, which have ranged from 0.162 × 10−9 cm/s to 11.6 × 10−9 cm/s.17,19 Higher Papp values (12,700 × 10−9 cm/s) were published by Lopez et al.,23 but it was determined that there was a mistake in the calculation of the permeability value. The actual Papp value from the Lopez study should have been calculated to be 9.82 × 10−9 cm/s, which would be considered to be in the range of acceptable values based on previously published studies.
Lopez et al.23 also did a study looking at transport of insulin across a cellular monolayer when using insulin-loaded microparticles. The apparent permeability was again incorrectly calculated and should have been 3.37 × 10−9 cm/s, instead of the published value of 4690 × 10−9 cm/s. This study is of value because it shows that loaded insulin can be released from the microparticles and transported across a cellular monolayer. As expected, the apparent permeability values are lower for the insulin-loaded microparticles because of the time it takes for the insulin to diffuse out of the polymer.
In comparing all of these different cellular studies, it should be noted that the microparticle concentration used in these studies was 1 mg/ml. Microparticle concentrations in the previous studies varied from 5.00 to 13.33 mg/ml.13,17,19,23 It is thought that insulin was loaded into the microparticles in the apical chamber during the transport experiment. ELISA was used to measure the initial and final apical insulin concentrations. The amount of insulin transported to the basolateral chamber does not account for the loss of insulin in the apical chamber, thus it is thought that insulin is loaded into the microparticles. Further evidence to support this theory was seen by Blanchette et al.9 when performing transport experiments with bleomycin and P(MAA-g-EG) nanospheres. Bleomycin was imbibed by the swollen nanospheres, thus limiting the amount of bleomycin available for transport across the cellular monolayer. Based on this conclusion, a higher concentration of microparticles in the apical chamber would further reduce the insulin concentration in the apical chamber over time, therefore reducing the amount of insulin that can be transported to the basolateral chamber. This is one possible explanation for why Papp values were slightly higher with this work.
It should be reiterated that cell culture conditions, the presence or absence of calcium, and microparticle size and concentration can all influence the permeation enhancement effect of P(MAA-g-EG).3,20 However, reliable comparisons can be made between different samples using the same experimental conditions.
Insulin transport studies were also carried out using a Caco-2/HT29-MTX monolayer with identical experimental conditions. Again, Papp increased for wells with P(MAA-g-EG) (15.01 ± 0.65 × 10−9 cm/s) and P(MAA-g-EG) WGA (15.20 ± 1.43 × 10−9 cm/s) as compared to an insulin only solution (2.98 ± 0.27 × 10−9 cm/s) (p<0.01), Table 1. This correlates to approximately a 5-fold increase in apparent permeability while the increase in apparent permeability with the Caco-2 cells was 9-fold.
In contrast to TEER results from the Caco-2 insulin transport studies, the microparticles did not cause a change in resistance across the monolayer as seen in Figure 10. These results are in agreement with the previous studies that evaluated a range of microparticle concentrations with the co-culture and saw that there was no significant change in TEER values. While the apparent permeability did increase significantly (~2-fold) for the insulin only solution between the Caco-2 (1.61 ± 0.38 × 10−9 cm/s) and Caco-2/HT29-MTX (2.98 ± 0.27 × 10−9 cm/s) cultures (p<0.01), permeability values remained the same for wells containing microparticles in both cellular models. This result is interesting because the microparticles had no affect on the TEER value in the co-culture system, yet the Papp value remained the same in both cellular models.
Figure 10.
Caco-2/HT29-MTX cells were seeded on a permeable Transwell® plate and cultured for 21 days. Cells were incubated with 1 mg/ml P(MAA-g-EG) + 0.2 mg/ml insulin (△), 1 mg/ml P(MAA-g-EG) WGA + 0.2 mg/ml insulin (○), and a 0.2 mg/ml insulin solution (□) for 3 hours. (Top) TEER values are represented as a percent of initial values as measured during an insulin transport experiment. (Bottom) Insulin concentration in the basolateral chamber was determined by ELISA. The presence of microparticles significantly enhanced insulin transport.
We would expect to see a reduction in TEER with an increase in insulin transport, but this was not the case for the co-culture. The presence of microparticles in the co-culture did increase insulin permeability by 5-fold as compared to an insulin only solution, but the increase in insulin permeability was more significant in the Caco-2 culture in the presence of microparticles (9-fold). It is possible that the microparticles still had a Ca2+ chelating effect in the co-culture, but due to overall lower resistances in the co-culture the reduction in TEER would not have been as drastic as in the Caco-2 culture. After 3 hours, the % of initial TEER values in the co-culture for P(MAA-g-EG) were 95.40% ± 0.05 and 96.67% ± 0.08 for P(MAA-g-EG) WGA, while the TEER values for the insulin solution were 106.75% ± 0.06.
