Abstract
OBJECTIVE
To evaluate the accuracy of a neonatal gentamicin nomogram to achieve therapeutic gentamicin serum concentrations without further adjustment, allowing for decreased serum drug monitoring
METHODS
Retrospective single center review of all gentamicin pharmacokinetic evaluations in patients ≤ 30 days of life from July 2005 – June 2007. Patients were evaluated for postnatal age, gestational age, weight, serum creatinine, dose/interval, serum drug peaks and troughs, results of discharge hearing test and recent use of indomethacin. Logistic regression was utilized to determine potential factors impacting overall dosing accuracy, potentially allowing for decreased therapeutic drug monitoring. Factors found to be significant were incorporated into new guidelines which were evaluated through pharmacokinetic modeling.
RESULTS
Overall accuracy rate was 84% when empiric dosing guidelines were utilized; 16% of all doses were changed due to supratherapeutic troughs and 1% were changed due to subtherapeutic peaks. Variables found to impact the necessity for dose changes incuded gestational age (p≤0.001), weight (p≤0.001), indomethacin use (p≤0.001), number of indomethacin doses used (p≤0.001 and p=0.009 for 1–3 and 4–6 doses, respectively), and SCr in patients ≥ 7 days old (p=0.028); however, only gestational age remained a significant predictor when all other factors were considered (p=0.008). The current guidelines were changed to account for increased troughs in patients ≤ 28 weeks gestation and examined through pharmacokinetic modeling. Pharmacokinetic modeling of the new guidelines predicted an overall accuracy of 94%.
CONCLUSIONS
From the data gathered regarding the accuracy in patients ≥ 35 weeks gestation, we recommend to decrease therapeutic drug monitoring within this cohort. Utilizing the results of regression analysis, the current guidelines have been adjusted to allow for increased clearance in patients ≤ 28 weeks gestation, although they still need to be prospectively evaluated.
Keywords: aminoglycosides, extended-interval, gentamicin, neonate, pharmacokinetics
BACKGROUND
Gentamicin is one of the most commonly used empiric antibiotics in the neonatal intensive care unit (NICU) setting.1–2 Due to its efficacy against pathogens of early life, namely Escherichia coli, gentamicin is often a first line agent against neonatal sepsis.3–4 It is estimated that true sepsis occurs in 1–5 per 1000 live births, although the risk may be higher in those born prematurely or with low birth weight.3,5 Due to the high associated mortality rate (5%–15%), there is a low threshold for treatment in the event of suspected sepsis, leading to the regular use of gentamicin within the neonatal population.3,5–6
Efficacy evaluations in adults have shown that a Cmax/MIC ratio of 8–10:1 increases the clinical response rate to 90%.7 In adults, oncedaily, extended-interval dosing such as in the Hartford nomogram is used to achieve these high Cmax/MIC ratios. In this nomogram, peaks over 20 mg/L are often sought to capitalize on the effect of concentration over MIC. With these elevated peaks, it is essential to allow complete clearance (e.g., undetectable troughs) to decrease the exposure of auditory and renal cells to aminoglycosides and emphasize the post-antibiotic effect.8–9 While these regimens have become the preferred method of aminoglycoside dosing in many adult patients, the increased volume of distribution (Vd) and renal immaturity of neonates10 prevent the same regimens from being utilized in this population.
