Abstract
Object
Intracranial monitoring for temporal lobe seizure localization to differentiate neocortical from mesial temporal onset seizures requires both neocortical subdural grids and hippocampal depth electrode implantation. There are 2 basic techniques for hippocampal depth electrode implantation. This first technique uses a stereotactically guided 8-contact depth electrode directed along the long axis of the hippocampus to the amygdala via an occipital bur hole. The second technique involves direct placement of 2 or 3 4-contact depth electrodes perpendicular to the temporal lobe through the middle temporal gyrus and overlying subdural grid. The purpose of this study was to determine whether one technique was superior to the other by examining monitoring success and complications.
Methods
Between 1997 and 2005, 41 patients underwent invasive seizure monitoring with both temporal subdural grids and depth electrodes placed in 2 ways. Patients in Group A underwent the first technique, and patients in Group B underwent the second technique.
Results
Group A consisted of 26 patients and Group B 15 patients. There were no statistically significant differences between Groups A and B regarding demographics, monitoring duration, seizure localization, or outcome (Engel classification). There was a statistically significant difference at the point in time at which these techniques were used: Group A represented more patients earlier in the series than Group B (p < 0.05). The complication rate attributable to the grids and depth electrodes was 0% in each group. It was more likely that the depth electrodes were placed through the grid if there was a prior resection and the patient was undergoing a new evaluation (p < 0.05). Furthermore, Group A procedures took significantly longer than Group B procedures.
Conclusions
In this patient series, there was no difference in efficacy of monitoring, complications, or outcome between hippocampal depth electrodes placed laterally through temporal grids or using an occipital bur hole stereotactic approach. Placement of the depth electrodes perpendicularly through the grids and middle temporal gyrus is technically more practical because multiple head positions and redraping are unnecessary, resulting in shorter operative times with comparable results.
Keywords: epilepsy surgery, subdural grid electrode, complication, depth electrode, electroencephalography
Temporal lobe epilepsy is the most common form of surgically remediable epilepsy.18 As surgical therapies for medically intractable epilepsies have evolved, so have the clinical definitions of these syndromes.12 In no place is this more clearly evident than the temporal lobe. As surgical therapies for mesial TLE applied generally to all TLE failed, it became evident that approximately 10% of all TLE cases are of neocortical origin, and outside the region resected in a standard anterior temporal lobectomy.7–16 Moreover, it is also apparent that even in lesional cases, surgical outcome in neocortical TLE is inferior to mesial temporal lobe treatments.1,18 Furthermore, the presence of eloquent cortex in the lateral neocortex often favors less aggressive resections in the dominant temporal lobe.6,19 In these difficult situations, it is often intracranial monitoring that guides mesial versus neocortical resection.6,8 This issue is particularly relevant to patients with normal MR imaging results in whom surgical outcomes are significantly less successful than those with lesional TLE. In addition, for patients with normal MR imaging and TLE originating from the dominant temporal lobe, a selected resection of neocortex or mesial temporal structures may yield better cognitive and memory outcomes.14
Intracranial monitoring for temporal lobe seizure localization may require both neocortical subdural grids and hippocampal depth electrode implantation when the case is not clearly mesiotemporal. Subdural grid and strip electrodes are required to obtain adequate coverage of the lateral temporal neocortex for neocortical seizure localization and functional mapping of language. There are 2 basic techniques for hippocampal depth electrode implantation. In the first (Technique A), a stereotactically frame-guided depth electrode is directed along the long axis of the hippocampus to the amygdala via an occipital bur hole. The second (Technique B) involves direct placement of short depth electrodes perpendicular to the temporal lobe via frameless stereotaxis through the middle temporal gyrus and overlying subdural grid, which has been proven safe and accurate by Murphy et al.15 Mehta et al.13 have shown that lateral temporal placement may be more accurate than occipital placement. The purpose of this study was to test the hypothesis that for cases of possible neocortical TLE, depth electrodes through the craniotomy site (Technique B) demonstrate equivalent outcomes and complications to occipital depth electrodes and a separate craniotomy (Technique A).
Methods
Patient Population
All adult and pediatric patients undergoing neocortical temporal grid electrode coverage and mesial temporal depth electrode insertion were identified from our epilepsy surgery database. Between 1997 and 2005, 41 patients underwent invasive seizure monitoring with both temporal neocortical coverage (subdural grid electrodes) and medial coverage (depth electrodes). All patients underwent a temporal craniotomy for placement of a sub-temporal neocortical grid and strips. All patients had medial temporal depth electrodes placed for recording from the mesial temporal structures, and were divided into 2 groups: Group A, which underwent Technique A, and Group B, which underwent Technique B (techniques are described below). This study excluded patients who underwent hippocampal depth electrode placement without simultaneous grid implantation.
