Summary
Background
High-dose dexamethasone is a mainstay of therapy for multiple myeloma. We studied whether low-dose dexamethasone in combination with lenalidomide is non-inferior to and has lower toxicity than high-dose dexamethasone plus lenalidomide.
Methods
Patients with untreated symptomatic myeloma were randomly assigned in this open-label non-inferiority trial to lenalidomide 25 mg on days 1–21 plus dexamethasone 40 mg on days 1–4, 9–12, and 17–20 of a 28-day cycle (high dose), or lenalidomide given on the same schedule with dexamethasone 40 mg on days 1, 8, 15, and 22 of a 28-day cycle (low dose). After four cycles, patients could discontinue therapy to pursue stem-cell transplantation or continue treatment until disease progression. The primary endpoint was response rate after four cycles assessed with European Group for Blood and Bone Marrow Transplant criteria. The non-inferiority margin was an absolute difference of 15% in response rate. Analysis was by modified intention to treat. This trial is registered with ClinicalTrials.gov, number NCT00098475.
Findings
445 patients were randomly assigned: 223 to high-dose and 222 to low-dose regimens. 169 (79%) of 214 patients receiving high-dose therapy and 142 (68%) of 205 patients on low-dose therapy had complete or partial response within four cycles (odds ratio 1·75, 80% CI 1·30–2·32; p=0.008). However, at the second interim analysis at 1 year, overall survival was 96% (95% CI 94–99) in the low-dose dexamethasone group compared with 87% (82–92) in the high-dose group (p=0·0002). As a result, the trial was stopped and patients on high-dose therapy were crossed over to low-dose therapy. 117 patients (52%) on the high-dose regimen had grade three or worse toxic effects in the first 4 months, compared with 76 (35%) of the 220 on the low-dose regimen for whom toxicity data were available (p=0·0001), 12 of 222 on high dose and one of 220 on low-dose dexamethasone died in the first 4 months (p=0·003). The three most common grade three or higher toxicities were deep-vein thrombosis, 57 (26%) of 223 versus 27 (12%) of 220 (p=0·0003); infections including pneumonia, 35 (16%) of 223 versus 20 (9%) of 220 (p=0·04), and fatigue 33 (15%) of 223 versus 20 (9%) of 220 (p=0·08), respectively.
Interpretation
Lenalidomide plus low-dose dexamethasone is associated with better short-term overall survival and with lower toxicity than lenalidomide plus high-dose dexamethasone in patients with newly diagnosed myeloma.
Introduction
For over three decades, the mainstay of therapy for multiple myeloma was melphalan and prednisone.1 Autologous stem-cell transplantation (ASCT) later prolonged survival compared with conventional chemotherapy.2–4 More recently, thalidomide,5 bortezomib,6 and lenalidomide7 have emerged as effective therapies.
High-dose dexamethasonc was first used in combination with infusional vincristine and doxorubicin for the treatment of refractory myeloma.8 Later, it was incorporated alone or in combination into various pre-transplant induction regimens for the treatment of newly diagnosed disease.9–11 Although effective, regimens containing high-dose dexamethasone are associated with significant toxicity 10,12,13 and a treatment-related early mortality rate of over 10% in some randomised trials.10,12
Lenalidomide is an analogue of thalidomide that has significant clinical activity in relapsed or refractory myeloma.14,15 In a phase 2 trial, lenalidomide plus standard high-dose pulse dexamethasone showed high response rates (91%) with lower toxicity than previously seen with thalidomide plus dexamethasone in patients with newly diagnosed myeloma.16 Preliminary results of a randomised trial showed the superiority of lenalidomide plus high-dose dexamethasone compared with dexamethasone alone in newly diagnosed myeloma.17 The purpose of this trial was to test the hypothesis that the efficacy of lenalidomide plus high-dose dexamethasone could be preserved, but toxicity reduced, with a lower dexamethasone dose.
Methods
Patients
Patients were eligible if they had previously untreated symptomatic multiple myeloma, bone marrow plasmacytosis (≥10% plasma cells or sheets of plasma cells) or a biopsy proven plasmacytoma, and measurable disease defined as serum monoclonal protein of more than 10 g/L or urine monoclonal protein of 0·2 g per day or more. Patients had to have haemoglobin of more than 70 g/L, platelet count of 75×109 per litre or higher, absolute neutrophil count of more than 1·0×109 per litre, serum creatinine of less than 25 mg/L, bilirubin 15 mg/L or lower, and alanine aminotransferase and aspartate aminotransferase less than or equal to two and a half times the upper limit of normal. Patients were excluded if they had grade 2 or higher peripheral neuropathy, active infection, current or prior deep vein thrombosis, or Eastern Cooperative Oncology Group (ECOG) performance score of 3 or 4. Pregnant or nursing women were not eligible. Women of child-bearing potential unwilling to use a dual method of contraception and men who were unwilling to use a condom were not eligible.
All patients provided written informed consent before entering the trial in accordance with the Declaration of Helsinki. The study was approved by the institutional review boards in the participating ECOG institutions. Patients were enrolled between Nov 3, 2004, and April 7, 2006, from participating institutions.