In order to utilize these principles in neonates while still reducing the risk of toxicity, extended interval dosing has been introduced in the neonatal population.11–26 While these methods vary significantly, they include once-daily regimens that administer the same total daily dose as traditional dosing, allowing for clearance over 24 hours, as well as extended interval regimens that administer a higher dose and allow it to clear completely before readministration, typically 36–48 hours, depending on gestational age and birthweight.26 Between these two options, extended interval regimens emphasize higher Cmax/MIC ratios and post-antibiotic effect, while once-daily methods emphasize the convenience and simplicity of regimens.14, 27 Within this population, similar Cmax/MIC ratios as those seen in adults have been suggested to improve efficacy with both regimens.28 Skopnik et al. found that when utilizing once-daily methods, higher peak concentrations with the same trough concentrations could be obtained in vitro.29 Although the actual desired Cmax will vary based on the institution and bacterial specific MIC, E. coli isolates are reported to have a MIC90 of 0.5–1, leading to minimum peak levels of 4–10 mg/L with once-daily methods.30 In several clinical trials, this has led to targeted peaks ranging from 5–12 mg/L and trough concentrations less than 2 mg/L when utilizing daily administration methods.11,13–15 Higher peak concentrations are often targeted with extended interval regimens.17
In order to achieve these recommended concentrations, several different regimens are cited in the pediatric literature, without clear evidence of one preferred method. A regimen of 2.5–5 mg/kg administered daily is typically recommended, often with provisions for different daily doses depending on gestational age.11,13–15,26 A metaanalysis in 2005 used pooled patient data from both once-daily and extended-interval regimens to evaluate the achievement of therapeutic peaks and troughs in six commonly used protocols.31 From their calculated findings, patients would only achieve a “therapeutic” concentration (defined as peak ≥ 5 mg/L and trough ≤ 1 mg/L) 53.2%–88.1% of the time, depending on the regimen used. When evaluating only the once-daily regimens, it was found that these were most likely to achieve peaks ≥ 5 mg/L and troughs ≤ 1.5 mg/L, which is the trough value initially targeted in these regimens. While this provided important information, these data were limited to those patients less than 7 days old and was evaluated using mathematical models without prospective evaluation.
In order to reduce the risk of complications (e.g., oto-and nephrotoxicity), therapeutic drug monitoring (TDM) is often conducted in patients receiving gentamicin.10 This often necessitates the collection of numerous blood samples at various time points to ensure that the recommended peak and trough concentrations are achieved. This monitoring not only increases the patient's pain, but can cause clinically important blood loss. Furthermore, 75% of the cost of gentamicin therapy is due to TDM.32 For these reasons, it is important that a dosing regimen achieve the targeted peak and trough concentrations without requiring additional dosage adjustment.. In order to achieve this goal, a unique dosing nomogram was developed at the University of California San Francisco.
It was felt that the use of a once-daily regimen over an extended-interval would improve ease of administration. Based on recommendations from the literature11,13–14 and internal pharmacokinetic data, this protocol aimed to achieve troughs ≤ 2 mg/L and peaks ≥ 6 mg/L without further dosage adjustment. It was hypothesized that the use of an accurate empiric dosing regimen could reduce the need for TDM within our institution. Secondary endpoints included the impact of inaccuracy on toxicity and the establishment of the effect of confounding factors on overall accuracy.
METHODS
Choice of Initial Extended-Interval Regimen
A review of the literature demonstrates that there is significant variance in the empiric dosing of gentamicin in neonates.11–26 Doses from 2.5 mg/kg to 5 mg/kg have been used to attain peaks ≥ 6 mg/L and troughs ≤ 2 mg/L. Within a single protocol the dose used for an individual patient may also change depending patient characteristics such as weight, age or gestation. Based on previously published reports utilizing once-daily administration11,13–14 and internal pharmacokinetic data, a simplified regimen of 5 mg/kg in patients ≥ 35 weeks gestation and 3.5 mg/kg in patients < 35 weeks gestation was developed to achieve therapeutic serum concentrations.
Patient Selection for Evaluation of Regimen
Data was collected from all pediatric patients admitted to the Intensive Care Nursery (NICU) from July 2005 to July 2007 who underwent gentamicin pharmacokinetic evaluation. The pharmacy prescription database, WORx Drug Therapy Management System (Mediware Information Systems; v.2.9.5) and the proprietary program (University Lab; OZtech Systems, Inc.) were used to identify all pharmacokinetic evaluations of gentamicin during this time. Patients were assessed for gestational age (GA), postnatal age, weight at time of dosing, prescribed dose/dosing interval (mg/kg) and results of ALGO (Natus Medical Incorporated) hearing testing at discharge. ALGO hearing testing is a standard method of testing newborn hearing employed upon discharge within the NICU. Results are reported as pass/fail based on standardized preset parameters.33 Subsequent peak and trough concentrations and any dosing adjustments made were also noted. In patients ≥ 7 days old, serum creatinine (SCr) was also evaluated; SCr measurement is unreliable in patients < 7 days old, and thus was omitted from the evaluation of these patients.34 Finally, due to known interactions between indomethacin received within 30 days of gentamicin, the number of indomethacin doses and time elapsed between indomethacin and gentamicin dosing were also noted.35–37
Patients older than 30 days, those without complete pharmacokinetic results or those utilizing doses outside the recommended protocol were excluded. Multiple pharmacokinetic evaluations from a single patient were included if they occurred as discrete dosing events (defined as secondary to separate positive cultures or potential infectious events). The study was approved by the Committee on Human Research.