Although there was no statistically significant difference between the TEER values, the average values are slightly reduced from initial values in wells containing microparticles, whereas TEER values increased slightly from initial values in wells containing an insulin solution. This suggests a slight increase in the paracellular space, which could possibly explain the increase in insulin transport. Further studies using immunofluorescence staining for proteins involved in the tight junction complex could help elucidate the mechanism by which microparticles enhance transport.
One important point to note is that P(MAA-g-EG) and P(MAA-g-EG) WGA both enhance insulin permeability in the co-culture while having little effect on tight junction integrity as measured by TEER. Methods to modify the paracellular space are often critiqued because of concerns over potential irreversible opening of the tight junctions. It is encouraging that the presence of the microparticles in the co-culture does not cause any irreversible damage to the integrity of the tight junctions.
As expected, the apparent permeability did increase for the insulin only solution in the co-cultured monolayer. Hilgendorf et al. previously compared the apparent permeability of several small molecular weight drugs (<350 Da) that are absorbed via the paracellular pathway in both a Caco-2 monolayer and a Caco-2/HT29-MTX monolayer.16 They concluded that Papp values increased from 1.8-fold (terbutaline) to 13.7-fold (mannitol) in the co-culture depending on the drug. While the increase in insulin transport wasn’t as high as it was for mannitol, it is important to keep in mind that insulin is a much larger molecule, both in molecular weight and three-dimensional shape. To our knowledge, this is the first study that has been done to compare the transport of a protein across a Caco-2 monolayer and a Caco-2/HT29-MTX monolayer.
It is also interesting to note that the presence of the mucus layer in the co-culture did not slow insulin diffusion across the cellular monolayer. This can be explained by thinking about the diffusional barriers to insulin, which can be separated into the mucus layer and the epithelial cell layer. The diffusional barrier that the mucus layer presents is relatively insignificant in comparison to the epithelial layer. Previous animal studies by Aoki et al. demonstrated that the mucus layer does not affect diffusive absorption, but instead acts as an enzymatic barrier to insulin absorption.1 In this work, the mucus layer of rat small intestine was removed by hyaluronidase pretreatment and transport of insulin and another paracellular marker, FITC-dextran (mw, 4.4 kDa) (FD-4) were observed before and after pre-treatment. FD-4 Papp was the same before and after hyaluronidase treatment, whereas Papp for insulin increased significantly after hyaluronidase treatment. This work then suggests that the mucus layer acts as an enzymatic barrier to insulin absorption and not a diffusional barrier.
In all transport studies with the Caco-2 and Caco-2/HT29-MTX cultures, there was no significant difference in Papp between P(MAA-g-EG) and P(MAA-g-EG) WGA. We did not expect to see any differences in insulin transport in this type of transport study. The advantage of using WGA functionalized microparticles is to increase residence time and a static cell culture set-up, like the one used, would not exploit this advantage. Other studies to test mucoadhesion and in vivo insulin absorption are better suited to determine the effects of WGA functionalization.
CONCLUSION
The cytotoxicity experiments that were conducted demonstrated that P(MAA-g-EG) and P(MAA-g-EG) WGA concentrations between 0.5 mg/mL to 2.5 mg/mL did not decrease cell viability after incubation with cells for two hours. In addition, it was determined that both P(MAA-g-EG) and P(MAA-g-EG) WGA were cytocompatible with Caco-2 and HT29-MTX cell lines.
Overall, the goal of this work is to create a replacement therapy for multiple daily insulin injections. Therefore, we must make certain that the continued administration of an oral dosage would not damage the intestinal epithelial barrier. Damage to the barrier could result in the passage of unwanted toxins and bacteria, possibly leading to systemic infections and toxicity. Therefore, cytotoxicity experiments are a good screening tool to use to eliminate carriers that could potentially cause damage to the intestinal epithelial barrier.
The presence of the HT29-MTX cell in the co-culture reduced the overall resistance of the cellular monolayer, thus cellular resistance values more closely mimicked small intestinal resistance values in vivo. A two-fold increase in Papp was observed for the insulin solution in the co-culture. In addition, the presence of the mucus layer did not create a significant diffusional barrier.
