Abstract
Non-typeable Haemophilus influenzae (NTHi) is the most common bacteria responsible for episodic acute otitis media (AOM; non-otitis prone), recurrent AOM (rAOM; otitis prone) and AOM treatment failure (AOMTF) in children. In this 3.5 years of prospective study, we measured the serum antibody response to outer membrane proteins D, P6 and OMP26 of NTHi in children with AOM (n= 26), rAOM (n= 32), AOMTF (n=27). The geometric mean titers (GMTs) of IgG at their acute AOM visit against protein D in otitis prone children were significantly lower compared to AOMTF (p value < 0.01) and non-otitis prone (p value <0.03) children; otitis prone children had significantly lower IgG levels to P6 compared to AOMTF children (p value < 0.02); otitis prone children had significantly lower IgG levels to OMP26 compared to AOMTF children (p value <0.04). Comparing acute to convalescent titers after AOM, otitis prone and AOMTF children had no significant change in total IgG against all the three proteins, while non-otitis prone children had significant increases to protein D. Anti-Protein D, P6 and OMP26 antibody levels measured longitudinally during NP colonization between age 6 and 24 months in 10 otitis prone children and 150 non-otitis prone children showed < 2-fold increases over time in otitis prone children compared to > 4 fold increases in the non-otitis prone children (p value < 0.001). We conclude that otitis prone children mount less of an IgG serum antibody response toward Protein D, P6 and OMP26 after AOM which may account for recurrent infections. The data on acute sera of otitis prone versus non-otitis prone children and the acute-to-convalescence response in non-otitis prone children point to a possible link of anti-PD to protection. Moreover, the data suggest that otitis prone children should be evaluated for their responses to Protein D, P6 and OMP26 vaccine antigens of NTHi.
Keywords: Non-typeable Haemophilus influenzae, P6 protein, protein D, protein OMP26, Acute otitis media, Otitis prone
Introduction
Acute otitis media (AOM) is the most common bacterial infection during early childhood.[1] By 3 years of age, 60-70% of children experience at least one episode of AOM. Some children are subject to recurrent episodes of otitis media (rAOM), defined as three or more episodes of AOM in six months or four or more episodes in a 12 month period.[2, 3] These children are often referred to as otitis prone.[4] AOM treatment failure (AOMTF) occurs when a child fails to achieve bacterial eradication and/or resolution of symptoms after at least 48 hours of appropriate antibiotic therapy [5, 6] or signs and symptoms of AOM return within 14 days of completing an antibiotic treatment course.
Non-typeable Haemophilus influenzae (NTHi) is frequently associated with otitis prone and AOMTF.[6-9] Infection with NTHi results in strain specific immunity.[10, 11] Because of heterogeneity in the outer membrane proteins (OMPs) of unencapsulated NTHi, the identification of potential vaccine candidates for NTHi has posed a significant challenge.[12] Several OMPs of NTHi have been proposed as potential vaccine antigens on the basis of their sequence conservation, immunogenicity and/or demonstration of significant protection in animal models following immunization.[13]. Three highly conserved proteins among NTHi strains have shown significant potential as vaccine candidates: Protein D, P6 and OMP26.[14-16]
Protein D is a 43 kilodalton surface-exposed lipoprotein that has shown protection against NTHi AOM in a chinchilla model.[17] It has the potential to protect children against NTHi AOM, shown in the randomized clinical trial of vaccine where Protein D as a carrier-protein was conjugated with pneumococcal capsular polysaccharides.[18] DeMaria et al has shown that immunization with P6 provides protection against AOM due to NTHi in the chinchilla model.[19] The antibodies in the chinchilla to P6 were shown to be bactericidal. Intranasal immunization with P6 was shown to confer antigen-specific mucosal immunity and enhance mucosal clearance of NTHi in a mouse model.[20] OMP26 is also associated with protection against NTHi infections as shown in a chinchilla and rat model.[21, 22]
Experimental data derived from humans and animal models indicate that serum antibodies play a critical role in host defense against NTHi infection.[23] It has been reported that otitis prone children develop a poor IgG response following AOM and poor anamnestic responses to P6 protein.[24, 25] Whether otitis prone children are similarly hyporesponsive to Protein D and OMP26 proteins of NTHi has not been studied previously. The objectives of this study were to evaluate and compare the serum IgG, IgM and IgA antibody response against outer membrane proteins D, P6 and OMP26 of NTHi in otitis prone, AOMTF and non-otitis prone children at the time of AOM and during asymptomatic NP colonization from 6 to 24 months of age.
Methods
Patient population
The samples collected and analyzed for this paper were obtained from a prospective study supported by the National Institutes of Deafness and Communication Disorders. Children were enrolled from a middle class, suburban sociodemographic pediatric practice in Rochester, NY (Legacy Pediatrics). The study was approved by the University of Rochester and Rochester General Hospital Research Subjects Review Board and written informed consent was obtained for participation and all procedures.