Pharmacokinetic Analysis
Dosing accuracy was defined as the achievement of a trough ≤ 2 mg/L and peak ≥ 6 mg/L with the first pharmacokinetic evaluation. An accuracy level of 95% was established as the point in which the continued monitoring of pharmacokinetic levels may be potentially discontinued. All levels were drawn at steady state as a trough, followed by a peak on the consecutive dose. Peaks drawn late (more than 30 minutes after the infusion finished) or troughs drawn early (more than 30 minutes prior to next dose) were adjusted based on the time drawn using the following equation, where t' is the difference between the measured concentration and the steady state concentration:
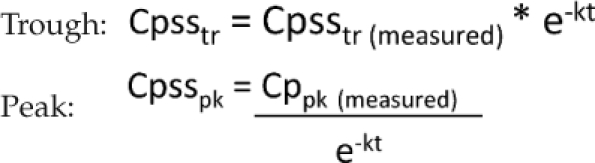 |
Depending on the results of the secondary endpoint evaluation, pharmacokinetic evaluation was further conducted to evaluate any changes necessary to the gentamicin dosing nomogram. This evaluation was conducted using standard pharmacokinetic calculations including the following formulas, where T = time between end of infusion and Cpsspk, t = infusion time, τ = dosing interval and Ko = dosing rate in mg/hr:
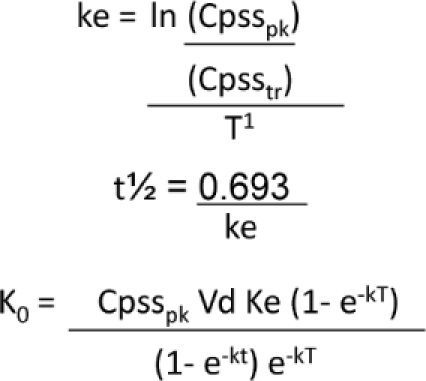 |
Pharmacokinetic calculations were performed using Microsoft Excel 2007 (Microsoft Corporation; c. 2006). Based on the results of this analysis, statistical testing was planned to predict the efficacy of changes made to the current nomogram.
Statistical Analysis
Dosing accuracy was analyzed through the use of descriptive percentages. Continuous variables were assessed using Student's t-test and categorical variables were with Fisher's exact test. Bivariate logistic regression was utilized to determine the impact on dosing inaccuracy of covariates (e.g., GA, weight, postnatal age, serum creatinine, and concurrent indomethacin use, including number of indomethacin doses and time between indomethacin and gentamicin administration). In order to adjust for confounding variables, these factors were also analyzed using multivariate logistic regression. In this analysis, it was found that weight and GA were highly correlated (r2=0.908), so only GA was utilized in the final regression model. Due to the small sample using indomethacin, this was analyzed as a binomial variable in the final model; separate evaluation of the effect of indomethacin doses and administration was conducted for those patients with indomethacin use. Classification tree analysis was used to determine cut points for statistically significant differences in dosing accuracy based on gestational age. Any differences in the current nomogram and an updated dosing scheme were conducted based on the accuracy of each predicted model using a Student's t-test. For all statistical analyses, α was set at 0.05 and analyses were completed using STATA statistical software (StataCorp LP; v.9).
RESULTS
Out of 616 pharmacokinetic evaluations, 289 were eligible for inclusion (Figure). Patient demographics and pharmacokinetic profiles are listed in Tables 1 and 2. Overall, there was an 84% dosing accuracy rate using the current guidelines (Table 3). Only 15% (n=44) of all doses were changed due to trough concentrations above the target value, while only 1% (n=3) were changed due to peaks below the target value.
Figure.
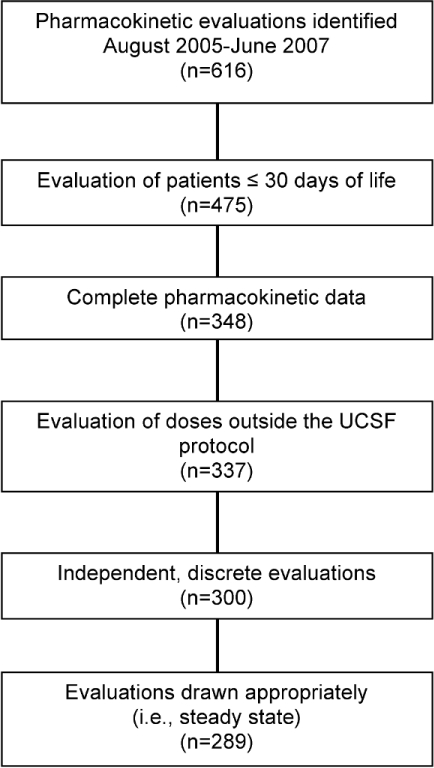
Patient selection.