Randomisation and masking
Patients were randomly assigned one-to-one to receive either lenalidomide plus high-dose dexamethasone or lenalidomide plus low-dose dexamethasone in this open-label trial. The randomised treatment codes were generated by the central ECOG coordinating centre randomisation unit with a computerised random number generator to produce permuted blocks. Dynamic balancing was used to maintain treatment balance within networks of affiliated centres, but no stratification was used. The block size in the permuted blocks and the balance keys for institutional balancing were not disclosed to investigators. The randomised treatment was then communicated by the ECOG coordinating centre to the investigator by a web-based registration system only after registration of the patient, guaranteeing concealment until registration was complete. Patients were enrolled by approved investigators in participating institutions.
Procedures
Patients received either oral lenalidomide 25 mg daily on days 1–21 plus oral dexamethasone 40 mg daily on days 1—4, 9–12, and 17–20 of each 28-day cycle or the same schedule of lenalidomide plus oral dexamethasone 40 mg daily on days 1, 8,15 and 22 of each 28-day cycle. After the first four cycles, patients could discontinue therapy to pursue stem-cell transplantation (or other treatment options) or continue therapy on study until disease progression. Patients were allowed lo interrupt therapy for growth-factor-supported stem-cell mobilisation; however, patients who received non-protocol therapy or transplantation were required to discontinue the study. Dose adjustments were allowed for toxicity. All patients were recommended to receive a bisphosphonate monthly (either pamidronate 90 mg over 2–4 h every 4 weeks or zoledronic acid 4 mg intravenously over 15 min every 4 weeks). Thromboprophylaxis was recommended but not mandated initially during this study. However, after the first 266 patients were enrolled, mandatory thrombo-prophylaxis was added for all patients due to high rates of deep-vein thrombosis.18 Patients who progressed or did not respond in the first four cycles were offered treatment with thalidomide instead of lenalidomide, keeping the dexamethasone dose constant.
The response and progression criteria used were standard European Group for Blood and Bone Marrow Transplant (Bladé) criteria except that responses were confirmed 4 weeks apart (instead of 6 weeks).19 Patients were also classified as having a very good partial response with the International Myeloma Working Group response criteria.13 A category of immunofixation negative complete response20 was defined as confirmed disappearance of the monoclonal protein in the serum and urine by immunofixation studies without the requirement for bone marrow studies. The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 3), was used to classify and grade adverse events.
The primary endpoint was overall response rate in the first four cycles among eligible patients (ie, on a modified intention-to-treat basis). Additional endpoints included best overall response (assessed in eligible patients only), time to progression, progression-free survival, and overall survival (assessed in patients for whom data were available). Time to progression was defined as time from randomisation to disease progression. Progression-free survival was defined as time from randomisation to disease progression or death due to any cause.
Statistical analysis
The primary purpose of this study was to determine if lenalidomide plus low-dose dexamethasone had a response rate that was not inferior to lenalidomide plus high-dose dexamethasone, while reducing toxicity. Anticipated response rate in the high-dose group was 70%, and an absolute difference in response rate of 15% between groups at 4 months was the margin of non-inferiority (ie, a response rate of 55% or lower in the low-dose group would indicate inferiority). This margin would give an odds ratio for response in the high-dose group of 1·91 or greater to indicate inferiority. The planned sample size was 196 eligible patients per arm with an overall one-sided type 1 error rate of 0·10 and type 2 error rate of 0·05. Statistical power was 95%. Preplanned interim analyses were done by an independent data monitoring committee when full data became available on 25%, 50%, and 75% of accrual, without adjustment for a spending. At the second interim analysis, the committee recommend release of study results.
Two-sided Fisher’s exact tests were used to test for differences between categorical variables. Two-sided Wilcoxon rank sum tests were used to compare continuous variables. Survival analysis was done with the Kaplan-Meier method.21 Differences between survival curves were tested for statistical significance with the two-sided log-rank test. The effect of confounding baseline variables on survival differences between the two arms was studied with a Cox proportional hazards model. SAS (version 9.2) was used for all statistical analyses. This trial is registered with ClinicalTrials.gov, number NCT00098475.
Role of the funding source
The NCI provided input on the design of the trial, but had no role in the analysis, interpretation, decision to publish, or writing of the report. The manufacturer of lenalidomide (Celgene Corporation, Summit, NJ, USA) was not involved in the design, analysis, interpretation, or writing of this trial. The corresponding author (SVR) and statistician (SJ) had full access to all the data in the study. All authors of this paper had the final responsibility for the decision to submit for publication.