Two cohorts of children were studied. Healthy children without prior AOM were enrolled at age 6 months and followed prospectively until 30 months of age. Serum, nasopharyngeal (NP) and oropharyngeal (OP) cultures were obtained seven times during the study period at age 6, 9, 12, 15, 18, 24, and 30 months, samples for the 30 month time point were excluded from this analysis as too few subjects had reached the 30 month visit. During the study period whenever a child in this group experienced an AOM, serum, NP and OP cultures were again obtained and middle ear fluid (MEF) was obtained by tympanocentesis. Three weeks following an AOM event, serum, NP and OP cultures were again obtained as convalescent samples. The majority of these children represent the group of non-otitis prone children who are studied at their first or second AOM episode; however, some of these children went on to meet the definitions of otitis prone and AOMTF and are included in those groups for analysis. A second cohort of children 6 to 36 months old, with rAOM or AOMTF, was studied at the time of an AOM event. Serum, NP and OP samples were collected acutely and in convalescence, 3 weeks following an AOM event, and MEF was obtained at the time of the AOM. A subset of these children was then followed prospectively until 30 months of age for detection of asymptomatic NP colonization with NTHi and additional AOMs.
To assure the diagnosis of AOM, children were examined by validated otoscopist pediatricians using the American Academy of Pediatrics AOM diagnostic guidelines.[26] A tympanocentesis was performed to confirm the presence of an otopathogens in MEF.[27] Middle ear fluid, NP, and OP samples were inoculated into trypticase soy broth, trypticase soy agar with 5% sheep blood plates, and chocolate agar plates. Bacteria were isolated according to the CLSI standard culture procedures. An isolate was further identified as NTHi on a similar basis as described by Murphy et al.[28] to include not only colony morphology, porphyrin reactivity, and growth requirement for hemin & nicotinamide adenine dinucleotide and Haemophilus ID Quad plates, but also by ompP6 sequencing to distinguish NTHi from H. haemolyticus.[28]
ELISA assay
Protein D was kindly provided as a gift from GlaxoSmithKline Biologicals, Rixensart Belgium, P6 plasmid was provided by Dr. Tim Murphy, University of Buffalo and OMP26 plasmid was provided by Dr. Jennelle Kyd, University of Canberra, Australia. The 96-well Nunc-Immulon 4 plates were coated with 0.25 μg-0.5 μg/ml of individual OMP antigens (100 μl/well) in bicarbonate [pH 9.4] coating buffer and incubated overnight at 4°C. After washing, the plates were blocked with 3% skim milk at 37°C for 1hr (200 μl per well). After five washes, 100 μl of serum at a starting dilution of 1:100 (in PBS-3% skim milk) was added to the wells and diluted serially 2 fold. The mixture was incubated at room temperature for 1 hr followed by the addition of affinity purified goat anti-human IgG, IgM or IgA antibody conjugated to horseradish-peroxidase (Bethyl Laboratories, Inc, Montgomery, TX) as a secondary antibody. The reaction products were developed with TMB Microwell Peroxidase Substrate System (KPL, Gaithersburg, MD), stopped by the addition of 1.0 molar phosphoric acid and read by an automated ELISA reader using a 450-nm filter.
To provide quantitative results on antibody concentrations, the level of the specific antibody present in the unknown sample was determined by comparison to an internal reference serum (pool of human serum with high anti-OMP titers). The levels of IgG, IgM and IgA in the reference serum were quantitatively measured by using a human IgG/IgA/IgM ELISA quantitation kit (Bethyl laboratories).
A Four-parameter logistic-log function was used to form the reference and sample curves. This ELISA was fully validated according to ICH Guidance. The assay lower limit of detection for protein D was 3.5 ng/ml for IgG, 4.5 ng/ml for IgM, and 8 ng/ml for IgA; for P6 it was at 1 ng/ml for IgG, 3 ng/ml for IgM, and 3 ng/ml for IgA; and for OMP26 it was at 4 ng/ml for IgG, 3 ng/ml for IgM, and 10.5 ng/ml for IgA. The inter-assay coefficient of variation was ≤20% for all antigens and secondary antibody combinations.
Statistical analysis
All the statistical analysis was performed on GraphPad Prism 5. Unpaired t test was used to compare the difference among three groups for the IgG, IgM and IgA antibody analysis. Paired t test was applied to compare acute vs convalescence serum samples. Mann-Whitney test was used to analyze the antibody level in relation to the culture results. One way ANOVA was used to evaluate the antibody rise over time. P values of < 0.05 were considered significant.
Results
Children were treated with recommended dosages of antibiotics after the diagnosis of AOM as included in the AAP 2004 guideline. If child AOM symptoms persist after at least 48 hours of appropriate antibiotic therapy or signs and symptoms of AOM return within 14 days of completing an antibiotic treatment course, he was considered treatment failure (AOMTF).