Table 1.
Patient Demographics
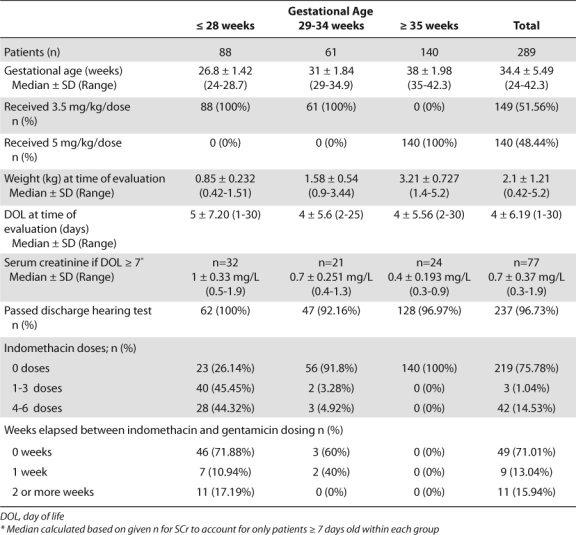
Table 2.
Patient Pharmacokinetic Profile
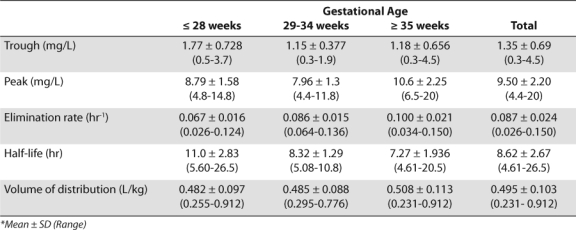
Table 3.
Accuracy of Current Gentamicin Dosing * Guidelines
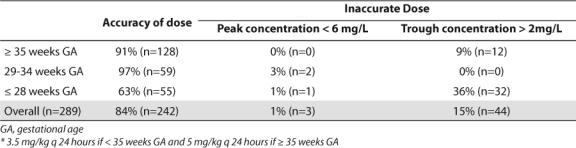
Bivariate subgroup analysis demonstrated that gestational age (p≤0.001), weight (p≤0.001), indomethacin use (p≤0.001), number of indomethacin doses (p≤0.001 and p=0.009 for 1–3 and 4–6 doses, respectively), and SCr in patients ≥ 7 days old (p=0.028) independently impacted the overall accuracy rate of the empiric dosing of gentamicin (Table 4). Adjusting for postnatal age and indomethacin use throughout the entire sample demonstrated that each week increase in gestational age decreased the odds of dose adjustment by 12% (p=0.008). This effect no longer remained significant when SCr was taken into account (Table 5). The timing and number of indomethacin doses also had no impact on dosing accuracy throughout the entire sample (Table 6).
Table 4.
Bivariate Analysis Evaluating Impact of Variables on Need to Change Dose
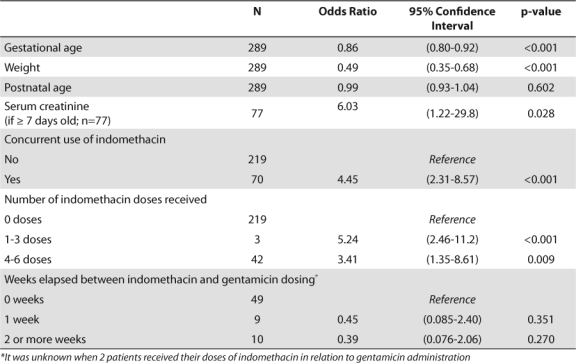
Table 5.
Multivariate Analysis Evaluating Impact of Variables on Need to Change Dose due to Inadequate Serum Gentamicin Peak or Trough Concentration
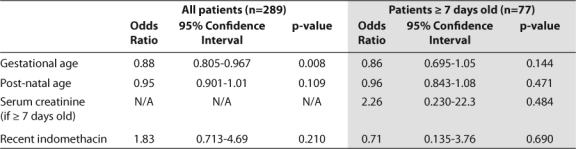
Table 6.