Results
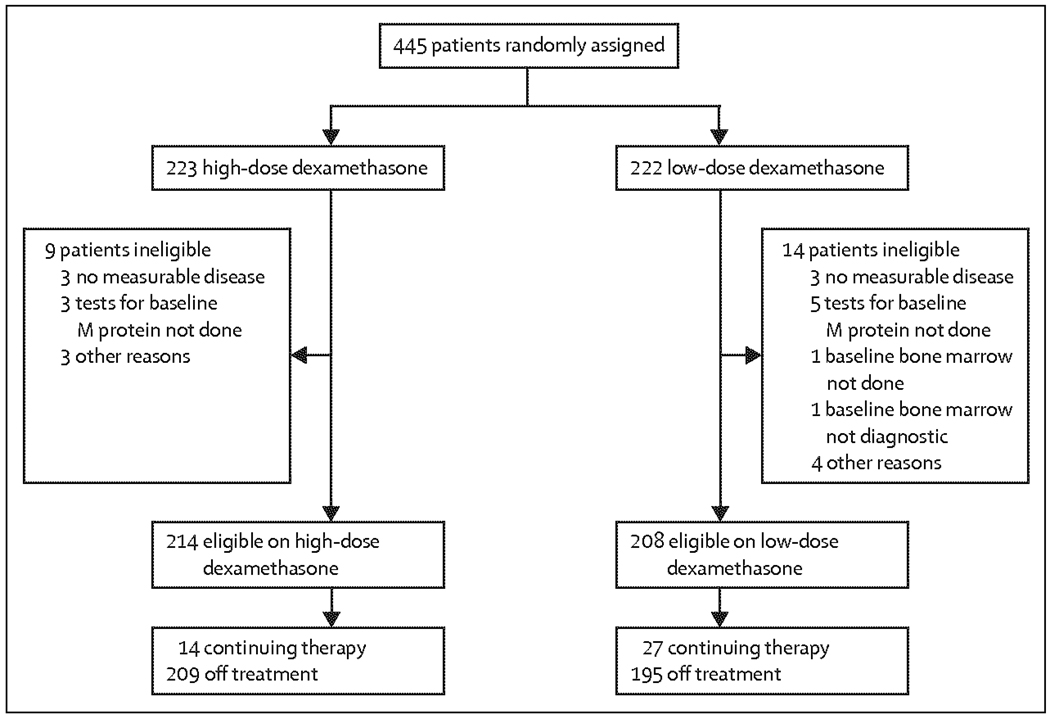
445 patients were accrued (table 1); 223 were randomly assigned to receive lenalidomide plus high-dose dexamethasone and 222 to receive lenalidomide plus low-dose dexamethasone. 149 patients (67%) in the high-dose group had bone disease at baseline compared with 127 (57%) of 222 in the low-dose group. 422 patients were eligible for analysis (figure 1). As of December, 2008, 404 (91%) of 445 patients are off study
Table 1.
Characteristics of participants
| Lenalidomide plus high-dose dexamethasone (n=223) | Lenalidomide plus low-dose dexamethasone (n=222) | |
|---|---|---|
| Age (years) | 66 (36–87) | 65 (35–85) |
| ≥65 | 119(53) | 114(51) |
| <65 | 104 (47) | 108 (49) |
| Sex, male | 132(59) | 121(55) |
| Race | ||
| White | 187(85) | 194(88) |
| Non-white | 33 (15) | 27(12) |
| Missing | 3 | 1 |
| International staging system | ||
| Stage 1 | 68 (33) | 70(33) |
| Stage 2 | 86 (41) | 87 (42) |
| Stage 3 | 55 (26) | 52 (25) |
| Missing | 14 | 13 |
| ECOG performance status | ||
| 0 | 99(44) | 110(50) |
| 1 | 104(47) | 91(41) |
| 2 | 20(9) | 21(9) |
| M protein | 9·2 (4·4–14·6) | 8·9 (5·2–13·7) |
| Serum M, present | 194 (100) | 190 (100) |
| Unknown | 29 (15) | 32 (17) |
| IgG (g/L) | 121(63) | 121(64) |
| IgA (g/L) | 46 (24) | 55(29) |
| Missing | 2 | 0 |
| Bone disease | ||
| Present | 149 (67) | 127 (57) |
| Absent | 74(33) | 95(43) |
| Haemoglobin | ||
| ≤110 g/L | 120 (54) | 111 (50) |
| >110 g/L | 103 (46) | 111 (50) |
| Serum creatinine | ||
| >15mg/L | 31 (14) | 30 (14) |
| ≤15 mg/L | 192 (86) | 192 (86) |
| Albumin (g/L) | 35 (4–52) | 36 (19–51) |
| Missing | 9 | 9 |
| Beta-2 microglobulin (mg/L) | 3·8 (0·8–29·7) | 3·5 (0·6–64·4) |
| Missing | 5 | 4 |
| Bone-marrow plasma cell percentage* | 40 (0–100) | 37(0–100) |
| Missing | 37 | 33 |
Data are median (range) or number (%). For variable with missing values, we have excluded missing value from calculations of percentages or medians.
Bone-marrow plasma cell percentages missing because exact percentages on a bone marrow exam are sometimes hard to determine on pathology.
Figure 1.
Study profile
Median duration of therapy was 4 months (95% CI ·7–4·7) in the high-dose group and 6 months (4·9–7·8) in the low-dose group. 21 (14%) of 223 patients in the high-dose group remained on treatment for more than 1 year compared with 66 (30%) of 222 patients in the low-dose group. The mean relative dose intensity of lenalidomide delivered in the first four cycles was 91·1% of the targeted dose in the high-dose group and 91·5% in the low-dose group; the intensity for dexamethasone was 87·2% and 95·7%, respectively. Because the study was designed as an induction trial and patients were allowed to go off-study to pursue autologous stem-cell transplantation, 167 patients interrupted or stopped treatment to have stem-cell harvest. Of these patients, 163 (98%) were successful and four (2%) were unsuccessful.