IgG, IgM and IgA antibody levels against Protein D, P6 and OMP26 NTHi antigens in three groups of children at the time of an AOM
IgG, IgM and IgA antibody levels against Protein D, P6 and OMP26 proteins of NTHi were measured at the time of an acute AOM in 32 otitis prone children, 27 children with AOMTF and 26 children with their first or second AOM as a non-otitis prone group (Figure 1).
Figure 1.
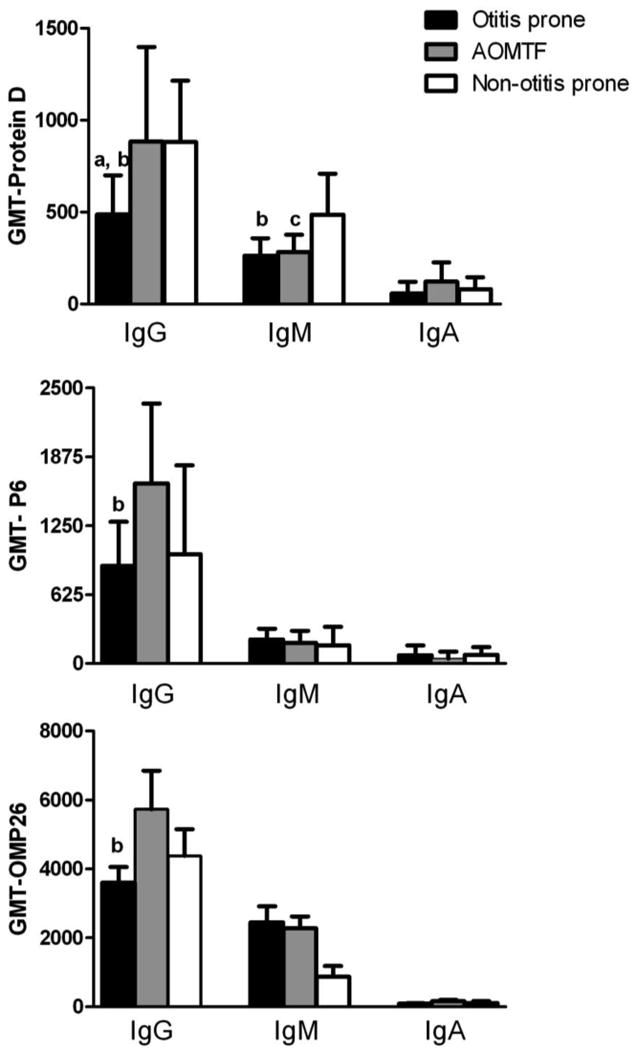
Comparison of IgG, IgM and IgA antibody against three antigens of NTHi in the serum samples of children at their acute visit of AOM in 32 otitis prone (black bar), 27 AOMTF (gray bar) and 26 Non-otitis prone (white bar) children.
Note: All the antibody concentrations against three proteins are in ng/ml.
The significant difference (p value <0.05) observed between the two groups is shown with a, b and c letters:
a: otitis prone vs non-otitis prone children
b: otitis prone vs AOMTF children
c: AOMTF vs non-otitis prone children
The geometric mean titers (GMTs) of IgG against protein D in otitis prone children were significantly lower compared to AOMTF (p value < 0.01) and non-otitis prone (p value <0.03) children. The IgG anti- P6 titers in otitis prone children were significantly lower compared to AOMTF children (p value < 0.02), but the difference was not significant compared to non-otitis prone children. The IgG anti-OMP26 antibody titers in otitis prone children were significantly lower compared to AOMTF children (p value <0.04), but the difference was not significant compared to non-otitis prone children.
The otitis prone children and AOMTF children had significantly lower IgM levels against protein D compared to non-otitis prone children (p value <0.05). No significant differences in IgM titers directed against P6 or OMP 26 were demonstrated between the 3 groups of children. Also, no significant differences were found for IgA in the serum samples of the three groups of children against the 3 studied proteins of NTHi.
Acute and convalescent antibody levels against Protein D, P6 and OMP26 NTHi antigens in three groups of children
Twenty-five otitis prone, 15 AOMTF and 21 non-otitis prone children had paired serum samples obtained at their acute (at the time of AOM) and convalescent stage (3 weeks later). Figure 2 shows the IgG antibody response (GMTs) against protein D, P6 and OMP26 in the paired samples from the 3 groups of children. In otitis prone and AOMTF children, there was no significant rise in the antibody titers to any of the three proteins in the acute vs. convalescence stage. In non-otitis prone children significant increases in IgG antibody to protein D (p value <0.05), but not to P6 or OMP26 in the convalescence stage was detected.
Figure 2.