Mulitvariate Analysis Evaluating Impact of Indomethacin Doses And Administration on Need to Change Dose Due to Inadequate Serum Gentamicin Peak or Trough Concentration
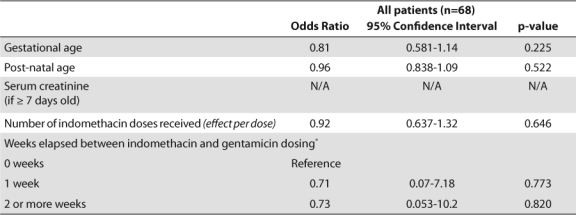
Indomethacin is primarily used in patients of low gestational age, thus confounders between these two terms were investigated. Significant collinearity was found (r2 = –0.6997), indicating that indomethacin use was most likely a surrogate for low gestational age. Excluding indomethacin from the regression model did not impact the effect of gestational age, postnatal age or serum creatinine on dose accuracy. Classification tree methods determined that a cutoff of 28 weeks gestation produced the most significant difference in dosing accuracy. An analysis of accuracy based on gestational age cutoffs is shown Tables 3 and 7.
Table 7.
Accuracy of Proposed Gentamicin Dosing * Guidelines

Using the results of the classification tree analysis, pharmacokinetic modeling demonstrated that extending the dosing interval to q 36 hours in patients ≤ 28 weeks gestation would improve overall dosing accuracy to 94% (p<0.001) and accuracy for those ≤ 28 weeks gestation to 96% (p=0.018) (Table 7). After this change there was a statistically significant decrease in supratherapeutic troughs for all gestation groups and those ≤ 28 weeks (Tables 3 and 7; p<0.001 for all gestation groups and p=0.045 for ≤ 28 weeks gestation). Extending the dosing interval led to no change in subtherapeutic peaks. Further evaluation of the incidence of subtherapeutic peaks based on dosing group revealed the lowest incidence with the near-term or term infants (GA ≥ 35 weeks) (< 0.01%; Table 3).
Safety evaluation demonstrated no statistically significant difference in the pass rate of the ALGO hearing test between patients whose doses were changed and those who had accurate empiric gentamicin doses (p = 0.859).
DISCUSSION
Numerous studies have evaluated the efficacy and safety of once-daily aminoglycosides in preterm and term infants.11,13–14,18,22,25–26 While many of these evaluate the attainment of therapeutic peaks and troughs, few look at the achievement of both without further adjustment. This gentamicin dosing guideline was designed to establish a simple nomogram in which an appropriate peak and trough were reached by the first pharmacokinetic evaluation to reduce the use of TDM. The results provide data to support the discontinuation of TDM in select neonatal populations. Overall, a high frequency (99%) of peaks was above 6 mg/L, especially within the ≥ 35 weeks gestation cohort. This provides confidence that these patients, when dosed at 5 mg/kg every 24 hours, will achieve a therapeutic peak. Clinically this may translate into decreased need for therapeutic monitoring of serum peak levels in near-term or term infants, which may decrease cost as well as the pain and blood loss associated with serum drug monitoring.
Although the current guideline does not yet provide confidence that TDM can be stopped in all patients, the results of the secondary analysis identified ways in which the current gentamicin nomogram can be improved. As shown in the literature, a significant impact of indomethacin on gentamicin clearance was found, although this was not the strongest predictor of accuracy in the multivariate model.13,17,34,36–38 Previous studies have demonstrated a decrease in glomerular filtration rate/aminoglycoside clearance by prostaglandin inhibition from indomethacin as well as low gestational age.13,21,34,37 Due to the primary use of indomethacin in low GA patients, it has been suggested that the effect of indomethacin may be confounded by gestational age, as seen in this evaluation.13 Once indomethacin use was removed from the regression model, it was clear that gestational age remained the most significant predictor of accuracy and the best target for guideline improvement.
The effect of measured renal function on serum drug concentrations is well established in the literature and clinical practice. The development of SCr as a marker of renal function is not well established in infants until 7–30 days of life, depending on gestational age.34,38 While this effect was minimized in our study through the evaluation of SCr as a predictor of accuracy only in patients ≥ 7 days old, it was most likely not eliminated. An evaluation of other measures of renal function (i.e., urine output) may provide further validation of the effect of neonatal renal function on the ability of empiric dosing regimens to attain therapeutic concentrations.