In evaluating TEER, P(MAA-g-EG) microparticles at a concentration of 1 mg/ml reversibly reduced initial resistance values in the Caco-2 culture by 30% after 3 hours. There was also a concentration dependent decrease in TEER values. This reduction in TEER was correlated to an opening of the tight junctions, which was demonstrated by a 9-fold increase in Papp for P(MAA-g-EG) as compared to the insulin only solution. Results were in good agreement with previously published studies examining insulin transport in the presence of P(MAA-g-EG).13,17,19
Microparticles did not seem to produce a significant reduction in TEER values in the Caco-2/HT29-MTX monolayers. It is possible that the presence of the mucus layer increased the distance of the microparticles from the apical side of the cells, thus reducing their effect on TEER. Although, in insulin transport experiments, the presence of microparticles still increased insulin transport 5-fold. The decrease in TEER in the presence of the microparticles might be less due to a lower overall resistance of the co-culture. TEER may not be the best way to measure modulation of tight junctions in the co-culture. Work using immunofluorescence staining for proteins involved in the tight junction complex could help elucidate the mechanism by which microparticles enhance transport.
Acknowledgments
This work was funded by NIH Grant EB No. 000246. Support was also provided by a Livingston Fellowship to KMW.
References
- 1.Aoki Y, Morishita M, Takayama K. Role of the mucous/glycocalyx layers in insulin permeation across the rat ileal membrane. Int J Pharm. 2005;297(1–2):98–109. doi: 10.1016/j.ijpharm.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175(3):880–885. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- 3.Balimane PV, Chong S. Cell culture-based models for intestinal permeability: A critique. Drug Discov Today. 2005;10(5):335–343. doi: 10.1016/S1359-6446(04)03354-9. [DOI] [PubMed] [Google Scholar]
- 4.Behravesh E, V, Sikavitsas I, Mikos AG. Quantification of ligand surface concentration of bulk-modified biomimetic hydrogels. Biomaterials. 2003;24(24):4365–4374. doi: 10.1016/s0142-9612(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 5.Behrens I, Kissel T. Do cell culture conditions influence the carrier-mediated transport of peptides in Caco-2 cell monolayers? Eur J Pharm Sci. 2003;19(5):433–442. doi: 10.1016/s0928-0987(03)00146-5. [DOI] [PubMed] [Google Scholar]
- 6.Behrens I, Stenberg P, Artursson P, Kissel T. Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells. Pharm Res. 2001;18(8):1138–1145. doi: 10.1023/a:1010974909998. [DOI] [PubMed] [Google Scholar]
- 7.Bell CL, Peppas NA. Water, solute and protein diffusion in physiologically responsive hydrogels of poly(methacrylic acid-g-ethylene glycol) Biomaterials. 1996;17(12):1203–1218. doi: 10.1016/0142-9612(96)84941-6. [DOI] [PubMed] [Google Scholar]
- 8.Besheer A, Wood KM, Peppas NA, Mader K. Loading and mobility of spin-labeled insulin in physiologically responsive complexation hydrogels intended for oral administration. J Controlled Release. 2006;111(1–2):80. doi: 10.1016/j.jconrel.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Blanchette J, Peppas NA. Cellular evaluation of oral chemotherapy carriers. J Biomed Mater Res, Part A. 2005;72(4):381–388. doi: 10.1002/jbm.a.30243. [DOI] [PubMed] [Google Scholar]
- 10.Blanchette J, Peppas NA. Oral chemotherapeutic delivery: design and cellular response. Ann Biomed Eng. 2005;33(2):149. doi: 10.1007/s10439-005-8973-8. [DOI] [PubMed] [Google Scholar]
- 11.Delie F, Rubas W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit Rev Ther Drug Carrier Syst. 1997;14(3):221–286. [PubMed] [Google Scholar]
- 12.Foss AC, Goto T, Morishita M, Peppas NA. Development of acrylic-based copolymers for oral insulin delivery. Eur J Pharm Biopharm. 2004;57(2):163–169. doi: 10.1016/S0939-6411(03)00145-0. [DOI] [PubMed] [Google Scholar]
- 13.Foss AC, Peppas NA. Investigation of the cytotoxicity and insulin transport of acrylic-based copolymer protein delivery systems in contact with caco-2 cultures. Eur J Pharm Biopharm. 2004;57(3):447–455. doi: 10.1016/j.ejpb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39(2):266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96(3):736–749. [PubMed] [Google Scholar]