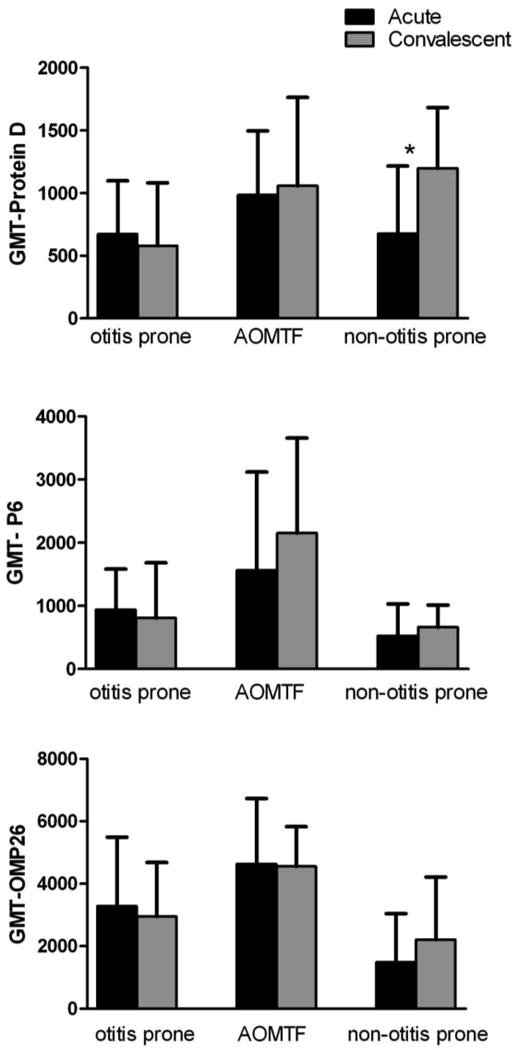
Comparison of IgG antibody in the serum samples of 25 otitis prone, 15 AOMTF and 21 non-otitis prone children at their acute vs convalescence stage.
y axis indicates the GMT with 95% confidence intervals.
* indicates a significant difference (p value <0.05) between the acute vs convalescence stage.
Antibody levels against Protein D, P6 and OMP26 NTHi antigens in relation to presence of AOM and NP colonization
The IgG antibody response against protein D, P6 and OMP26 was determined in the three groups of children in three AOM scenarios: (1 NTHi causing the AOM event (2) NTHi NP colonization at the time of an AOM caused by another otopathogen and (3) no NTHi isolated from the NP or MEF at the time of an AOM episode caused by another otopathogen (Table 1) There was no significant difference in antibody titers against protein D and P6 in otitis prone and AOMTF children for the three scenarios. In contrast, non-otitis prone children had significantly higher anti-protein D and anti-P6 antibody levels when NTHi was present in the MEF or NP (p value < 0.05) compared to children with no NTHi isolated from the MEF or NP. No differences were detected for anti-OMP26 antibody levels for the three child groups and three scenarios.
Table 1.
Geometric mean titers (GMTs) of serum IgG against Protein D, P6 and OMP26 in relation to isolation of NTHi in children with acute otitis media.
| Antigens | Children group | # of samples | NTHi AOM | NTHi NP colonized | No NTHi in MEF or NP |
|---|---|---|---|---|---|
| GMT (95% confidence intervals) | |||||
| Protein D | Otitis prone | 20, 11, 12 | 790 (404-1545) |
451f (181-1120) |
440g (200-965) |
| AOMTF | 8, 5, 7 | 904 (525-1557) |
715 (102-5004) |
1215e,g (650-2271) |
|
| Non-otitis prone | 13, 9, 34 | 1100a (685-1768) |
1645b,f (599-4519) |
563a,b,e (387-819) |
|
| P6 | Otitis prone | 20, 11, 12 | 1392 (938-2064) |
601c,d (217-1665) |
919 (499-1692) |
| AOMTF | 8, 5, 7 | 1956 (1022-3747) |
2611d (502-13581) |
1046 (481-2273) |
|
| Non-otitis prone | 13, 9, 34 | 1899a (1319-2733) |
1742b,c (348-8723) |
666a,b (415-1067) |
|
| OMP26 | Otitis prone | 20, 11, 12 | 3529 (2527-4928) |
3551f (1744-7228) |
3454c (1958-6091) |
| AOMTF | 8, 5, 7 | 3853h (2368-6270) |
3907e (2391-6382) |
6371e (3549-11438) |
|
| Non-otitis prone | 13, 9, 34 | 1582h (772-3244) |
1496e,f (833-2688) |
1240c,e (785-1959) |
|
Significant difference (p value < 0.05):
NTHi AOM vs no NTHi in MEF or NP
NTHi NP colonized vs no NTHi in MEF or NP
Otitis prone vs Non-otitis prone
Otitis prone vs AOMTF
AOMTF vs Non-otitis prone
Trend: (p value between 0.05-0.1)
Otitis prone vs Non-otitis prone
Otitis prone vs AOMTF
AOMTF vs Non-otitis prone
Antibody level in non-otitis prone and otitis prone children with age
Figure 3 shows the IgG antibody levels against Protein D, P6 and OMP26 at the time of routine non-AOM visits in prospectively followed non-otitis prone children and otitis prone children at different ages (6-24 months). The data shown are from 150 non-otitis prone children and 10 otitis prone children. The 150 non-otitis prone children include the 27 AOM children (non-otitis prone) and 101 children don't have any AOM episode. In the non-otitis prone children, between 6 months and 24 months of age, the IgG antibody levels rose almost four fold from 500 to 2003 ng/ml for protein D, 475 to 1950 ng/ml for P6 and 677 to 2159 ng/ml for OMP26 protein. The increase in IgG antibody level with age was significant for all the three proteins (p value < 0.001 for each antigen). In comparison, otitis prone children had less than two fold rise in IgG antibody over time (781 to 1141 ng/ml for protein D, 1050 to 1620 ng/ml for P6 and 588 to 1195 ng/ml for OMP26 protein); this rise in antibody level with age in otitis prone children was not significant; p value =0.54 for protein D, p value =0.69 for P6 and p value =0.33 for OMP26.