Supratherapeutic troughs were the most common cause of inaccurate dosing within the study; previous reports have shown this may affect ototoxicity.10,39–40 Safety evaluation of the impact of dosing inaccuracy demonstrated no effect on the pass rate of the ALGO hearing test, similar to results seen in previous analyses.18,41 This indicates that although the overall accuracy rate has not be achieved in these patients, no adverse effects are apparent at the time of discharge.
From the results of the primary and secondary analyses it was established that the gentamicin dosing nomogram currently used achieves adequate accuracy to discontinue TDM in a select group of patients. Patients ≥ 35 weeks GA demonstrate a low rate of subtherapeutic levels; therefore, it is proposed that routine monitoring of serum peak concentrations be discontinued in this population. Alteration of the current nomogram to further divide patients based on GA improved the nomogram to an overall accuracy of 96%. Within the sub-categorization of patients with gestational age < 35 weeks, the overall accuracy rate for patients in the 29–34 weeks GA cohort was 97%, although this is limited by a small sample size. This further demonstrates the ability of the current regimen to accurately provide a dose for these patients. Although the revision of the current guideline to extend the interval has improved the achievement of therapeutic peaks/troughs, it also potentially decreases the convenience associated with once-daily dosing.
While these results provide an accurate representation of the overall dosing accuracy of this gentamicin nomogram, the results are limited by single-center retrospective evaluation. Furthermore, while the changes made in the nomogram are promising for decreased TDM in a wider range of patients, this has been demonstrated only through pharmacokinetic calculations and will need to be prospectively evaluated to determine true accuracy. Finally, the effect of acute clinical changes and other potential drug interactions (i.e., diuretic use) on serum gentamicin concentrations was not studied and may also present an impact on dosing accuracy that needs to be further evaluated.
CONCLUSION
Gentamicin is a commonly used antibiotic in the NICU for suspected or confirmed neonatal infections. Through evaluation of the accuracy of current guidelines, modifications were made to improve the overall dosing accuracy and allow for decreased therapeutic drug monitoring. From this data, a new gentamicin dosing regimen (3.5 mg/kg every 36 hours for GA ≤ 28 weeks; 3.5 mg/kg every 24 hours for GA 29–34 weeks; 5 mg/kg every 24 hours for ≥ 35 weeks GA) is proposed to achieve therapeutic peaks and troughs without further adjustment. From the data gathered regarding accuracy in patients ≥ 35 weeks gestation, we recommend to decrease TDM within this cohort. The remainder of the nomogram still needs to be prospectively evaluated, but future evaluation will hopefully allow for decreased monitoring in patients with GA < 35 weeks.
Acknowledgments
This research was presented as a poster presentation at the University of California Department of Clinical Pharmacy 10th Annual Spring Research Seminar on May 5, 2007. This research was summarized in a 10-minute platform presentation during the Western States Residency Conference on May 22, 2007.
ABBREVIATIONS
- Cmax
maximum concentration
- GA
gestational age
- MIC
minimum inhibitory concentration
- NICU
neonatal intensive care unit
- SCr
serum creatinine
- TDM
therapeutic drug monitoring
- Vd
volume of distribution
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Clark R, Bloom B, Spitzer A, Gerstmann D. Reported medication use in the neonatal intensive care unit: Data from a large national data set. Pediatrics. 2006;117:1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 2.Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL, et al. Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr Infect Dis J. 2005;24:766, 773. doi: 10.1097/01.inf.0000178064.55193.1c. [DOI] [PubMed] [Google Scholar]