Data Collection
All charts were retrospectively reviewed. Data pertaining to technique of insertion, operative time, blood loss, duration of monitoring, complications, number of electrodes, and outcome were recorded.
Presurgical Workup
All patients were referred for invasive monitoring after extensive Phase I noninvasive evaluations. Prior to electrode implantation, patients had undergone noninvasive techniques to localize the epileptogenic region that included the following: outpatient scalp EEG, inpatient prolonged video EEG monitoring to record habitual seizures, structural neuroimaging (MR imaging), functional neuroimaging (subtraction ictal to interictal SPECT coregistered to MR imaging), PET, and neuropsychological testing. Magnetic resonance imaging was performed in all patients with either 1.5-T, or later, 3-T magnets according to the epilepsy protocol as described by Jack.5 These studies and clinical history were presented at our multidisciplinary epilepsy surgical conference, with neurologists, neuroradiologists, and neurosurgeons present to discuss surgical options and approaches.
Surgical Method
Subdural Grid Insertion
Subdural grid insertion was performed under general anesthesia; a craniotomy and dural opening were performed in all cases. Placement of electrodes was guided by noninvasive testing, according to the recommendations of the attendants at the epilepsy surgery conference. All grids, strips, and depth electrodes were manufactured by Adtech Medical Instrument Corporation. The electrode cables were tunneled away from the incision to the skin in separate stab incisions per lead, and the dura was reapproximated. Postoperatively, the patients were monitored in the intensive care unit with continuous video and intracranial EEG. Postoperative spiral CT was performed to confirm electrode locations and rule out occult hemorrhage in all cases. Prophylactic antibiotics, either cefazolin or vancomycin, were given as scheduled intravenous dosing while grids were in place and for 3 doses after removal. Head dressings were applied postoperatively and were left in place throughout monitoring. Anticonvulsants were tapered, with variable rates of taper depending on the severity of seizures and the anticonvulsant. When monitoring failed to reveal the ictal onset zone or seizure onsets colocalized with eloquent function (such as language), the patient underwent grid removal, wound irrigation, and primary closure without resection.
Depth Electrode Insertion Technique
For the patients in Group A, after receiving a general anesthetic and prior to craniotomy and subdural grid placement as described above, the patients were placed in a COMPASS frame (COMPASS International Inc.).9 Patients then underwent MR imaging for data acquisition. Data was then registered and the trajectory planned by choosing a 2-point target consisting of the center of the amygdala and the long axis of the hippocampus. Upon return to the operative theater, the patient was positioned prone, and the phantom arc was used to plan the incision. After sterile preparation and draping of the patient, a stab incision was made and twist-drill craniotomy was performed. An 8-contact depth electrode (10 mm between contacts) was then passed along the long axis of the hippocampus again (with the amygdala as the target) to a preset depth on the side ipsilateral to the craniotomy and grid placement; this occurred in all 26 patients. The 8-contact depth electrode was then secured to the scalp and connected to the data collection system. This process was repeated on the opposite side for bilateral depth electrode implantation, which occurred in 22 patients (Fig. 1).
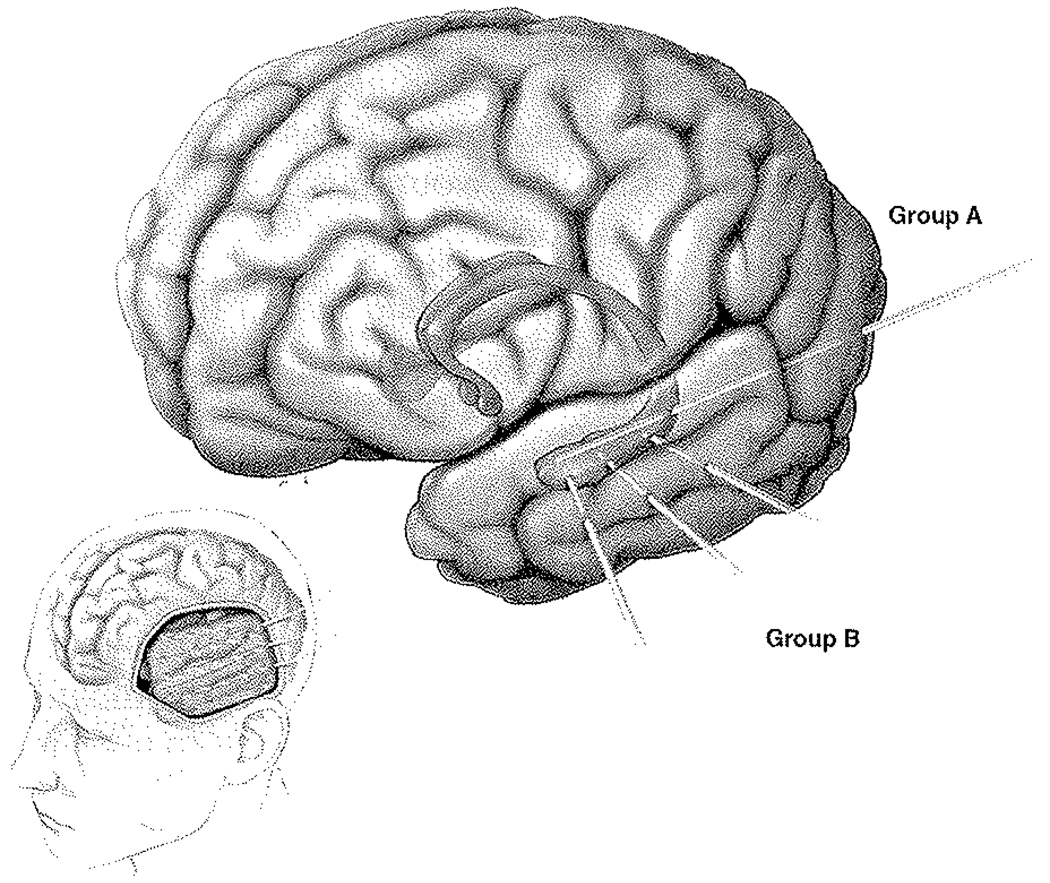
FIG. 1.
