Abstract
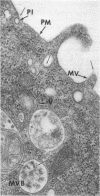
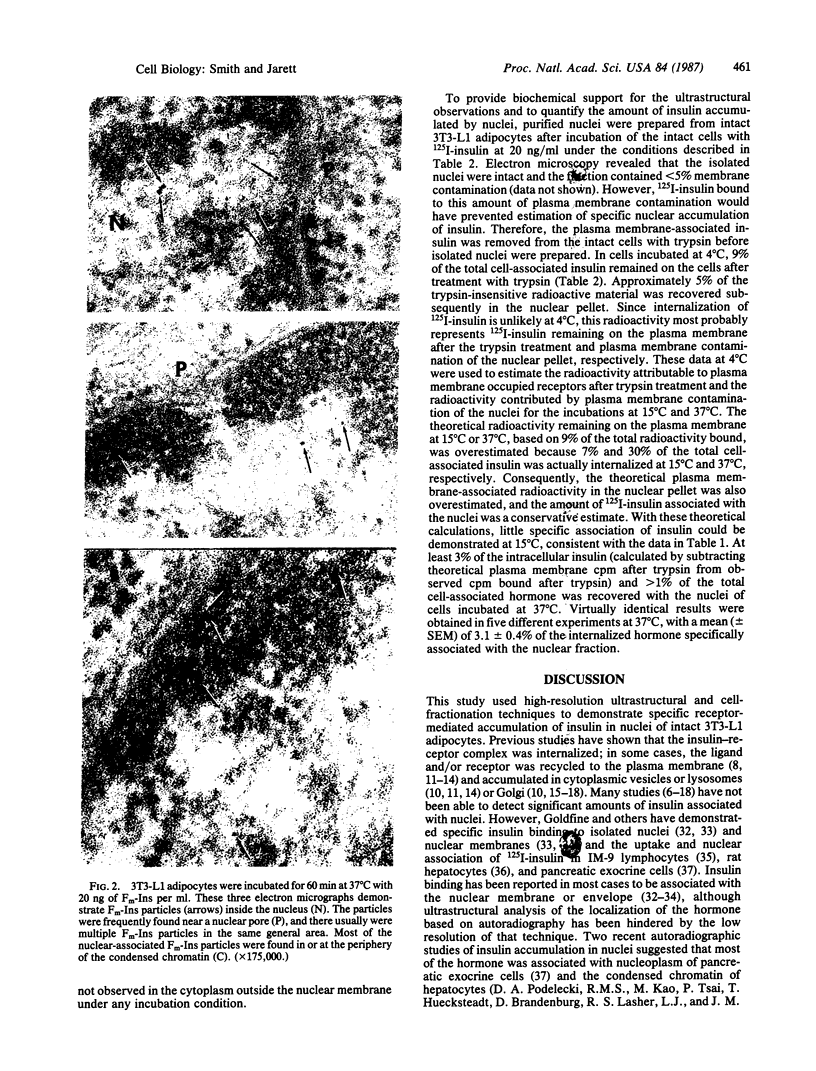
Monomeric ferritin-labeled insulin (Fm-Ins), a biologically active, electron-dense marker of occupied insulin receptors, was used to characterize the internalization of insulin in 3T3-L1 adipocytes. Fm-Ins bound specifically to insulin receptors and was internalized in a time- and temperature-dependent manner. Fm-Ins was found in cytoplasmic vesicles within 5-10 min at 37 degrees C and subsequently was observed in multivesicular bodies and lysosomes. In addition, small amounts of Fm-Ins were associated with nuclei after 30 min. The number of Fm-Ins particles observed in nuclei continued to increase in a time-dependent manner until at least 90 min. In the nucleus, several Fm-Ins particles usually were found in the same general location--near nuclear pores, associated with the periphery of the condensed chromatin. Addition of a 250-fold excess of unlabeled insulin or incubation at 15 degrees C reduced the number of Fm-Ins particles found in nuclei after 90 min by 99% or 92%, respectively. Nuclear accumulation of unlabeled ferritin was only 2% of that found with Fm-Ins after 90 min at 37 degrees C. Biochemical experiments utilizing 125I-labeled insulin and subcellular fractionation indicated that intact 3T3-L1 adipocytes internalized insulin rapidly and that approximately equal to 3% of the internalized ligand accumulated in nuclei after 1 hr. These data provide biochemical and high-resolution ultrastructural evidence that 3T3-L1 adipocytes accumulate potentially significant amounts of insulin in nuclei by an insulin receptor-mediated process. The transport of insulin or the insulin-receptor complex to nuclei in this cell or in others may be directly involved in the long-term biological effects of insulin--in particular, in the control of DNA and RNA synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergeron J. J., Sikstrom R., Hand A. R., Posner B. I. Binding and uptake of 125I-insulin into rat liver hepatocytes and endothelium. An in vivo radioautographic study. J Cell Biol. 1979 Feb;80(2):427–443. doi: 10.1083/jcb.80.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson-Lougarre A., Blum C. J. Sites de liaison nucléaires à l'insuline dans les noyaux isolés de thyroïde bovine. C R Seances Acad Sci D. 1979 Jul 9;289(2):129–132. [PubMed] [Google Scholar]
- Carpentier J. L., Gazzano H., Van Obberghen E., Fehlmann M., Freychet P., Orci L. Intracellular pathway followed by the insulin receptor covalently coupled to 125I-photoreactive insulin during internalization and recycling. J Cell Biol. 1986 Mar;102(3):989–996. doi: 10.1083/jcb.102.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Barazzone P., Freychet P., Le Cam A., Orci L. Intracellular localization of 125I-labeled insulin in hepatocytes from intact rat liver. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2803–2807. doi: 10.1073/pnas.76.6.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J., Posner B. I., Bergeron J. J. Receptor-mediated endocytosis of [125I]insulin into pancreatic acinar cells in vivo. Endocrinology. 1984 Nov;115(5):1996–2008. doi: 10.1210/endo-115-5-1996. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Willeput J., Huet de Froberville A. Receptor-mediated internalisation of insulin in intact rat liver. A biochemical study. FEBS Lett. 1979 Oct 15;106(2):338–344. doi: 10.1016/0014-5793(79)80528-1. [DOI] [PubMed] [Google Scholar]
- Fakan S., Puvion E. The ultrastructural visualization of nucleolar and extranucleolar RNA synthesis and distribution. Int Rev Cytol. 1980;65:255–299. doi: 10.1016/s0074-7696(08)61962-2. [DOI] [PubMed] [Google Scholar]