Figure 3.
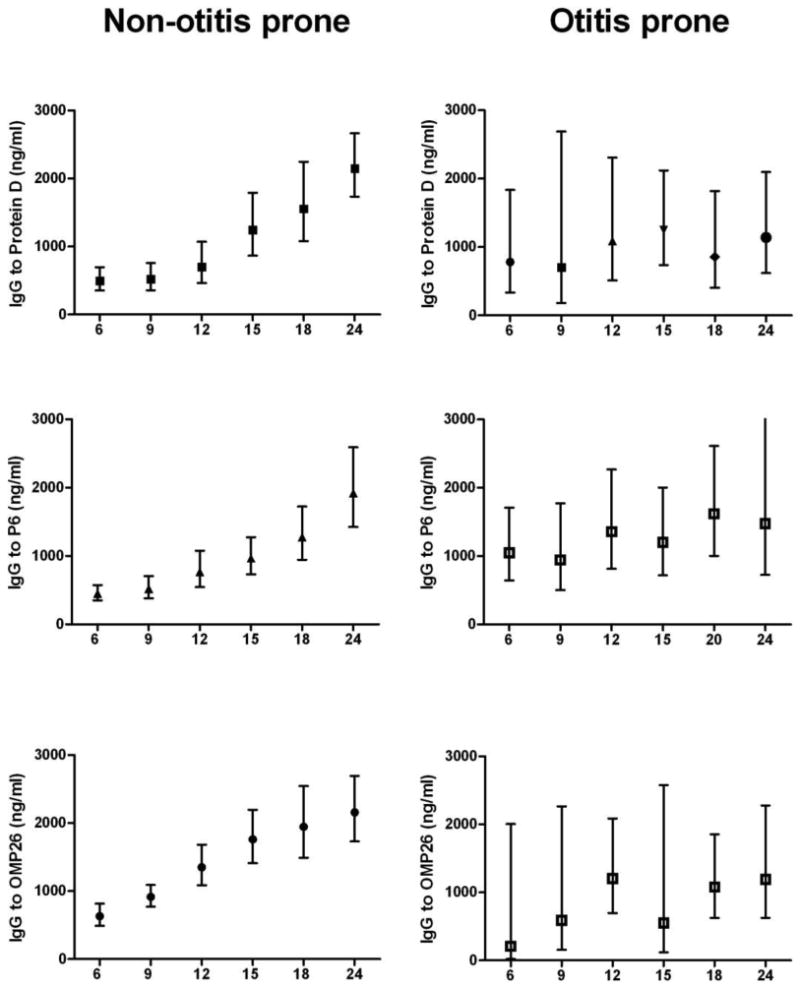
Comparison of IgG antibody level with age (6-24 months) against three proteins of NTHi in Non-otitis prone and otitis prone children. The IgG antibody data at each age is presented as geometric mean average with 95% confidence intervals. Significant difference for all the three proteins (p value<0.001), comparing relative rise in IgG serum antibody over time was found in non-otitis prone children while the difference was not significant in otitis prone children (p value =0.54 for protein D, p value=0.69 for P6 and p value=0.33 for OMP26).
Discussion
The focus of this study was to examine the antibody response of otitis prone children in comparison with non-otitis prone children to vaccine candidates protein D, P6 and OMP26 of NTHi. For the first time, to our knowledge we also were able to study the immune responses of children who meet the definition of AOMTF. The results confirm and extend the observations of others for otitis prone children [23-29], contradict some earlier reports [29, 30] and provide much new data.
We observed that otitis prone children generally mount lower serum IgG antibody responses over time against the three antigens we studied compared to non-otitis prone children, a finding previously made by Faden et al for P6 [23], although anti-OMP26 and anti-P6 was not different in acute-to convalescent sera, whilst there was a significant difference for anti-PD. Also, anti-PD levels were low in acute sera of otitis versus non-otitis prone children. The above findings suggest a link between anti-PD and protection.