Graphic illustration of depth electrode insertion. Computerized lateral view of the left cerebral hemisphere (lower left) shows typical exposure and grid placement for temporal and frontal lobe coverage. Larger cerebrum in isolation (upper right) shows a shadow illustration of the deep amygdala and hippocampus. The 2 different techniques are illustrated: Group A, consisting of the typical occipital insertion and longitudinal depth electrode coverage of the hippocampus; and Group B, composed of the orthogonally directed depth electrodes typically inserted through the grid.
For the patients in Group B, after the patient was placed under general anesthesia, the head was secured to the Mayfield pinion and positioned for craniotomy. The Stealth Station (Medtronic) fiducials were digitized and registered. A craniotomy was performed as described above. After grid implantation, the Stealth wand was used to plan the trajectories of the 4-contact depth electrodes (5-mm spacing between contacts) into the amygdala and hippocampus. In 15 patients, 4-contact depth electrodes were placed into the amygdala and the head of the hippocampus. In an additional 4 patients, an extra 4-contact depth electrode was placed into the body of the hippocampus (Fig. 1). A stab incision was made in the planned entry point through the silastic grid, the pia was coagulated, and a separate stab incision made. Using the predetermined targets, the depth of insertion was measured. The depths were individually measured by hand at the back table (sterile surgical table) and a Steri-Strip used to mark the depth electrode and to prevent it from over-insertion beyond the target site. These depth electrodes were secured to the dura at closure and to the skin after skin closure. Typically, the depth electrodes were inserted into the amygdala (15 patients), middle of the head of the hippocampus (15 patients), and posteriorly in the body of the hippocampus itself (4 patients). The depth electrodes and grid wires were then tunneled out through the scalp separately and connected. After functionality was confirmed, the dura was then closed primarily with the aid of a small bovine pericranial graft. The craniotomy was then closed in a typical fashion.
Outcome Assessment
Outcome was assessed using modified guidelines recommended by Engel et al.4 and our previous publications.21 This classification system is summarized in Table 1. We grouped modified Engel Classes I and II together to represent a “favorable” outcome category, and Engel Classes III and IV as an “unfavorable” outcome category, as we have in previous publications.20,21 The outcome classes “favorable” and “unfavorable” used here correspond to International League Against Epilepsy (ILAE) classifications 1–4 and 5–6, respectively.22
TABLE 1.
Classification system used to define outcome*
| Class | Characteristics |
|---|---|
| I | free of disabling seizures |
| IA | seizure free |
| IB | nondisabling simple partial seizures only |
| IC | some disabling seizures after surgery, but free of disabling seizures for <2 yrs |
| ID | generalized convulsions w/ AED discontinuation |
| II | rare disabling seizures |
| IIA | initially free of disabling seizures but has rare seizures now |
| IIB | rare disabling seizures since op |
| IIC | rare seizures for the last 2 yrs |
| IID | nocturnal seizures only |
| III | worthwhile improvement, defined as >50% reduction in seizures to 90% reduction in disabling seizures |
| IV | no worthwhile improvement |
Statistical Analysis
The statistical software JMP version 6.0 (SAS Institute Inc.) was used for statistical analysis. Univariate associations with complications and outcomes were assessed using chi-square or Fisher exact tests as appropriate. Where continuous variables were reported, ANOVA was used. Statistical significance was set at p < 0.05. Our study was approved by the Mayo Clinic’s Institutional Review Board, and patients gave consent to participate.