The overall (complete plus partial) response to therapy after four cycles was higher with high-dose dexamethasone than with low-dose, 169 (79%) of 214 patients on high-dose dexamethasone had an overall response (complete or partial) compared with 142 (68·3%) of 208 on low-dose (p=0·008). The difference in response rates between high-dose and low-dose was 10·7% (two-sided asymptotic 80% CI 6·8–20·8). Although this is lower than 15%, the odds ratio for response of 1·75 (80% CI 1·30–2·32) indicates that low-dose therapy is inferior in terms of overall response rate after four cycles because the preplanned inferiority odds ratio of 1·91 is well within the CI. 90 (42%) patients achieved complete response or very good partial response in the high-dose dexamethasone group in the first four cycles of therapy compared with 49 (24%) patients in the low-dose treatment group (p<0·0001; webappendix). Disease progression within the first four cycles of therapy was low in both groups, noted in eight (4%) of 214 patients receiving lenalidomide plus high-dose dexamethasone and five (2%) of 208 receiving lenalidomide plus low-dose dexamethasone. 20 patients (five from the high-dose group and 15 from the low-dose group) who progressed or did not achieve a response in either group were enrolled to treatment with thalidomide plus dexamethasone; only one minor response was observed among 11 eligible patients (two and nine from the two groups). Nine patients were ineligible because they were enrolled to receive thalidomide incorrectly.
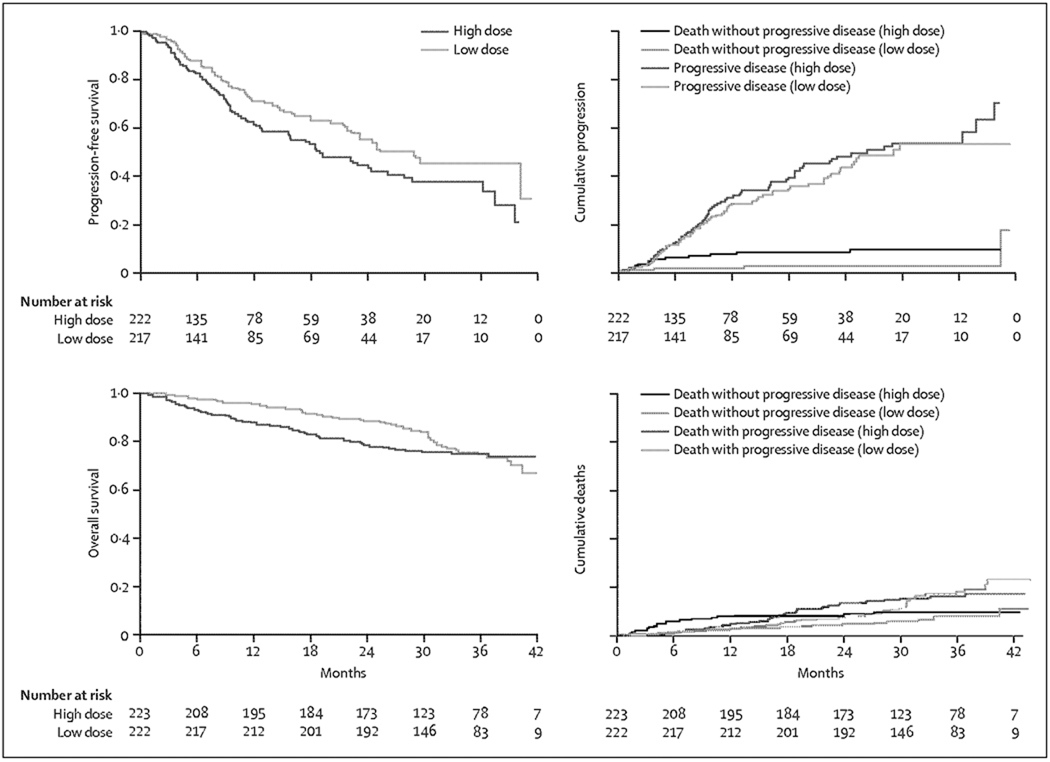
Overall survival was not a protocol-specified endpoint in this study. However, the study was stopped on recommendations of the independent data monitoring committee at a median follow-up of 12·5 months (95% CI 11·5–14·6) because overall survival was significantly higher with low dose than with high-dose dexamethasone (figure 2; log-rank p=0·0002). The 1-year overall survival was 96% (95% CI 94–99) in the low-dose group compared with 87% (82–92) in the high-dose group; 2-year overall survival was 87% (81–93) and 75% (68–93), respectively. We studied the effect of the following variables on survival in univariate analysis: treatment group, international staging system (stage 2 or 3 vs 1 and missing vs stage 1), ECOG performance status (>0 vs 0), presence or absence of bone disease, race (white vs other), haemoglobin (≤110 g/l. vs >110 g/l.), serum creatinine (>15 mg/L vs ≤15 mg/L), and age (<65 years vs ≥65 years). These analyses, including outcome assessment by race and sex, were done post hoc and were not prespecified in the protocol. Bone disease, haemoglobin, serum creatinine, and race were not significant on univariate analysis. On a multiple regression analysis of variables significant (p<0·05) in the univariate analysis (treatment arm, international staging system, ECOG performance status, and age) and race, which was borderline significant (p=0·08), the difference in overall survival between the treatment groups remained significant (p=0·001; table 2). Differences in overall survival were noted in patients age less than 65 years (p=0·01) and those age 65 years and older (p=0·004). Among those age less than 65 years, 1 year overall survival rate was 91% (95% CI 85–97) with high-dose dexamethasone and 98% (92–99) with low-dose dexamethasone. 1-year overall survival rates for those age 65 years and older were 83% (76–90) with high-dose and 94% (89–99) with low-dose. All patients in the high-dose group were instructed to cross-over to low-dose immediately (March 27, 2007).
Figure 2.
Overall survival in patients receiving lenalidomide and either high-dose or low-dose dexamethasone
Table 2.