- 16.Hilgendorf C, Spahn-Langguth H, Regardh CG, Lipka E, Amidon GL, Langguth P. Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J Pharm Sci. 2000;89(1):63–75. doi: 10.1002/(SICI)1520-6017(200001)89:1<63::AID-JPS7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Ichikawa H, Peppas NA. Novel complexation hydrogels for oral peptide delivery: in vitro evaluation of their cytocompatibility and insulin-transport enhancing effects using Caco-2 cell monolayers. J Biomed Mater Res, Part A. 2003;67(2):617. doi: 10.1002/jbm.a.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson J, Wikman A, Artursson P. The mucus layer as a barrier to drug absorption in monolayers of human intestinal epithelial HT29-H goblet cells. Int J Pharm. 1993;99(2–3):209–218. [Google Scholar]
- 19.Kavimandan NJ, Losi E, Peppas NA. Novel delivery system based on complexation hydrogels as delivery vehicles for insulin-transferrin conjugates. Biomaterials. 2006;27(20):3846. doi: 10.1016/j.biomaterials.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Lacaz-Vieira F. Calcium site specificity. Early Ca2+-related tight junction events. J Gen Physiol. 1997;110(6):727–740. doi: 10.1085/jgp.110.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990;50(19):6334–6343. [PubMed] [Google Scholar]
- 22.Lesuffleur T, Porchet N, Aubert JP, Swallow D, Gum JR, Kim YS, Real FX, Zweibaum A. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J Cell Sci. 1993;106:771–783. doi: 10.1242/jcs.106.3.771. [DOI] [PubMed] [Google Scholar]
- 23.Lopez JE, Peppas NA. Cellular evaluation of insulin transmucosal delivery. J Biomater Sci, Polym Ed. 2004;15(4):385–396. doi: 10.1163/156856204323005262. [DOI] [PubMed] [Google Scholar]
- 24.Madsen F, Peppas NA. Complexation graft copolymer networks: swelling properties, calcium binding and proteolytic enzyme inhibition. Biomaterials. 1999;20(18):1701–1708. doi: 10.1016/s0142-9612(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 25.Meaney C, O’Driscoll C. Mucus as a barrier to the permeability of hydrophilic and lipophilic compounds in the absence and presence of sodium taurocholate micellar systems using cell culture models. Eur J Pharm Sci. 1999;8(3):167–175. doi: 10.1016/s0928-0987(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 26.Peppas NA, Klier J. Controlled release by using poly(methacrylic acid-g-ethylene glycol) hydrogels. J Controlled Release. 1991;16(1–2):203–214. [Google Scholar]
- 27.Pontier C, Pachot J, Botham R, Lenfant B, Arnaud P. HT29-MTX and Caco-2/TC7 monolayers as predictive models for human intestinal absorption: role of the mucus layer. J Pharm Sci. 2001;90(10):1608–1619. doi: 10.1002/jps.1111. [DOI] [PubMed] [Google Scholar]
- 28.Powell DW. Barrier function of epithelia. Am J Physiol. 1981;241(4):G275–G288. doi: 10.1152/ajpgi.1981.241.4.G275. [DOI] [PubMed] [Google Scholar]
- 29.Schilling RJ, Mitra AK. Intestinal mucosal transport of insulin. Int J Pharm. 1990;62(1):53–64. [Google Scholar]
- 30.Sipahigil O, Gursoy A, Cakalagaoglu F, Okar I. Release behaviour and biocompatibility of drug-loaded pH sensitive particles. Int J Pharm. 2006;311(1–2):130–138. doi: 10.1016/j.ijpharm.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Torres-Lugo M, Garcia M, Record R, Peppas NA. pH-Sensitive hydrogels as gastrointestinal tract absorption enhancers: transport mechanisms of salmon calcitonin and other model molecules using the Caco-2 cell model. Biotechnol Prog. 2002;18(3):612–616. doi: 10.1021/bp0101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres-Lugo M, Garcia M, Record R, Peppas NA. Physicochemical behavior and cytotoxic effects of p(methacrylic acid-g-ethylene glycol) nanospheres for oral delivery of proteins. J Controlled Release. 2002;80(1–3):197–205. doi: 10.1016/s0168-3659(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 33.Walter E, Janich S, Roessler BJ, Hilfinger JM, Amidon GL. HT29-MTX/Caco-2 cocultures as an in vitro model for the intestinal epithelium: in vitro-in vivo correlation with permeability data from rats and humans. J Pharm Sci. 1996;85(10):1070–1076. doi: 10.1021/js960110x. [DOI] [PubMed] [Google Scholar]
- 34.Wikman-Larhed A, Artursson P. Co-cultures of human intestinal goblet (HT29-H) and absorptive (Caco-2) cells for studies of drug and peptide absorption. Eur J Pharm Sci. 1995;3(3):171–183. [Google Scholar]