Results
Patient Demographics
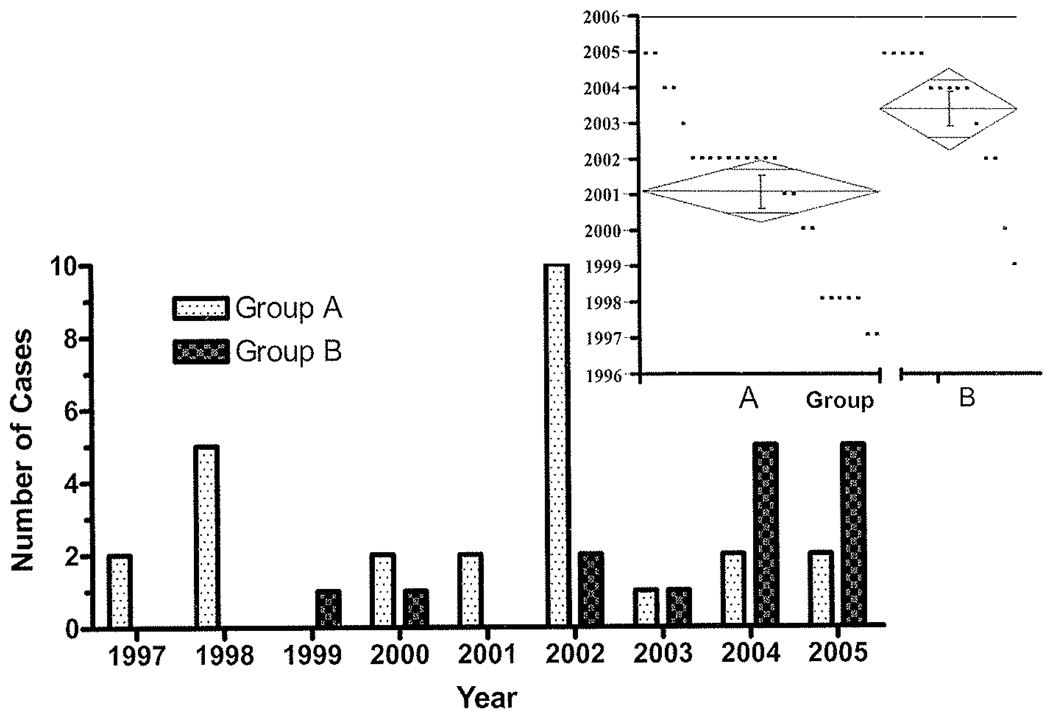
Between July 1997 and December 2005, there were 41 sessions of intracranial monitoring using neocortical temporal grid electrodes and mesial temporal depth electrode placement. Overall, there were 193 grid monitoring sessions during this period.20,21 Over that period of time, approximately 400 epilepsy surgical cases for either therapeutic or diagnostic purposes were performed excluding vagus nerve stimulator implantation. Figure 2 illustrates the case distribution timeline with respect to the technique used. It is clear that Technique B occurred more frequently later in our study compared with Technique A. A 1-way ANOVA of Group A compared with Group B was performed. Group A cases (26) occurred primarily in 2001 (95% CI 2000–2002), and Group B cases in 2003 (95% CI 2002–2004; p = 0.0022). Demographic information is represented in Table 2. The average age of the study population was 33 ± 12 years (range 8–62 years). The average age of the patients in Group A was 35 ± 14 years and in Group B was 31 ± 8 years, a statistically nonsignificant difference. There were 20 females and 21 males.
FIG. 2.
Bar graph showing the distribution of cases according to operative date. The Group B patients occur more frequently later in the study than Group A patients. The inset demonstrates results of the 1-way ANOVA of Group A (left) compared with Group B (right). Individual points within the graph represent individual cases according to year. The diamond shape represents the mean (center line) and the points of the diamond represent the 95% CIs. The 26 Group A cases occurred primarily in 2001 (95% CI 2000–2002), and the Group B cases in 2003 (95% CI 2002–2004), a statistically significant difference (p = 0.0022).
TABLE 2.