- Fan J. Y., Carpentier J. L., Van Obberghen E., Blackett N. M., Grunfeld C., Gorden P., Orci L. The interaction of 125I-insulin with cultured 3T3-L1 adipocytes: quantitative analysis by the hypothetical grain method. J Histochem Cytochem. 1983 Jul;31(7):859–870. doi: 10.1177/31.7.6343480. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Carpentier J. L., Van Obberghen E., Freychet P., Thamm P., Saunders D., Brandenburg D., Orci L. Internalized insulin receptors are recycled to the cell surface in rat hepatocytes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5921–5925. doi: 10.1073/pnas.79.19.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goidl J. A. Insulin binding to isolated liver nuclei from obese and lean mice. Biochemistry. 1979 Aug 21;18(17):3674–3679. doi: 10.1021/bi00584a006. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Clawson G. A., Smuckler E. A., Purrello F., Vigneri Action of insulin at the nuclear envelope. Mol Cell Biochem. 1982 Oct 1;48(1):3–14. doi: 10.1007/BF00214816. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D. Insulin receptors and the site of action of insulin. Life Sci. 1978 Dec 31;23(27-28):2639–2648. doi: 10.1016/0024-3205(78)90643-4. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Jones A. L., Hradek G. T., Wong K. Y. Electron microscope autoradiographic analysis of [125I]iodoinsulin entry into adult rat hepatocytes in vivo: evidence for multiple sites of hormone localization. Endocrinology. 1981 May;108(5):1821–1828. doi: 10.1210/endo-108-5-1821. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Jones A. L., Hradek G. T., Wong K. Y., Mooney J. S. Entry of insulin into human cultured lymphocytes: electron microscope autoradiographic analysis. Science. 1978 Nov 17;202(4369):760–763. doi: 10.1126/science.715440. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J. Binding of insulin to isolated nuclei. Proc Natl Acad Sci U S A. 1976 May;73(5):1427–1431. doi: 10.1073/pnas.73.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk W. K., Jarett L. Intracellular mediators of insulin action. Diabetes Metab Rev. 1985;1(3):229–259. doi: 10.1002/dmr.5610010302. [DOI] [PubMed] [Google Scholar]
- Hammons G. T., Smith R. M., Jarett L. Inhibition by bacitracin of rat adipocyte plasma membrane degradation of 125I-insulin is associated with an increase in plasma membrane bound insulin and a potentiation of glucose oxidation by adipocytes. J Biol Chem. 1982 Oct 10;257(19):11563–11570. [PubMed] [Google Scholar]
- Heidenreich K. A., Brandenburg D., Berhanu P., Olefsky J. M. Metabolism of photoaffinity-labeled insulin receptors by adipocytes. Role of internalization, degradation, and recycling. J Biol Chem. 1984 May 25;259(10):6511–6515. [PubMed] [Google Scholar]
- Horvat A. Insulin binding sites on rat liver nuclear membranes: biochemical and immunofluorescent studies. J Cell Physiol. 1978 Oct;97(1):37–47. doi: 10.1002/jcp.1040970106. [DOI] [PubMed] [Google Scholar]
- Izzo J. L., Roncone A. M., Helton D. L., Izzo M. J. Subcellular distribution of intraportally injected 125I-labeled insulin in rat liver. Arch Biochem Biophys. 1979 Nov;198(1):97–109. doi: 10.1016/0003-9861(79)90399-0. [DOI] [PubMed] [Google Scholar]
- Jarett L., Schweitzer J. B., Smith R. M. Insulin receptors: differences in structural organization on adipocyte and liver plasma membranes. Science. 1980 Dec 5;210(4474):1127–1128. doi: 10.1126/science.7003710. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. Ultrastructural localization of insulin receptors on adipocytes. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3526–3530. doi: 10.1073/pnas.72.9.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser N., Vlodavsky I., Tur-Sinai A., Fuks Z., Cerasi E. Binding, internalization, and degradation of insulin in vascular endothelial cells. Diabetes. 1982 Dec;31(12):1077–1083. doi: 10.2337/diacare.31.12.1077. [DOI] [PubMed] [Google Scholar]
- Locke M., Huie P. The nucleolus during epidermal development in an insect. Tissue Cell. 1980;12(1):175–195. doi: 10.1016/0040-8166(80)90060-9. [DOI] [PubMed] [Google Scholar]