Multivariate analysis of overall survival
| Hazard ratio (95% CI) |
p value | |
|---|---|---|
| Low dose vs high dose | 0·40 (0·23–0·70) | 0·001 |
| ISS stage* | 0· 02 | |
| Stage II/III vs stage I | 3·51 (1·49–8·28) | |
| Stage missing vs stage I | 2·68 (0·80–8·95) | |
| ECOG performance status (1 or 2 vs 0) | 1·65 (0·95–2·89) | 0·08 |
| Age (≥65 vs <65 years) | 2·02 (1·15–3· 57) | 0·02 |
| Race (white vs non-white) | 2·69(0·97–7· 47) | 0·06 |
Global p value. ISS-International staging system.
With the current median follow-up of 35·8 months (95% CI 35·1–36·3) as of December, 2008, the best overall response rates on each group (table 3) show better response with lenalidomide plus high-dose dexamethasone (median response duration 21·4 months, 95% CI 19·7–27·8) than with lenalidomide plus low-dose dexamethasone (24·1 months, 21·5–28·1). Among patients who responded, median time to partial response or better was 1 month. Only five patients in the study (two in the high-dose group and three in the low-dose group) who achieved minor response by four cycles converted to partial response or better with longer therapy. Although the overall response rate did not improve, the level of response of patients with partial response improved with longer duration of therapy (data not shown). The higher response rates for high-dose dexamethasone did not translate into superior progression-free survival (figure 3): median progression-free survival was 19·1 months (15·7–26·3) with high dose versus 25·3 months (22·3–not reached) with low-dose (p=0·026). 93 patients progressed in the high-dose group compared with 70 in the low-dose group. Over 2 years of follow-up, 16 of 222 patients died without progression in the high-dose group compared with four of 217 in the low-dose group. 77 patients in the high-dose group progressed compared with 66 of 217 in the low-dose group. Median times to progression were 22·3 months (15·9–36·4) in the high-dose group and 26·1 months (22·3–not reached) in the low-dose group (p=0·298). After 24 months, additional follow-up since crossover to low-dose dexamethasone was done, overall survival curves converge at 3 years (figure 3; p=0·467). Median overall survival has not been reached. 56 (25%) of 223 patients in the high-dose group and 53 (24%) of 222 in the low-dose group have died.
Table 3.
Best overall response to therapy
| High dose (n=214) |
Low dose (n=208) |
Total (n=422) |
p value | |
|---|---|---|---|---|
| Overall response rate (partial response or better)* | 174 (81) | 146 (70) | 320(76) | 0·009 |
| Complete plus very good partial response | 108 (50) | 84(40) | 192(45) | 0·040 |
| Complete response | 10(5) | 9(4) | 19(5) | ‥ |
| Immunofixation-negative complete response | 27(13) | 21(10) | 48(11) | ‥ |
| Very good partial response | 71(33) | 54(26) | 125(30) | ‥ |
| Partial response | 66(31) | 62(30) | 128(30) | ‥ |
| Minimal response | 11(5) | 26(13) | 37(9) | ‥ |
| No response/stable disease | 9(4) | 17(8) | 26(6) | ‥ |
| Progressive disease | 8(4) | 5(2) | 13(3) | ‥ |
| Unevaluable | 12(6) | 14(7) | 26(6) |
Data are number (%).
Odds ratio for difference in response 1·85 (80% CI 1·37–2·49).
Figure 3.
Survival, progression, and death during extended follow-up in patients receiving lenalidomide and either high-dose or low-dose dexamethasone
The most common cause of death was progressive disease, which caused 35 (63%) of 56 deaths in the high-dose group and 37 (70%) of 53 deaths in the low-dose group. Other common causes of death were thromboembolic events, which caused five (9%) deaths in the high-dose group and one (2%) in the low-dose group; infection, which caused four (7%) and three (6%), respectively; and cardiac complications, which caused six (11%) and two (4%), respectively (webappendix).
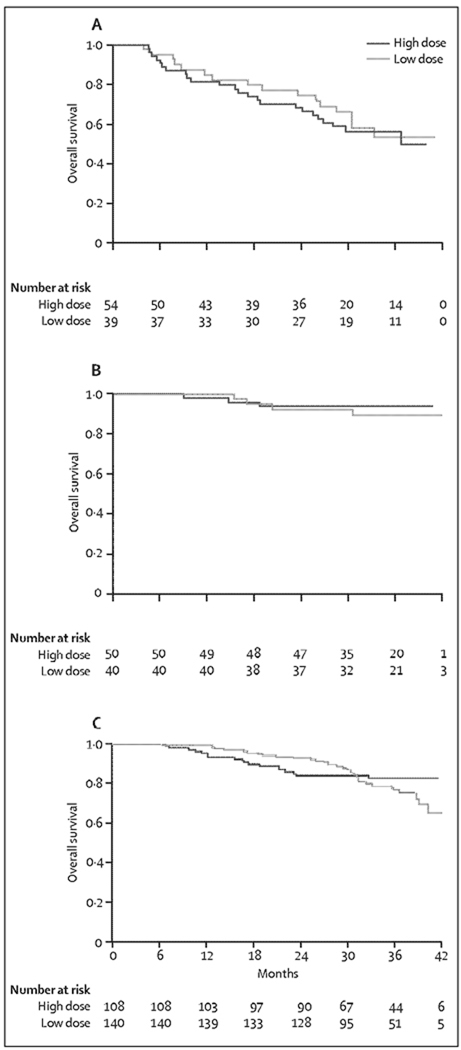
We did landmark analyses to determine the effect of stem-cell transplantation and outcome of patients who continued the primary therapy in either group. Of 431 patients alive at the 4-month landmark analysis point, 183 discontinued from the study, whereas 248 continued primary therapy beyond 4 months. Of the 183 patients who discontinued from the study at 4 months, 93 (median age 69 years, range 38–87) did not pursue stem-cell therapy as recommended by the protocol (group 1); 3-year overall survival in this group was 55% and did not differ between those receiving high-dose and low-dose therapy (log-rank p=0·631; figure 4A). Of the 93 patients, 16 of 54 in the high-dose group and 14 of 39 in the low dose group pursued other treatment options with bortezomib or alkylator-based therapy; the rest stopped therapy at that point and had not received alternative treatment at the time of analysis. 2-year progression-free survival was 25% in both groups. The remaining 90 patients (57 years, 37–53) had autologous stem-cell transplantation and are a cohort of patients who had four cycles of induction with lenalidomide plus dexamethasone followed by transplantation (group 2). 3-year overall survival in this group was 92% and did not differ between treatment groups (log-rank p=0·528; figure 4B). 2-year progression-free survival was 63% with high dose and 65% with low-dose dexamethasone. Among the 431 patients in the landmark analysis, 50 (24%) of 212 patients on high-dose and 40 (18%) of 219 on low-dose dexamethasone received autologous stem-cell transplantation at 4 months.
