Abstract
Aromatase inhibitors (AIs) have now been shown to be more effective than the antiestrogen (AE) tamoxifen and have few side effects in ER+ breast cancer patients. However, some patients may not respond and resistance to treatment may develop in others. To investigate the mechanisms involved in the loss of sensitivity of the tumors to AIs, we have studied athymic mice with tumors grown from human estrogen receptor (ER) positive breast cancer cells (MCF-7) stably transfected with aromatase (MCF-7Ca). Treatment with letrozole upregulated Her-2 after four weeks despite continued responsiveness of tumor growth to letrozole. Furthermore, the level of Her-2 protein in letrozole refractory tumors was found to be six fold higher than the control tumors. Cells isolated from these tumors also had increased levels of Her-2 along with lower expression of ERα and aromatase and apparent estradiol independent growth. When Her-2 was inhibited by trastuzumab (antibody against Her-2) ERα levels in the cells were restored indicating that Her-2 is a negative regulator of ERα. This interaction between Her-2 and ER suggests that inhibition of both the Her-2 and estrogen signaling pathways is required to prolong the responsiveness of the tumors to endocrine therapies. Thus, when treatment with trastuzumab and letrozole was combined, ER was restored and tumor growth markedly inhibited compared to treatment with either drug alone. These findings demonstrate that tumor cells under the stress of treatment can adapt and utilize alternate pathways. Thus, when letrozole treatment was stopped, tumor Her-2 levels declined and ER levels were restored to those of hormone sensitive tumors. A second course of letrozole treatment inhibited tumors growth to the same extent and for as long as the initial treatment. These and other strategies to restore aromatase and ERα resulting in sensitivity to hormone therapy could be of substantial benefit to patients who have acquired resistance to AIs.
Keywords: aromatase inhibitors, estrogen, resistance, breast cancer
2. Introduction
Over the last few years aromatase inhibitors (AIs) have proved to be effective treatment for ER+ breast cancer. However despite their benefits, some patients may eventually relapse during treatment while others may never respond. In order to address the problem of resistance to aromatase inhibitor treatment, a clearer understanding is needed of how the tumor adapts to survive the pressure of repressive treatment. To investigate the mechanisms involved in the tumor’s resistance to AIs, we have utilized the xenograft mouse model we initially developed as a model for postmenopausal breast cancer to study the anti-tumor effects of sequencing and combining AIs and antiestrogens [1]. Tumors of human estrogen receptor (ER) positive breast cancer cells (MCF-7) stably transfected with aromatase (MCF-7Ca) are grown in mice and treated with letrozole for an extended period of time (2–3). In addition, we have isolated cells from these tumors after 56 weeks of letrozole treatment when tumors have regained the ability to grow despite the treatment. These cells will form tumors in immunosuppressed ovariectorized mice without estrogen stimulation and are unaffected by AI administration. We have previously reported that AI resistant cells and tumors become dependent on alternate signaling pathways. For example, HER-2/MAPK are key regulators of the growth of letrozole refractory cells (1,4). Signaling proteins in the MAPK cascade were all increased in letrozole resistant tumors. When LTLT cells, isolated from the letrozole resistant tumors were treated with inhibitors of the MAPKinase pathway, cell proliferation was reduced, MAPK activity was decreased and ER expression was restored to control levels. These results suggest that crosswalk occurs between ER and tyrosine kinase receptor signaling. Inhibitors of EGFR/Her-2 also restored the sensitivity of LTLT cells and tumors to the aromatase inhibitor. Utilizing knowledge gained from these studies, our goal is to identify ways to reverse the resistance and restore sensitivity of the tumors to AIs thereby delaying the need for the patient to be subjected to chemotherapy. In this report, we describe several approaches to meet this objective.
3. Materials and Methods
Antibodies against estrogen receptor (ERα) and progesterone receptor (PGR) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), ErbB-2 from Upstate Biotechnology (Lake Placid, NY), Grb2 from BD Transduction Laboratories (San Jose, CA) and Shc, p-Shc, MAPK, p-MAPK, p-ER (Ser167), p-Raf, p-MEK1/2, p90RSK, p-p90RSK, and p-Elk from Cell Signaling Technology (Beverly, MA), MEK1/2 inhibitors U0126 and PD98059 as well as in vitro protein kinase assay. Beta-actin antibody was from Oncogene Research Products (Boston, MA); other supplies included 10xTGS (Tris.glycine/SDS buffer)j 10% SDS, N,N,N,NV-tetra-methylethylenediamine, nonfat dry milk, and loading buffer and were from Bio-Rad (Hercules, CA).