Characteristics of 41 craniotomies with subdural grid monitoring and depth electrode implantation for seizure localization*
| Depth Electrode Placement |
||||
|---|---|---|---|---|
| Characteristic | Total | Group A | Group B | p Value† |
| no. of patients | 41 | 26 | 15 | |
| mean age in yrs (range) | 33 ± 12 (8 –62) | 35 ± 14 | 31 ± 8 | NS |
| no. of children (≤18 yrs) | 3 (7%) | 2 | 1 | NS |
| M/F | 21:20 | 16:10 | 5:10 | NS |
| electrode coverage | ||||
| rt side | 26 (63%) | 16 | 10 | NS |
| It side | 15 (37%) | 10 | 5 | NS |
| lobe | ||||
| frontal | 23 (56%) | 13 | 10 | NS |
| temporal | 41 (100%) | 26 | 15 | NS |
| subtemporal | 39 (95%) | 25 | 14 | NS |
| parietal | 11 (27%) | 7 | 4 | NS |
| implants | ||||
| mean no. of grids (range) | 1.1 ± 0.4 (1–2) | 1.2 ± 0.5 | 1.1 ± 0.4 | NS |
| mean no. of strips (range) | 1.5 ± 1.8 (0–6) | 0.7 ± 1.1 | 2.9 ± 2.1 | <0.01 |
| mean no. of cables (range) | 8.2 ± 3.1 (4–18) | 7.6 ± 2.8 | 9.3 ± 3.2 | NS |
| mean no. of electrodes (range) | 55 ± 23 (32–144) | 51 ± 22 | 61 ± 25 | NS |
| mean op time (min) | 297 ± 18 | 327 ± 20 | 227 ± 28 | <0.01 |
| mean blood loss (ml) | 253 ± 44 | 258 ± 62 | 248 ± 65 | NS |
Mean values are presented ± SDs.
Abbreviation: NS = nonsignificant.
Chi-square analysis.
Surgical Results
A craniotomy was performed in all patients; 26 patients were implanted with grids on the right side, and 15 on the left side. Of these 41 patients, Group A consisted of 26 patients, and Group B 15 patients. All patients had depth electrode coverage of the hippocampus as confirmed on postoperative CT, and there were no misplacements. All patients had temporal lobe coverage; additional coverage is detailed in Table 2. Overall and group implantation numbers are further detailed in Table 2. There was a statistically significant difference in the number of strips placed in Group B patients (2.9 ± 2.1 strips) compared with Group A patients (0.7 ± 1.1 strips; p < 0.01). It was more likely that the depth electrodes were placed through the grid if the patient had undergone a prior resection and was undergoing a new evaluation (p < 0.05). Furthermore, operative time was significantly longer (by approximately 1 hour and 40 minutes) in those patients undergoing Technique A (Group A 327 ± 20 minutes vs 227 ± 28 minutes in Group B; p < 0.01). Blood loss was similar between the 2 groups. Hospitalization lasted an average of 12 ± 5 days (range 5–25 days).
Invasive Monitoring
Results of the invasive EEG monitoring are summarized in Table 3. There were 11 patients who had undergone prior resective surgery for seizures—4 in Group A and 7 in Group B, a statistically significant difference (p = 0.03). Identification of the seizure focus was accomplished in 32 patients (78%), and 27 of these patients underwent resection of this focus. Identification of a focal epileptogenic focus occurred in 20 patients in Group A and 12 in Group B; of these patients, 16 (Group A) and 11 (Group B) underwent resection. Five patients did not undergo resection due to localization of the seizure onset zone arising from an area with functional (eloquent) brain. Postoperative head CT was performed in all patients. Duration of monitoring was 8 ± 4 days overall; the group durations were similar.
TABLE 3.
Results of invasive EEG monitoring sessions using depth electrodes and grids*
| Depth Electrode Placement |
||||
|---|---|---|---|---|
| Characteristic | Total | Group A | Group B | p Value † |
| no. of patients | 41 | 26 | 15 | |
| mean hospitalization days (range) | 12 ± 5 (5–25) | 12 ± 5 | 11 ± 5 | NS |
| mean days of monitoring (range) | 8 ± 4 (2–24) | 9 ± 4 | 8 ± 5 | NS |
| no. w/fever (≥38.4°C) | 3 (7%) | 2 | 1 | NS |
| no. w/ leukocytosis (>10.5 WBCs) | 11 (27%) | 7 | 4 | NS |
| no. receiving periop corticosteroids | 20 (49%) | 11 | 9 | NS |
| mean follow-up (mos) | 49 ± 5 | 58 ± 6 | 32 ± 7 | <0.01 |
| no. w/ focal identification of epileptic focus | 32 (78%) | 20 | 12 | NS |
| no. w/ resection of epileptic focus | 27 (66%) | 16 | 11 | NS |
| no. w/ prior resective op for epilepsy | 11 (27%) | 4 | 7 | 0.03 |
Mean values are presented ± SDs.
Abbreviation: WBCs = white blood cells.
Chi-square analysis.
Patient Complications
The complication rate attributable to the difference in techniques was 0%. Within Groups A and B, 2 patients experienced complications, neither resulting in permanent morbidity. One patient in Group A experienced a coagulase negative superficial wound infection postoperatively that resolved with antibiotic treatment, and the craniotomy flap was not compromised. One patient in Group A experienced status epilepticus during monitoring, defined as a persistent seizure longer than 5 minutes. This seizure resolved with medical treatment without pervasive deficit. These complications were believed to be independent of the depth electrode technique.