Figure 4. Landmark analysis of overall survival.
Patients who went off-therapy after four cycles but did not have stem-cell transplants (A). Patients who went off-therapy after four cycles and had stem-cell transplants (B). Patients who continued primary therapy beyond 4 months (C; landmark analysis after 4 months of treatment).
248 patients (median age 66 years, range 35–87) continued on primary therapy beyond 4 months (group 3), 108 in the high-dose dexamethasone group (65 years, 36–87) and 140 in the low-dose dexamethasone group (66 years, 36–84). 3-year overall survival in these 248 patients was 79% (figure 4C). 3-year progression-free survival in this group was 46% with high-dose and 50% with low dose dexamethasone. Of 140 patients who received primary therapy with low-dose dexamethasone (median duration of therapy 11·2 months, range 10·2–12·1), 119 (91%) of 131 eligible patients responsed, 29 (22%) had immunofixation-negative complete response, and 75 (57%) had either complete or very good partial responses. The decision point at 4 months on discontinuing the study and pursuing autologous stem-cell transplantation was made by patients’ choice and physician discretion on the basis of age and other factors including response status and toxicity rate. At 4 months, 15 (18%) of 84 patients in group 1, 29 (33%) of 89 in group 2, and 92 (39%) of 237 in group 3 had complete or very good partial response. Grade 4 or higher toxicity was recorded in 16 (17%) of 92 patients in group 1, four (4%) of 90 group 2, and 17 (7%) of 248 in group 3.
Toxicities were most common with high-dose dexamethasone. Table 4 shows the most common grade 3 or higher adverse events anytime during the course of therapy for the 443 patients assessed for toxicity. 56 (27%) of 223 patients in the high-dose group and 37 (19%) of 222 in the low-dose group discontinued treatment due to adverse events (table 5). 57 (26%) of 223 patients in the high-dose group and 27 (12%) of 220 in the low-dose group had deep-vein thrombosis (p=0·0003); 20 (9%) and nine (4%) of these patients had pulmonary embolism. The incidence of deep-vein thrombosis in patients treated on the protocol after the start of mandatory prophylaxis was unchanged, and might be related to the fact that most patients were already compliant about prophylaxis even before the amendment. Most thromboembolic events occurred in the first 4 months; 45 (20%) of 223 in the high-dose group and 19 (9%) of 220 in the low-dose group had deep-vein thrombosis within the first four treatment cycles.
Table 4.
Major grade 3 or higher toxicity
| High dose (n=223) | Low dose (n=220)* | p value | |
|---|---|---|---|
| Haematological | |||
| Haemoglobin | 18(8) | 15(7) | 0·72 |
| Platelets | 13(6) | 11(5) | 0·83 |
| Neutrophils | 26(12) | 44(20) | 0·02 |
| Non-haematological | |||
| Deep-vein thrombosis or pulmonary embolism | 57(26) | 27(12) | 0·0003 |
| Infection or pneumonia | 35(16) | 20(9) | 0·04 |
| Hyperglycaemia | 25(11) | 14(6) | 0·09 |
| Cardiac ischaemia | 7(3) | 1 | 0·07 |
| Atrial fibrillation or flutter | 6(3) | 1 | 0·12 |
| Fatigue | 33(15) | 20(9) | 0·08 |
| Neuropathy | 5(2) | 4(2) | 0·1 |
| Non-neuropathic weakness | 25(11) | 9(4) | 0·01 |
| Summary | |||
| Any grade 3 or higher in first 4 months | 117(52) | 76(35) | 0·0001 |
| Any grade 3 or higher non-haematological toxicity at anytime during therapy |
146(65) | 106(48) | 0·0002 |
| Any grade 4 or higher non-haematological toxicity at anytime during therapy |
46(21) | 18(14) | 0·0002 |
| Early mortality (first 4 months) | 12(5) | 1 | 0·003 |
Data are number (%).
Data unavailable for two patients.
Table 5.