- 3.Gerdes JS. Diagnosis and management of bacterial infections in the neonate. Pediatr Clin North Am. 2004;51:939–959. doi: 10.1016/j.pcl.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Klein JO, Feigin RD, McCracken GH., Jr. Report of the task force on diagnosis and management of meningitis. Pediatrics. 1986;78:959–982. [PubMed] [Google Scholar]
- 5.Cohen Wolkowiez M, Moran C, Benjamin D, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052–1056. doi: 10.1097/inf.0b013e3181acf6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347:240–247. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 7.Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: Importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Nicolau DP, Freeman CD, Belliveau PP, et al. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother. 1995;39:650–655. doi: 10.1128/AAC.39.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman CD, Nicolau DP, Belliveau PP, Nightingale CH. Once-daily dosing of aminoglycosides: review and recommendations for clinical practice. J Antimicrob Chemother. 1997;39:677–686. doi: 10.1093/jac/39.6.677. [DOI] [PubMed] [Google Scholar]
- 10.Touw D, Westerman EM, Sprij AJ. Therapeutic drug monitoring of aminoglycosides in neonates. Clin Pharmacokinet. 2009;48:71–88. doi: 10.2165/00003088-200948020-00001. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal G, Rastogi A, Pyati S, et al. Comparison of once-daily versus twice daily gentamicin dosing regimens in infants ≥ 2500 g. J Perinatol. 2002;22:268–274. doi: 10.1038/sj.jp.7210704. [DOI] [PubMed] [Google Scholar]
- 12.Avent ML, Kinney JS, Istre GR, Whitfield JM. Gentamicin and tobramycin in neonates: Comparison of a new extended dosing interval regimen with a traditional multiple daily dosing regimen. Amer J Perinatol. 2002;19:413–420. doi: 10.1055/s-2002-36836. [DOI] [PubMed] [Google Scholar]
- 13.DiCenzo R, Forrest A, Slish JC, et al. A gentamicin pharmacokinetic population model and once-daily dosing algorithm for neonates. Pharmacotherapy. 2003;23:585–591. doi: 10.1592/phco.23.5.585.32196. [DOI] [PubMed] [Google Scholar]
- 14.Hansen A, Forbes P, Arnold A, O'Rourke E. Once-daily gentamicin dosing for the preterm and term newborn: Proposal for a simple regimen that achieves target levels. J Perinatol. 2003;23:635–639. doi: 10.1038/sj.jp.7210996. [DOI] [PubMed] [Google Scholar]
- 15.Hayani KC, Hatzopoulos FK, Frank AL, et al. Pharmacokinetics of once-daily dosing of gentamicin in neonates. J Pediatr. 1997;131:76–80. doi: 10.1016/s0022-3476(97)70127-6. [DOI] [PubMed] [Google Scholar]
- 16.Hoff D, Wilcox R, Tollefson L, et al. Pharmacokinetic outcomes of a simplified, weightbased, extended-interval gentamicin dosing protocol in crtitically ill neonates. Pharmacotherapy. 2009;29:1297–1305. doi: 10.1592/phco.29.11.1297. [DOI] [PubMed] [Google Scholar]
- 17.Lanao JM, Calvo MV, Mesa JA, et al. Pharmacokinetic basis for the use of extended interval dosage regimens of gentamicin in neonates. J Antimicrob Chemother. 2004;54:193–198. doi: 10.1093/jac/dkh261. [DOI] [PubMed] [Google Scholar]
- 18.Lundergan FS, Glasscock GF, Kim EH, Cohen RS. Once-daily gentamicin dosing in newborn infants. Pediatrics. 1999;103:1228. doi: 10.1542/peds.103.6.1228. [DOI] [PubMed] [Google Scholar]
- 19.Mercado MCK, Brodsky NL, McGuire MK, Hurt H. Extended interval dosing of gentamicin in preterm infants. Am J Perinatol. 2004;21:73–77. doi: 10.1055/s-2004-820515. [DOI] [PubMed] [Google Scholar]
- 20.Ohler KH, Menke JA, Fuller L. Use of higher dose extended interval aminoglycosides in a neonatal intensive care unit. Am J Perinatol. 2000;17:285–290. doi: 10.1055/s-2000-13436. [DOI] [PubMed] [Google Scholar]
- 21.Romero CdA, Castillo EG, Secades CM, et al. Once daily gentamicin dosing in neonates. Pediatr Infect Dis J. 1998;17:1169–1171. doi: 10.1097/00006454-199812000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Skopnik H, Heimann G. Once daily aminoglycoside dosing in full term neonates. Pediatr Infect Dis J. 1995;14:71–72. doi: 10.1097/00006454-199501000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Stickland MD, Kirkpatrick CMJ, Begg EJ, et al. An extended interval dosing method for gentamicin in neonates. J Antimicrob Chemother. 2001;48:887–893. doi: 10.1093/jac/48.6.887. [DOI] [PubMed] [Google Scholar]