Otitis prone children also had significantly lower IgG antibody response compared to children with AOMTF against all three antigens suggesting that immunologically these two groups of children behave differently. This was a novel observation. We did not observe any difference in the serum IgA antibody level in the otitis prone, AOMTF and non-otitis prone children. Otitis prone and AOMTF children had significantly lower IgM levels compared to non-otitis prone children for protein D, no difference for P6, and a trend for higher levels against OMP26. This is the first study to examine the responses to the three Ig classes of antibody to three NTHi antigens. We interpret these results to indicate that the different antigens elicit different antibody response profiles, possibly reflecting their different antigenicity in young children when the OMP is presented to the child host in a natural way by asymptomatic colonization or AOM infection.
Our evaluation of anti Protein D, P6 and OMP26 antibody responses in otitis prone children after AOM supports the generally held explanation for the otitis prone state: These children have a specific immunologic deficiency in antibody response to NTHi antigens during an AOM. After an AOM, the child may be left with an inadequate immune response and thereby remain as susceptible as before the infection to yet another infection. The mechanism for this impairment in the immune respnse – B cell immunity, T cell immunity or antigen presenting cells – is a topic under study by our group. We have recently shown that anti-protein D and P6 but not OMP 26 contribute to bactericidal activity against NTHi in non-otitis prone children.[20, 27] We are now studying whether there are differences in bactericidal activity specific to these antigens in otitis prone children.
Otitis prone children and children with AOMTF appeared to be similar immunologically but different from non-otitis prone children when we evaluated antibody levels to protein D and P6 in children after AOM and after asymptomatic NP colonization compared to children where colonization was not detected. This analysis was hampered by small subject numbers in some of the groupings and is not considered conclusive at this time. Also the group classified as not NTHi colonized almost certainly includes many children who were colonized but the colonization duration was sufficiently short that we failed to detect the colonization.
The acquisition of antibody over time to antigens expressed by NTHi and Streptococcus pneumoniae has been previously studied.[25, 31-34] Our finding of gradual acquisition of antibody to NTHi OMPs D, P6 and 26 is consistent with those reports. What is unique about our study design was the prospective collection of data on children beginning at 6 months of age that allowed us to have serum samples months to years before they became identified as otitis prone. The observation that these children are acquiring antibody to the three vaccine antigens we studied at a significantly slower rate than non-otitis prone children raises the question of whether these highest risk children will respond to parenteral vaccination sub-optimally as well.
The major strengths of our study include the prospective enrollment and collection of data and samples over a prolonged time interval. Addithionally, this is the first study to evaluate three vaccine candidates for NTHi simultaneously. We had a unique opportunity to collect MEF from children commencing with their very first AOM as well as subsequent AOMs. Our study also had limitations. We collected data on several epidemiologic factors associated with the otitis prone condition such as early in life onset of AOM, having siblings with rAOM, enrollment in daycare, and absence of breast feeding.[1, 5, 8, 35, 36] However, we currently have an insufficient sample size to include these covariates in our analysis. The children we studied from a higher income lifestyle may not be representative of low socioeconomic populations in developing countries. Collection of NP samples and serum occurred at 3 to 6 month intervals at the child's scheduled well visits. As such we missed some NP colonization episodes. Despite best efforts, we did not obtain blood samples from every child at every acute and convalescence AOM visit or every 3 to 6 month periodic sampling as designed. This created windows of missing data that we addressed as we could statistically.
In conclusion we have confirmed previous work in identifying the otitis prone child as immunologically deficient in response to three vaccine antigens of NTHi, following AOM and asymptomatic NP colonization. We found that children with AOMTF behave immunologically like otitis prone children in some respects and like non-otitis prone children in others. Patterns of IgG and IgM responses among otitis prone vs. non-otitis prone children differ and differ among OMP antigens, thereby indicating a need for continued study to understand the mechanisms for these observations. The presentation of these vaccine candidate antigens by the parenteral route (probably with an adjuvant) may mitigate the immunological hyporesponsiveness that we have described here following natural exposure of this otopathogen. However, this will require study because a vaccine that is less effective in the most vulnerable group of children would not be an optimal outcome.