Reason for discontinuation
| High dose (n=223) |
Low dose (n=222) |
Total (n=445) |
|
|---|---|---|---|
| Treatment completed per protocol | 52(25) | 49(25) | 101(25) |
| Disease progression | 33(16) | 35(18) | 68(17) |
| Adverse events or complications | 56(27) | 37(19) | 93(23) |
| Death on study | 8(4) | 5(3) | 13(3) |
| Patient withdrawal or refusal | 11(5) | 10(5) | 21(5) |
| Alternative therapy | 30(14) | 40(21) | 70(17) |
| Other complicating disease | 2(1) | 1(<1) | 3(1) |
| Other | 17(8) | 16(8) | 33(8) |
Data are number (%).
Discussion
Despite high response rates, the use of high-dose dexamethasone did not result in superior time to progression, progression-free survival, or overall survival compared with low-dose dexamethasone in newly diagnosed myeloma. Overall survival at 1 year was significantly better with low-dose than with high-dose dexamethasone, resulting in early closure of the study and crossover to low-dose dexamethasone. The lack of correlation between response and overall survival has been previously reported in myeloma.12,22 High-dose dexamethasone in a community-setting seems more toxic than low-dose dexamethasone, with more early deaths in the first 4 months, increased risk of thromboembolic complications, and higher overall risk of serious adverse events, particularly in patients older than 65 years. In conjunction with other studies,17,23,24 this study shows the efficacy of lenalidomide plus dexamethasone as initial therapy for myeloma. The response rates observed are better than those reported for thalidomide plus dexamethasone,10 and are achieved with lower toxicity, and with better survival at 3 years.
The cause of inferior overall survival with high-dose dexamethasone seems to be related to increased deaths due to toxicity, particularly in the first 4 months and in elderly patients. Whether additional factors, such as the immunosuppressive effect of high-dose dexamethasone on the immunomodulatory effect of lenalidomide, contribute is unclear. Also serious adverse events associated with high-dose dexamethasone might have had a deleterious effect on the performance status of patients and the ability to tolerate subsequent salvage therapy. With longer follow-up, the survival curves do converge, perhaps showing the effect of crossover.
On landmark analysis, the 3-year overall survival of patients who received four cycles of induction with either dose followed by autologous stem-cell transplantation was 92%, suggesting that lenalidomide plus dexamethasone is a good option for pretransplant induction therapy. This finding also suggests that autologous stem-cell transplantation should remain part of the therapeutic strategy in patients eligible for the procedure, even as new drugs are developed. Although no problems with stem-cell mobilisation were noted, other reports suggest problems with mobilisation with growth factor alone after lenalidomide therapy, and that chemomobilisation might be needed.25The landmark analysis also showed that the overall survival of patients taking primary therapy with low-dose dexamethasone is similar to that in the original intention-to-treat analysis, and responses match results published previously by the Mayo Clinic.23 Thus, low-dose dexamethasone seems to be an effective front-line regimen for myeloma, particularly in elderly patients, given the 3-year overall survival of 68% and good tolerability.
Deep-vein thrombosisis is a major concern with lenalidomide-based combinations. However, the rate of this complication was low in the low-dose group compared with that in the high-dose group. The International Myeloma Working Group has provided detailed guidelines on the appropriate thromboprophylaxis for patients receiving therapy with lenalidomide or thalidomide.26 All patients receiving these agents should be on routine thromboprophylaxis.
There are some important limitations of the study. First, because the dose of dexamethasone was the main study question, the trial was designed to not lower the dose of dexamethasone after four cycles in the high-dose group. The inferior overall survival with high-dose therapy might therefore have occurred because patients received inappropriately high-dose steroids beyond the first four cycles. The higher early mortality in the first 4 months with high-dose dexamethasone suggests that even short courses carry significant risk. Second, the study did not mandate thromboprophylaxis or antibiotic prophylaxis and this could have contributed to the higher treatment-related mortality in the high-dose group. High-dose dexamethasone might, therefore, be safe for patients less than 65 years of age with appropriate prophylaxis, and in centres that have experience with this regimen. Third, the inferior survival outcome with high-dose dexamethasone was greatest in patients 65 years and older, and this regimen might be safe in patients less than 65 years of age, who might benefit from the greater response rates with high doses. These possibilities require further study. Fourth, the trial was designed as an induction trial, since it was expected that patients would proceed to autologous stem-cell transplantation after four cycles of induction. Thus, accurate determination of the efficacy and safety of long-term primary therapy with low-dose dexamethasone is difficult, and the trial by itself does not establish the regimen as a new standard of care and needs to be compared with other active regimens, such as bortezomib plus dexamethasone. Furthermore, there are limited data on the efficacy of stem-cell transplantation as salvage therapy after long-term primary therapy with low-dose dexamethasone. More randomised trials are therefore needed to address these questions. Finally, because the trial was designed before routine use of cytogenetics, we cannot assess the effect of cytogenetic abnormalities. However, we have recently shown that the adverse effect of high-risk cytogenetic abnormalities on progression-free survival is not overcome by lenalidomide therapy.27
The role of high-dose dexamethasone in combination with thalidomide or other drags in myeloma is not addressed by this trial and needs further study. The trial also does not address the role of high-dose dexamethasone in relapsed or refractory myeloma. Preliminary analysis of pivotal studies with lenalidomide plus dexamethasone in relapsed myeloma show that patients whose dexamethasone dose was reduced because of toxicity had a better outcome compared with patients who continued on high-dose dexamethasone.28 High-dose dexamethasone might still have a role in the treatment of patients with acute renal failure caused by myeloma cast nephropathy, cord compression from myeloma, or aggressive refractory disease.