Postresection Seizure Outcomes
Twenty-seven (66%) of 41 patients underwent resection. Table 4 details the outcome of patients after resection with respect to Engel Class. Overall, 16 patients (59%) were seizure free after resection (Engel Class I), and an additional 5 patients (19%) demonstrated a significant reduction in seizures (Engel Class II).4 In Group A, 56% of the patients were designated Engel Class I with 69% showing a good outcome (Engel Class I or II), whereas in Group B 64% were designated Engel Class I with 91% demonstrating a good outcome. Although there appears to be better seizure control using Technique B (91% with good outcome), the small sample size did not allow statistical significance. Only 22% experienced no significant reduction in seizure frequency or severity (Engel Class IV).4 Mean overall follow-up duration was 49 ± 5 months—58 ± 6 months for patients in Group A and 32 ± 7 months for those in Group B, a statistically significant difference (p < 0.01).
TABLE 4.
Postresection seizure outcomes*
| Depth Electrode Placement |
|||
|---|---|---|---|
| Seizure Frequency† | Total | Group A | Group B |
| Class I (seizure free or aura only) | 16 (59) | 9 (56) | 7 (64) |
| Class II (significant improvement) | 5 (19) | 2 (13) | 3 (27) |
| Classes I & II combined (good outcome) | 21 (78) | 11 (69) | 10 (91) |
| Class III (improvement) | 0 | 0 | 0 |
| Class IV (no significant reduction) | 6 (22) | 5 (31) | 1 (9) |
| Classes III & IV combined (poor outcome) | 6 (22) | 5 (31) | 1 (9) |
All data presented as number of patients (%).
None of the Engel Class differences were significant between groups using chi-square analysis.
Discussion
Neocortical TLE is a surgically remediable disorder if the region of seizure onset can be identified and safely resected.11 In comparison with medial TLE, surgical outcomes in neocortical TLE are less favorable, even in cases in which a lesion exists.11,18 Therefore, it is imperative in circumstances in which a neocortical seizure onset is suspected that the seizure origin is investigated thoroughly. In many cases this investigation requires intracranial EEG. Intracranial investigation becomes additionally important in cases of dual pathology, in which there may be both mesial and neocortical structural or microscopic pathology.2 It has been demonstrated that investigation using intracranial EEG can improve results with neocortical TLE—approaching results noted in mesial TLE—with a 67% seizure-free rate and 97% good outcomes (Engel Class I or II) after resection of the ictal onset zone determined by intracranial EEG and a tailored temporal corticectomy.8 Our study revealed an approximately 60% seizure-free rate and a good outcome in nearly 80%; however, Group B patients had a 64% seizure-free rate and 91% rate of good outcome, similar to results published by Jung et al.8
The first description of the use of mesial temporal depth electrodes was published in 1963 by Crandall et al.3 In this study, the electrodes were placed in a similar manner to Technique B using the Talairach atlas and plain radiograph pneumoencephalograms to guide depth electrode placement. Later, Kelly and colleagues9,10 introduced longitudinally placed depth electrodes via a medial occipital bur hole. In either circumstance there are theoretical differences that would cause one to favor a certain technique over the other. For instance, orthogonally placed electrodes (Technique B) are more accurate and provide some limited neocortical coverage, but using this technique has been criticized for less hippocampal sampling, which may be overcome by inserting more than 1 depth electrode.13 It should be noted that the orthogonally placed depth electrodes yield very limited coverage of the neocortex, which is inadequate for stimulation mapping for language. Medial temporal depth electrodes (Techniques A and B) combined with subdural grid and strip electrodes for detailed mesial and neocortical temporal lobe coverage have never been directly compared. It has become apparent that frameless systems are seemingly similar to frame-based systems in terms of accuracy of either placement of the depth electrodes.13,15 Our study provides data supporting that the 2 techniques are equivalent in a small group of patients in terms of complications and outcome. However, there was a significant difference in total operative time. In addition, concordant with the dominance of frameless systems, there was a shift in preference at our institution from Technique A to B during the course of this study.
Regarding technique, it is notable that when placing orthogonal depth electrodes through a grid, some shift can occasionally occur when the skin flap is closed. Our practice has been to place the bone flap in sterile cold storage to prevent potential mass effect and cortical compression from the grids. The orthogonal depth electrodes are measured based on stereotaxis, and then marked with a Steri-Strip “tail.” When the depth electrodes are passed through the grid, this tail helps prevent further insertion of the depth electrode into the brain when the skin flap is closed. Additional sutures are used to secure the depth electrodes along the margin of the grid, dura, and skin. Despite these precautions, the depth electrodes can occasionally be pushed in by the skin flap closure. Placement of several 4-contact subtemporal strips will offer some additional monitoring of the mesial temporal lobe structures in case a shift of the orthogonal depth electrodes occurs. However, in this series there were no complications attributable to depth electrode placement with either technique.