- 24.Vervelde ML, Rademaker CMA, Krediet TG, et al. Population pharmacokinetics of gentamicin in preterm neonates: Evaluation of a once-daily dosage regimen. Ther Drug Monit. 1999;21:514–519. doi: 10.1097/00007691-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Langlass T, Mickle T. Standard gentamicin dosage regimen in neonates. Am J Health-Syst Pharm. 1999;56:440–443. doi: 10.1093/ajhp/56.5.440. [DOI] [PubMed] [Google Scholar]
- 26.Rao S, Ahmed M, Hagan R. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev. 2006;1:CD005091. doi: 10.1002/14651858.CD005091.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Lutsar I, M T. Understanding pharmacokinetics/pharmacodynamics in managing neonatal sepsis. Curr Opin Infect Dis. 2010;23(3):201–207. doi: 10.1097/QCO.0b013e328337bb42. [DOI] [PubMed] [Google Scholar]
- 28.Sherwin C, Broadbent R, Medlicott N, Reith D. Individualised dosing of amikacin in neonates: a pharmacokinetic/pharmacodynamic analysis. Eur J Clin Pharmacol. 2009;65:705–713. doi: 10.1007/s00228-009-0637-4. [DOI] [PubMed] [Google Scholar]
- 29.Skopnik H, Wallraf R, Nies B, et al. Pharmacokinetics and antibacterial activity of daily gentamicin. Arch Dis Child. 1992;67:57–61. doi: 10.1136/adc.67.1_spec_no.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cha R. In vitro activity of cefepime, imipenem, tigecycline, and gentamicin, alone and in combination, against extended-spectrum b-lactamase–producing Klebsiella pneumoniae and Escherichia coli. Pharmacotherapy. 2008;28:295–300. doi: 10.1592/phco.28.3.295. [DOI] [PubMed] [Google Scholar]
- 31.Murphy JE. Prediction of gentamicin peak and trough concentrations from six extended-interval dosing protocols for neonates. Am J Health-Syst Pharm. 2005;62:823–827. doi: 10.1093/ajhp/62.8.823. [DOI] [PubMed] [Google Scholar]
- 32.Thureen PJ, Reiter PD, Gresores A, et al. Once-versus twice-daily gentamicin dosing in neonates >/= 34 weeks' gestation: Cost-effectiveness analyses. Pediatrics. 1999;103:594–598. doi: 10.1542/peds.103.3.594. [DOI] [PubMed] [Google Scholar]
- 33.ALGO 3i Newborn Hearing Screener. 2010. Available at: http://www.natus.com/index.cfm?page=products_brand&crid=303&contentid=118. Accessed February 28, 2010.
- 34.Drukker A, Guignard J-P. Renal aspects of the term and preterm infant: a selective update. Curr Opin Pediatr. 2002;14:175–182. doi: 10.1097/00008480-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Gagliardi L. Possible indomethacin-aminoglycoside interaction in preterm infants. J Pediatr. 1985;107:991–992. doi: 10.1016/s0022-3476(85)80220-1. [DOI] [PubMed] [Google Scholar]
- 36.Jerome M, Davis J. The effects of indomethacin on gentamicin serum levels. Proc West Pharmacol Soc. 1987;30:85–87. [PubMed] [Google Scholar]
- 37.Zarfin Y, Koren G, Maresky D, et al. Possible indomethacin-aminoglycoside interaction in preterm infants. J Pediatr. 1985;106:511–513. doi: 10.1016/s0022-3476(85)80693-4. [DOI] [PubMed] [Google Scholar]
- 38.Keyes PS, Johnson CK, Rawlins TD. Predictors of trough serum gentamicin concentrations in neonates. Am J Dis Child. 1989;143:1419–1423. doi: 10.1001/archpedi.1989.02150240041014. [DOI] [PubMed] [Google Scholar]
- 39.Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007;13:119–126. doi: 10.2174/138161207779313731. [DOI] [PubMed] [Google Scholar]
- 40.García VP, Martínez FA, Agustí EB, et al. Drug-induced otoxicity: current status. Acta Oto-Laryngologica. 2001;121:569–572. doi: 10.1080/00016480121545. [DOI] [PubMed] [Google Scholar]
- 41.de Hoog M, van Zanten BA, Hop WC, et al. Newborn hearing screening: Tobramycin and vancomycin are not risk factors for hearing loss. J Pediatr. 2003;142:41–46. doi: 10.1067/mpd.2003.mpd037. [DOI] [PubMed] [Google Scholar]