Acknowledgments
This study was supported by NIH NIDCD RO1 08671. We thank Tim Murphy, MD, University of Buffalo for P6 plasmid; Jennelle Kyd, PhD, University of Canberra, Australia for OMP26 plasmid; and Jan Poolman, PhD, GSK Biologicals, Rixensart Belgium for recombinant protein D. We also thank Sally Thomas, LPN, CCRC, the nurses and staff of Legacy Pediatrics and the collaborating pediatricians from Sunrise Pediatrics, Westfall Pediatrics, Lewis Pediatrics and Long Pond Pediatrics and the parents who consented and the children who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160(1):83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Alho OP, Koivu M, Sorri M. What is an ‘otitis-prone’ child? Int J Pediatr Otorhinolaryngol. 1991;21(3):201–9. doi: 10.1016/0165-5876(91)90001-r. [DOI] [PubMed] [Google Scholar]
- 3.Alho OP, Laara E, Oja H. What is the natural history of recurrent acute otitis media in infancy? J Fam Pract. 1996;43(3):258–64. [PubMed] [Google Scholar]
- 4.Howie VM, Ploussard JH, Sloyer J. The “otitis-prone” condition. Am J Dis Child. 1975;129(6):676–8. doi: 10.1001/archpedi.1975.02120430016006. [DOI] [PubMed] [Google Scholar]
- 5.Pelton SI, Leibovitz E. Recent advances in otitis media. Pediatr Infect Dis J. 2009;28(10 Suppl):S133–S137. doi: 10.1097/INF.0b013e3181b6d81a. [DOI] [PubMed] [Google Scholar]
- 6.Pichichero ME, Casey JR, Hoberman A, Schwartz R. Pathogens causing recurrent and difficult-to-treat acute otitis media, 2003-2006. Clin Pediatr (Phila) 2008;47(9):901–6. doi: 10.1177/0009922808319966. [DOI] [PubMed] [Google Scholar]
- 7.Harrison CJ, Marks MI, Welch DF. Microbiology of recently treated acute otitis media compared with previously untreated acute otitis media. Pediatr Infect Dis. 1985;4(6):641–6. doi: 10.1097/00006454-198511000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Teele DW, Pelton SI, Klein JO. Bacteriology of acute otitis media unresponsive to initial antimicrobial therapy. J Pediatr. 1981;98(4):537–9. doi: 10.1016/s0022-3476(81)80755-x. [DOI] [PubMed] [Google Scholar]
- 9.Barkai G, Leibovitz E, Givon-Lavi N, Dagan R. Potential contribution by nontypable Haemophilus influenzae in protracted and recurrent acute otitis media. Pediatr Infect Dis J. 2009;28(6):466–71. doi: 10.1097/inf.0b013e3181950c74. [DOI] [PubMed] [Google Scholar]
- 10.Shurin PA, Pelton SI, Tager IB, Kasper DL. Bactericidal antibody and susceptibility to otitis media caused by nontypable strains of Haemophilus influenzae. J Pediatr. 1980;97(3):364–9. doi: 10.1016/s0022-3476(80)80182-x. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein JM, Faden HS, Loos BG, Murphy TF, Ogra PL. Recurrent otitis media with non-typable Haemophilus influenzae: the role of serum bactericidal antibody. Int J Pediatr Otorhinolaryngol. 1992;23(1):1–13. doi: 10.1016/0165-5876(92)90074-y. [DOI] [PubMed] [Google Scholar]
- 12.Poolman JT, Bakaletz L, Cripps A, et al. Developing a nontypeable Haemophilus influenzae (NTHi) vaccine. Vaccine. 2000;19 1:S108–S115. doi: 10.1016/s0264-410x(00)00288-7. [DOI] [PubMed] [Google Scholar]
- 13.Kyd J, Cripps A. Nontypeable Haemophilus influenzae: challenges in developing a vaccine. J Biotechnol. 1999;73(2-3):103–8. doi: 10.1016/s0168-1656(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 14.Forsgren A, Riesbeck K, Janson H. Protein D of Haemophilus influenzae: a protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clin Infect Dis. 2008;46(5):726–31. doi: 10.1086/527396. [DOI] [PubMed] [Google Scholar]
- 15.Nelson MB, Munson RS, Jr, Apicella MA, Sikkema DJ, Molleston JP, Murphy TF. Molecular conservation of the P6 outer membrane protein among strains of Haemophilus influenzae: analysis of antigenic determinants, gene sequences, and restriction fragment length polymorphisms. Infect Immun. 1991;59(8):2658–63. doi: 10.1128/iai.59.8.2658-2663.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.el Adhami W, Kyd JM, Bastin DA, Cripps AW. Characterization of the gene encoding a 26-kilodalton protein (OMP26) from nontypeable Haemophilus influenzae and immune responses to the recombinant protein. Infect Immun. 1999;67(4):1935–42. doi: 10.1128/iai.67.4.1935-1942.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakaletz LO, Kennedy BJ, Novotny LA, Duquesne G, Cohen J, Lobet Y. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect Immun. 1999;67(6):2746–62. doi: 10.1128/iai.67.6.2746-2762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367(9512):740–8. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 19.DeMaria TF, Murwin DM, Leake ER. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect Immun. 1996;64(12):5187–92. doi: 10.1128/iai.64.12.5187-5192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabirov A, Kodama S, Sabirova N, Mogi G, Suzuki M. Intranasal immunization with outer membrane protein P6 and cholera toxin induces specific sinus mucosal immunity and enhances sinus clearance of nontypeable Haemophilus influenzae. Vaccine. 2004;22(23-24):3112–21. doi: 10.1016/j.vaccine.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 21.Kyd JM, Cripps AW. Potential of a novel protein, OMP26, from nontypeable Haemophilus influenzae to enhance pulmonary clearance in a rat model. Infect Immun. 1998;66(5):2272–8. doi: 10.1128/iai.66.5.2272-2278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyd JM, Cripps AW, Novotny LA, Bakaletz LO. Efficacy of the 26-kilodalton outer membrane protein and two P5 fimbrin-derived immunogens to induce clearance of nontypeable Haemophilus influenzae from the rat middle ear and lungs as well as from the chinchilla middle ear and nasopharynx. Infect Immun. 2003;71(8):4691–9. doi: 10.1128/IAI.71.8.4691-4699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenfors LE, Raisanen S, Mogi G, Honjo I, Isaii T, Tomonori T, editors. The role of bacterial opsonization and phagocytosis in otitis media. Amsterdam: Kugler Publications; 1994. Recent advances in otitis media; pp. 535–8. [Google Scholar]
- 24.Bernstein JM, Bronson PM, Wilson ME. Immunoglobulin G subclass response to major outer membrane proteins of nontypable Haemophilus influenzae in children with acute otitis media. Otolaryngol Head Neck Surg. 1997;116(3):363–71. doi: 10.1016/S0194-59989770275-4. [DOI] [PubMed] [Google Scholar]
- 25.Yamanaka N, Faden H. Antibody response to outer membrane protein of nontypeable Haemophilus influenzae in otitis-prone children. J Pediatr. 1993;122(2):212–8. doi: 10.1016/s0022-3476(06)80115-0. [DOI] [PubMed] [Google Scholar]
- 26.Subcommittee on Management of Acute Otitis Media Diagnosis and Management of Acute Otitis Media. Pediatrics. 2004;113(5):1451–65. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 27.Pichichero ME, Kaur R, Casey JR, Sabirov A, Khan MN, Almudevar A. Antibody Response to Haemophilus influenzae Outer Membrane Protein D, P6, and OMP26 After Nasopharyngeal Colonization and Acute Otitis Media in Children. Vaccine. 2010;28(44):7184–92. doi: 10.1016/j.vaccine.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy TF, Brauer AL, Sethi S, Kilian M, Cai X, Lesse AJ. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007;195(1):81–9. doi: 10.1086/509824. [DOI] [PubMed] [Google Scholar]
- 29.Berman S, Lee B, Nuss R, Roark R, Giclas PC. Immunoglobulin G, total and subclass, in children with or without recurrent otitis media. J Pediatr. 1992;121(2):249–51. doi: 10.1016/s0022-3476(05)81197-7. [DOI] [PubMed] [Google Scholar]
- 30.Jorgensen F, Andersson B, Hanson LA, Nylen O, Eden CS. Gamma-globulin treatment of recurrent acute otitis media in children. Pediatr Infect Dis J. 1990;9(6):389–94. doi: 10.1097/00006454-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Faden H. The microbiologic and immunologic basis for recurrent otitis media in children. Eur J Pediatr. 2001;160(7):407–13. doi: 10.1007/s004310100754. [DOI] [PubMed] [Google Scholar]
- 32.Hotomi M, Yamanaka N, Saito T, et al. Antibody responses to the outer membrane protein P6 of non-typeable Haemophilus influenzae and pneumococcal capsular polysaccharides in otitis-prone children. Acta Otolaryngol. 1999;119(6):703–7. doi: 10.1080/00016489950180667. [DOI] [PubMed] [Google Scholar]
- 33.Soininen A, Pursiainen H, Kilpi T, Kayhty H. Natural development of antibodies to pneumococcal capsular polysaccharides depends on the serotype: association with pneumococcal carriage and acute otitis media in young children. J Infect Dis. 2001;184(5):569–76. doi: 10.1086/322794. [DOI] [PubMed] [Google Scholar]
- 34.Veenhoven R, Rijkers G, Schilder A, et al. Immunoglobulins in otitis-prone children. Pediatr Res. 2004;55(1):159–62. doi: 10.1203/01.PDR.0000099776.66136.39. [DOI] [PubMed] [Google Scholar]
- 35.Bluestone CD. Management of otitis media in infants and children: current role of old and new antimicrobial agents. Pediatr Infect Dis J. 1988;7(11 Suppl):S129–S136. doi: 10.1097/00006454-198811001-00002. [DOI] [PubMed] [Google Scholar]
- 36.Carlin SA, Marchant CD, Shurin PA, Johnson CE, Murdell-Panek D, Barenkamp SJ. Early recurrences of otitis media: reinfection or relapse? J Pediatr. 1987;110(1):20–5. doi: 10.1016/s0022-3476(87)80281-0. [DOI] [PubMed] [Google Scholar]