Antiestrogen Tamoxifen, Matrigel and 0.3% hydroxypropyl cellulose were purchased from Sigma Co. (St. Louis, MO). Herceptin was provided by Dr. K. Tkaczuk (UMB). Letrozole (CGS 20267) was kindly donated by Dr. D. Evans (Novartis Pharma A.G., Basel, Switzerland). Anastrozole and the pure antiestrogen fulvestrant (ICI 182, 780) were supplied by Drs. A. Wakeling and E. Anderson (AstraZeneca Pharmaceuticals, Macclesfield, United Kingdom).
MCF-7Ca human breast cancer cells stably transfected with the human aromatase gene were kindly provided by Dr. S. Chen, City of Hope, Duarte, CA [5]. These cells were routinely maintained in DMEM with 5% fetal bovine serum, 1% penicillin/streptomycin solution, and 750 µg/mL F418. Long-term letrozole treated cells (LTLT) were isolated from tumors of MCF-7Ca cells after the mice were treated for 56 weeks [2].
3.1 Cell Proliferation Assay
MCF-7Ca and LTLT-Ca cells were transferred into steroid-free medium for 3 days before plating (1 × 104 cells per well) into 24-well plates. The next day, cells were washed with DPBS and treated with steroid-free medium containing androstenedione (25nmol/L) and a range of concentrations of letrozole, anastrozole, tamoxifen, fulvestrant, gefitinib, U0126, or PD98059. The medium was changed every 3 days, and the cells were counted 9 days later using the MTT assay. The results were expressed as a percentage of the number of treated cells compared with the vehicle-treated cells (control) [6].
3.2 Tumor Xenograft Model
Ovariectomized BALB/c athymic nude mice 4–6 weeks of age were obtained from the National Cancer Institute (Frederick, MD). The animals were housed in a pathogen-free environment under controlled conditions of light and humidity and received food and water ad libitum. All animal studies were carried out according to the guidelines approved by the Animal Care Committee of the University of Maryland, School of Medicine.
Subconfluent MCF-7Ca cells were suspended in Matrigel (10 mg/mL) at 2.5 × 107 cellls/mL and 100 µL of cell suspension injected s.c. into each flank of the mouse. Tumor volumes were measured with calipers weekly as reported previously [7]. Treatments began when the tumors reached a measurable size (`300 mm3). Mice were assigned to groups for treatment, so that there was no statistically significant difference in mean tumor volume among the groups at the beginning of treatment. Mice were injected s.c. 5 days per week with the test compounds in 0.3% hydroxypropyl cellulose, except for herceptin which was used in solution as supplied. At the times indicated in the figures, mice were killed by decapitation under anesthesia and the trunk blood was collected. Tumors and uteri were excised, cleaned, weighed, and stored in liquid nitrogen for analysis later [6].
3.3 Western Blotting
Tumor tissues were homogenized in ice-cold DPBS containing protease inhibitors. Total 50µg of protein from each sample was analyzed by SDS-PAGE as described previously [6]. Bands were quantitated by densitometry using Molecular Dynamics Software (ImageQuant®, Sunnyvale, CA). The densitometric values were corrected for loading control.
3.4 The 3H2O release assay for measuring aromatase activity
The radiometric 3H2O release assay was performed as described previously [8]. For pretreatment studies, cells were treated with the indicated agent for 24 hours before incubating with 1µCi [1-β3H]androstenedione for 18 hours. For measuring aromatase activity in tumor samples, the tumors were homogenized in ice-cold DPBS and incubated with 1µCi [1-β3H]androstenedione for 2h. Aromatase activity was determined from the aromatization of [1β3H]androstenedione with specific release of 3H from the C-1β and formation of 3H2O. The activity of the enzyme was corrected for protein concentration in the tumor homogenates and cells [8].
3.5 Competitive Binding Studies
The competitive binding assay was used as described previously to assess binding of trastuzumab to ER in MCF-7Ca cells [6]. MCF-7Ca cells were transferred to steroid depleted medium for 1 day before plating for the binding assay. The radio labeled ligand was [1,2,6,73H]estradiol and excess non-radio-labeled estradiol was used to determine non-specific binding. Triamcinolone (1µM) was used to saturate glucocorticoid receptors.
3.6 ERα trans-activation assay
To measure activation of ERα, an ELISA based ER trans-activation assay was performed as per manufacturer’s guidelines (Panomics, Fremont, CA). Briefly activated ER, from nuclear extracts of cells and tumors were allowed to bind to an ER consensus binding site (ER Probe) on a biotinylated oligonucleotide. These oligonucleotides were then immobilized on a streptavidin coated 96-well plate. The ER bound to the oligonuclotide, was then detected by an antibody directed against ER. Addition of horseradish peroxidase (HRP)-conjugated secondary antibody provided colorimetric readout quantified at 450 nm absorbance.