- Nelson D. M., Smith R. M., Jarett L. Nonuniform distribution and grouping of insulin receptors on the surface of human placental syncytial trophoblast. Diabetes. 1978 May;27(5):530–538. [PubMed] [Google Scholar]
- Olefsky J. M., Marshall S., Berhanu P., Saekow M., Heidenreich K., Green A. Internalization and intracellular processing of insulin and insulin receptors in adipocytes. Metabolism. 1982 Jul;31(7):670–690. doi: 10.1016/0026-0495(82)90197-4. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M., Groyer-Picard M. T., Logeat F., Milgrom E. Ultrastructural localization of the progesterone receptor by an immunogold method: effect of hormone administration. J Cell Biol. 1986 Apr;102(4):1191–1199. doi: 10.1083/jcb.102.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas C., Desbuquois B. Receptor-mediated insulin degradation and insulin-stimulated glycogenesis in cultured foetal hepatocytes. Biochem J. 1982 Feb 15;202(2):333–341. doi: 10.1042/bj2020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner B. I., Patel B., Verma A. K., Bergeron J. J. Uptake of insulin by plasmalemma and Golgi subcellular fractions of rat liver. J Biol Chem. 1980 Jan 25;255(2):735–741. [PubMed] [Google Scholar]
- Purrello F., Vigneri R., Clawson G. A., Goldfine I. D. Insulin stimulation of nucleoside triphosphatase activity in isolated nuclear envelopes. Science. 1982 May 28;216(4549):1005–1007. doi: 10.1126/science.6281885. [DOI] [PubMed] [Google Scholar]
- Schumm D. E., Webb T. E. Effect of physiological concentrations of insulin and antidiabetic drugs on RNA release from isolated liver nuclei. J Cell Biochem. 1983;23(1-4):223–229. doi: 10.1002/jcb.240230119. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Cobb M. H., Rosen O. M., Jarett L. Ultrastructural analysis of the organization and distribution of insulin receptors on the surface of 3T3-L1 adipocytes: rapid microaggregation and migration of occupied receptors. J Cell Physiol. 1985 May;123(2):167–179. doi: 10.1002/jcp.1041230204. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Jarett L. A simplified method of producing biologically active monomeric ferritin-insulin for use as a high resolution ultrastructural marker for occupied insulin receptors. J Histochem Cytochem. 1982 Jul;30(7):650–656. doi: 10.1177/30.7.7050238. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Jarett L. Quantitative ultrastructural analysis of receptor-mediated insulin uptake into adipocytes. J Cell Physiol. 1983 May;115(2):199–207. doi: 10.1002/jcp.1041150215. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Jarett L. Ultrastructural basis for chloroquine-induced increase in intracellular insulin in adipocytes: alteration of lysosomal function. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7302–7306. doi: 10.1073/pnas.79.23.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Internalization and degradation of fat cell-bound insulin. Separation and partial characterization of subcellular vesicles associated with iodoinsulin. J Biol Chem. 1979 Oct 10;254(19):9786–9794. [PubMed] [Google Scholar]
- Ueda M., Robinson F. W., Smith M. M., Kono T. Effects of monensin on insulin processing in adipocytes. Evidence that the internalized insulin-receptor complex has some physiological activities. J Biol Chem. 1985 Apr 10;260(7):3941–3946. [PubMed] [Google Scholar]
- Vigneri R., Goldfine I. D., Wong K. Y., Smith G. J., Pezzino V. The nuclear envelope. The major site of insulin binding in rat liver nuclei. J Biol Chem. 1978 Apr 10;253(7):2098–2103. [PubMed] [Google Scholar]
- Wang C. C., Sonne O., Hedo J. A., Cushman S. W., Simpson I. A. Insulin-induced internalization of the insulin receptor in the isolated rat adipose cell. Detection of the internalized 138-kilodalton receptor subunit using a photoaffinity 125I-insulin. J Biol Chem. 1983 Apr 25;258(8):5129–5134. [PubMed] [Google Scholar]