One interesting aspect to our case series is the significant difference in time that patients experience with Technique A. The surgical times reported did not include anesthesia time for induction, frame placement, and MR imaging. This significantly increased total time under anesthesia, but this exact time was elusive in the charts and was not reported due to inherent inaccuracies. The difficulty with frame-based passage of depth electrodes and repositioning has also been described by Mehta et al.13 and Murphy et al.;15 thus, for this reason both groups have advocated frameless techniques. The implications of this finding are obvious: fewer complications in the patient from reduced anesthesia times and less operative cost if depth electrodes are placed laterally through the craniotomy site.
One of the only significant differences between Groups A and B was the difference in previous resective surgery. This previous surgery was more common in Group B patients. This finding is important because repeat resections are notorious for difficult-to-treat epilepsies. Siegel et al.17 reported a 39% seizure-free outcome after repeat resection in the largest series of its kind in 64 patients. Despite this difficult-to-treat group, with 7 of the 15 patients in Group B in this category, the results were comparable to Group A. If reoperated patients are excluded, Group A would have 10 patients with good outcome and 3 with poor outcomes, and Group B patients would have 6 patients with good out-comes and none with poor outcomes, a statistically nonsignificant difference.
Our current approach for patients with medically resistant TLE who require intracranial EEG is that patients in whom the lateralization of temporal lobe seizures is not clear from Phase I monitoring and are suspected to have medial TLE undergo bilateral intracranial depth electrode implantation via occipital bur holes. These patients are not included in the study reported here. However, this approach does not differentiate mesial temporal from neocortical onset seizures. In particular, for neocortical seizures originating from the dominant temporal lobe where good coverage of the temporal neocortex is required for localization and stimulation mapping, a craniotomy and subdural electrodes are required. If the lateralization of seizure onset is established adequately during Phase I evaluation and the clinical question is medial versus lateral neocortical temporal localization, we use Technique B combined with subdural grids and strips due to the reduced operative times. However, Technique A appears to be comparable in terms of efficacy and outcome.
Conclusions
There is no difference in efficacy of monitoring, complications, or outcome between hippocampal depth electrodes placed laterally through lateral temporal subdural grids (Group B) or depth electrodes placed stereotactically via an occipital bur hole (Group A) in this small series. If lateralization of TLE is not in question, then placement of the depth electrodes perpendicularly through the subdural grid and middle temporal gyrus is technically more practical because multiple head positions and redraping are unnecessary and it is quicker in terms of operative time. Furthermore, this technique has been proven to be more accurate by other authors.13
Acknowledgments
The authors thank Michael King, M.F.A., for his artwork expertise (Figure 1); Steven D. Thalacker, R.N., and Bruce A. Kall for data support and database preparation; and Dr. Corey Raffel for his surgical care of a portion of these patients within this series.
Abbreviations used in this paper
- EEG
electroencephalography
- TLE
temporal lobe epilepsy
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: JJ Van Gompel, FB Meyer, WR Marsh, KH Lee, GA Worrell. Acquisition of data: JJ Van Gompel, FB Meyer, GA Worrell. Analysis and interpretation of data: JJ Van Gompel, FB Meyer, WR Marsh, KH Lee, GA Worrell. Drafting the article: JJ Van Gompel, FB Meyer, WR Marsh, KH Lee, GA Worrell. Critically revising the article: JJ Van Gompel, FB Meyer, WR Marsh, KH Lee, GA Worrell. Reviewed final version of the manuscript and approved it for submission: JJ Van Gompel, FB Meyer, WR Marsh, KH Lee, GA Worrell. Statistical analysis: JJ Van Gompel. Administrative/technical/material support: JJ Van Gompel, GA Worrell. Study supervision: JJ Van Gompel, GA Worrell.