This trial in conjunction with other similar studies that show activity of lenalidomide,17,23,24 shows that low-dose dexamethasone in conjunction with lenalidomide is an active regimen for newly diagnosed myeloma with acceptable toxicity and low early mortality.
Acknowledgments
This study was funded and sponsored by the US National Cancer Institute (NCI). This study was coordinated by the Eastern Cooperative Oncology Group (Chair Robert I, Comis) and supported by Public Health Service Grants CA23318, CA66636, CA21115, CA13650, and CA93842 from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Funding National Cancer Institute, Rockville, MD, USA.
Footnotes
Contributors
SVR served as principal investigator, was involved in the original idea and design of the study, writing of the protocol, recruitment, and data analysis and interpretation. SJ was involved in data analysis. NSC, RF, DHV, MEW, RA, DSS, and PRG were involved in the study design, recruitment, and data interpretation. MK was involved in the original idea and design of the study. All authors reviewed and approved the paper.
Conflicts of interest
DHV has served on the speaker’s bureau for Millennium, Celgene, and Ortho-Biotech and on advisory boards for Celgene and Amgen. MEW has received research support from Celgene. RA has received research support and honoraria from Celgene, Millennium, and Novartis. RF has served as a consultant for Amgen, Halozyme, Otsuka, BMS, and Genzyme, has received research funding from Cylene and Pfizer, and has received honoraria from Celgene and Millennium. NSC has received research support from Millennium. The other authors declared no conflicts of interest.
Contributor Information
Prof SV Rajkumar, Mayo Clinic, Rochester, Minnesota, USA.
Prof P R Greipp, Mayo Clinic, Rochester, Minnesota, USA.
S Jacobus, Dana Farber Cancer Institute, Boston, MA, USA.
N S Callander, University of Wisconsin, Madison, WI, USA.
Prof R Fonseca, Mayo Clinic Arizona, Scottsdale, AZ, USA.
Prof DH Vesole, St Vincent’s Hospital, New York, NY, USA.
Prof ME Williams, University of Virginia Health System, Charlottesville, VA, USA.
R Abonour, Indiana University Simon Cancer Center, Indianapolis, IN, USA.
Prof DS Siegel, Hackensack University Medical Center, Hackensack, NJ, USA.
M Katz, International Myeloma Foundation, North Hollywood, CA, USA.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myćlomc. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 3.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoictic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 4.Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 5.Singhal S, Mehta J, Desikan R, et al. Antirumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 6.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 7.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlogie B, Smith L, Alexanian R. Effective treatment of advanced multiple myeloma refractory to alkylating agents. N Engl J Med. 1984;310:1353–1356. doi: 10.1056/NEJM198405243102104. [DOI] [PubMed] [Google Scholar]
- 9.Alexanian R, Dimopoulos MA, Delasalle K, Barlogie B. Primary dexamethasone treatment of multiple myeloma. Blood. 1992;80:887–890. [PubMed] [Google Scholar]
- 10.Rajkumar SV, Blood E, Vesole DH, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 11.Cavo M, Zamagni E, Tosi P, et al. Superiority of thalidomide and dexamethasone over vincristine-doxorubicindexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood. 2005;106:35–39. doi: 10.1182/blood-2005-02-0522. [DOI] [PubMed] [Google Scholar]
- 12.Facon T, Mary J-Y, Pegourie B, et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood. 2006;107:1292–1298. doi: 10.1182/blood-2005-04-1588. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Rosiñol L, Hussein M, et al. A multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone versus dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26:2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 15.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zonder JA, Crowley J, Hussein MA, et al. Superiority of lenalidomide (Len) plus high-dose dexamethasone (HD) compared to HD alone as treatment of newly-diagnosed multiple myeloma (NDMM): results of the randomized, double-blinded, placebo-controlled SWOG trial S0232. ASH Ann Meeting Abstr. 2007;110:A77. [Google Scholar]
- 18.Rajkumar SV, Blood E. Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med. 2006;354:2080. [PubMed] [Google Scholar]
- 19.Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 20.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Myeloma Trialists’ Collaborative Group. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6633 patients from 27 randomized trials. J Clin Oncol. 1998;16:3832–3842. doi: 10.1200/JCO.1998.16.12.3832. [DOI] [PubMed] [Google Scholar]
- 23.Lacy MQ, Gertz MA, Dispenzieri AA, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82:1179–1184. doi: 10.4065/82.10.1179. [DOI] [PubMed] [Google Scholar]
- 24.Niesvizky R, Jayabalan DS, Christos PJ, et al. BiRD (Biaxin(R) [clarithromycin]/Revlimid(R)[lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2007;111:1101–1109. doi: 10.1182/blood-2007-05-090258. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2032. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 26.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–423. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 27.Kapoor P, Kumar S, Fonseca R, et al. Impact of risk stratification on outcome among patients with multiple myeloma receiving initial therapy with lenalidomide and dexamethasone. Blood. 2009;114:518–521. doi: 10.1182/blood-2009-01-202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.San Miguel JF, Dimopoulos M, Weber D, et al. Dexamethasone dose adjustments seem to result in better efficacy and improved tolerability in patients with relapsed/refractory multiple myeloma who are treated with lenalidomide/dexamethasone (MM009/010 sub-analysis) ASH Ann Meeting Abstr. 2007;110 A2712 (abstr) [Google Scholar]