References
- 1.Burgerman RS, Sperling MR, French JA, Saykin AJ, O’Connor MJ. Comparison of mesial versus neocortical onset temporal lobe seizures: neurodiagnostic findings and surgical outcome. Epilepsia. 1995;36:662–670. doi: 10.1111/j.1528-1157.1995.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 2.Cascino GD, Jack CR, Jr, Parisi JE, Sharbrough FW, Schreiber CP, Kelly PJ, et al. Operative strategy in patients with MRI-identified dual pathology and temporal lobe epilepsy. Epilepsy Res. 1993;14:175–182. doi: 10.1016/0920-1211(93)90022-y. [DOI] [PubMed] [Google Scholar]
- 3.Crandall PH, Walter RD, Rand RW. Clinical applications of studies on stereotactically implanted electrodes in temporal-lobe epilepsy. J Neurosurg. 1963;20:827–840. doi: 10.3171/jns.1963.20.10.0827. [DOI] [PubMed] [Google Scholar]
- 4.Engel J, Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. ed 2. New York: Raven Press; 1993. pp. 609–621. [Google Scholar]
- 5.Jack CR., Jr Magnetic resonance imaging in epilepsy. Mayo Clin Proc. 1996;71:695–711. doi: 10.1016/S0025-6196(11)63008-5. [DOI] [PubMed] [Google Scholar]
- 6.Janszky J, Pannek HW, Fogarasi A, Bone B, Schulz R, Behne F, et al. Prognostic factors for surgery of neocortical temporal lobe epilepsy. Seizure. 2006;15:125–132. doi: 10.1016/j.seizure.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Janszky J, Pannek HW, Janszky I, Schulz R, Behne F, Hoppe M, et al. Failed surgery for temporal lobe epilepsy: predictors of long-term seizure-free course. Epilepsy Res. 2005;64:35–44. doi: 10.1016/j.eplepsyres.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Jung WY, Pacia SV, Devinsky R. Neocortical temporal lobe epilepsy: intracranial EEG features and surgical outcome. J Clin Neurophysiol. 1999;16:419–425. doi: 10.1097/00004691-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kelly PJ. Computer-assisted stereotaxis: new approaches for the management of intracranial intra-axial tumors. Neurology. 1986;36:535–541. doi: 10.1212/wnl.36.4.535. [DOI] [PubMed] [Google Scholar]
- 10.Kelly PJ, Sharbrough FW, Kall BA, Goerss SJ. Magnetic resonance imaging-based computer-assisted stereotactic resection of the hippocampus and amygdala in patients with temporal lobe epilepsy. Mayo Clin Proc. 1987;62:103–108. doi: 10.1016/s0025-6196(12)61877-1. [DOI] [PubMed] [Google Scholar]
- 11.Madhavan D, Kuzniecky R. Temporal lobe surgery in patients with normal MRI. Curr Opin Neurol. 2007;20:203–207. doi: 10.1097/WCO.0b013e328042baba. [DOI] [PubMed] [Google Scholar]
- 12.Maillard L, Vignal JP, Gavaret M, Guye M, Biraben A, McGonigal A, et al. Semiologic and electrophysiologic correlations in temporal lobe seizure subtypes. Epilepsia. 2004;45:1590–1599. doi: 10.1111/j.0013-9580.2004.09704.x. [DOI] [PubMed] [Google Scholar]
- 13.Mehta AD, Labar D, Dean A, Harden C, Hosain S, Pak J, et al. Frameless stereotactic placement of depth electrodes in epilepsy surgery. J Neurosurg. 2005;102:1040–1045. doi: 10.3171/jns.2005.102.6.1040. [DOI] [PubMed] [Google Scholar]
- 14.Mintzer S, Sperling MR. When should a resection sparing mesial structures be considered for temporal lobe epilepsy? Epilepsy Behav. 2008;13:7–11. doi: 10.1016/j.yebeh.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Murphy MA, O’Brien TJ, Cook MJ. Insertion of depth electrodes with or without subdural grids using frameless stereotactic guidance systems—technique and outcome. Br J Neurosurg. 2002;16:119–125. doi: 10.1080/02688690220131886. [DOI] [PubMed] [Google Scholar]
- 16.Schramm J, Kral T, Grunwald T, Blümcke I. Surgical treatment for neocortical temporal lobe epilepsy: clinical and surgical aspects and seizure outcome. J Neurosurg. 2001;94:33–42. doi: 10.3171/jns.2001.94.1.0033. [DOI] [PubMed] [Google Scholar]
- 17.Siegel AM, Cascino GD, Meyer FB, McClelland RL, So EL, Marsh WR, et al. Resective reoperation for failed epilepsy surgery: seizure outcome in 64 patients. Neurology. 2004;63:2298–2302. doi: 10.1212/01.wnl.0000147476.86575.a7. [DOI] [PubMed] [Google Scholar]
- 18.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 19.Van Gompel JJ, Rubio J, Cascino GD, Worrell GA, Meyer FB. Electrocorticography-guided resection of temporal cavernoma: is electrocorticography warranted and does it alter the surgical approach? J Neurosurg. 2009;110:1179–1185. doi: 10.3171/2008.10.JNS08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Gompel JJ, Stead SM, Giannini C, Meyer FB, Marsh WR, Fountain T, et al. Phase I trial: safety and feasibility of intracranial electroencephalography using hybrid subdural electrodes containing macro- and microelectrode arrays. Neurosurg Focus. 2008;25(3):E23. doi: 10.3171/FOC/2008/25/9/E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Gompel JJ, Worrell GA, Bell ML, Patrick TA, Cascino GD, Raffel C, et al. Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery. 2008;63:498–506. doi: 10.1227/01.NEU.0000324996.37228.F8. [DOI] [PubMed] [Google Scholar]
- 22.Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, et al. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42:282–286. [PubMed] [Google Scholar]