3.7 RNA Extraction and Reverse Transcription (RT)
RNA was extracted and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA) as per manufacturer’s protocol. Total RNA concentration and purity were determined at 260 nm and 280 nm absorbances. RNA was diluted with water to 0.08 µg/µl and reverse transcribed as described by Kazi et al [9].
PCR: Analysis of ERα, CYP-19 and pS2 mRNA expression were carried out by conventional PCR [6].
3.8 ChIP Assay
The ChIP assay was performed as reported [6]. The promoter analyzed by the ChIP assay was I.3.II, which is the main aromatase promoter utilized in breast cancer cells lines such as MCF-7 [10] and thus measures the effect of trastuzumab on endogenous aromatase in MCF-7 cells. The gene transfected in MCF-7Ca cells is placental aromatase and does not contain promoter 1.3/II. Immunocleared supernatants of formalin fixed tissue and cell extracts were incubated overnight at 4C with anti-ERα antibody. Protein A or G Sepharose beads and salmon sperm DNA were added and incubated for 1 h at 4C. The beads were then washed sequentially with 1 ml each of wash buffers. The protein-DNA complexes were eluted by twice incubating the beads in elution buffer for 10 min at room temperature with vigorous mixing. To separate immunoprecipitated protein and DNA, the pooled elutes were incubated at 65C overnight. The DNA was purified using the Qiaquick PCR purification kit (Qiagen). Alternatively, immunoprecipitated ERα on the beads was subjected to western immunoblotting. The boiled (denatured) protein samples were resolved by SDS-PAGE and membranes were probed for Histone H3 and RNA Polymerase II.
3.9 Statistics
For in vivo studies, mixed-effects models were used. The tumor volumes were analyzed with S-PLUS (7.0, Insightful Corp.) to estimate and compare an exponential parameter (βi) controlling the growth rate for each treatment groups. The original values for tumor volumes were log transformed. It was observed that the piecewise (knot at 15 weeks) model fits the data reasonably well (on groups switched after week 15 to another drug). The spline model with a single knot at time = week-15 weeks was used to accommodate the non-linearity with a piece-wise linear model. All p values less than 0.05 were considered statistically significant. The graphs are presented as mean ± standard error of the mean (SEM).
4. Results and Discussion
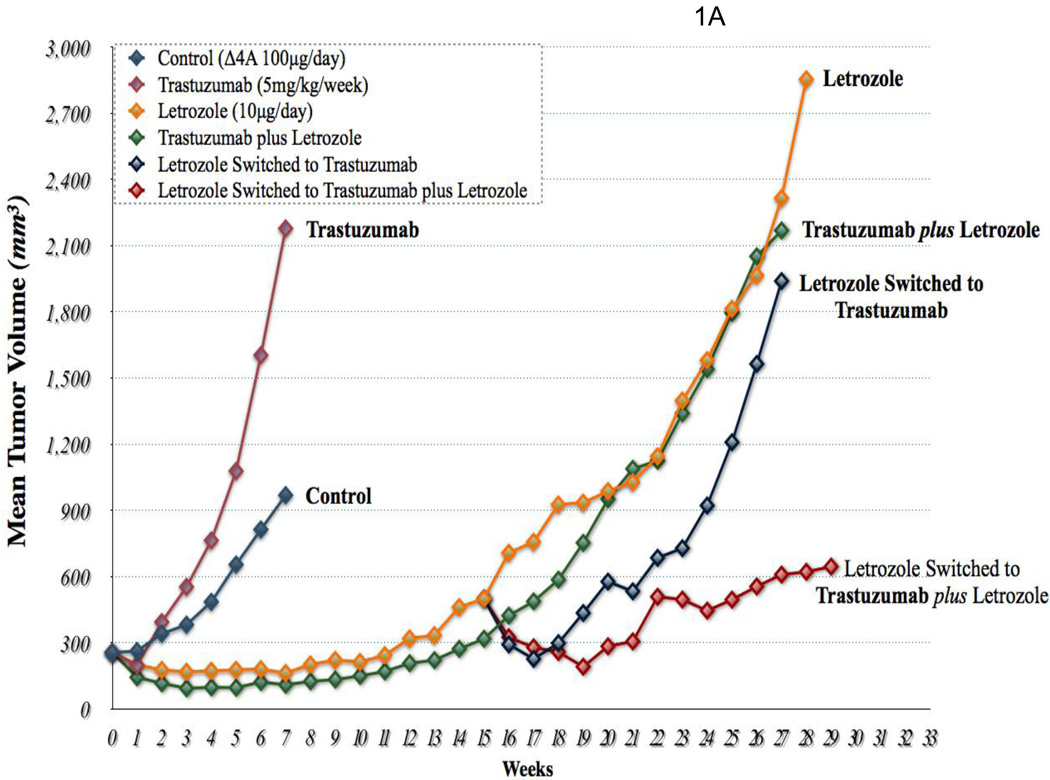
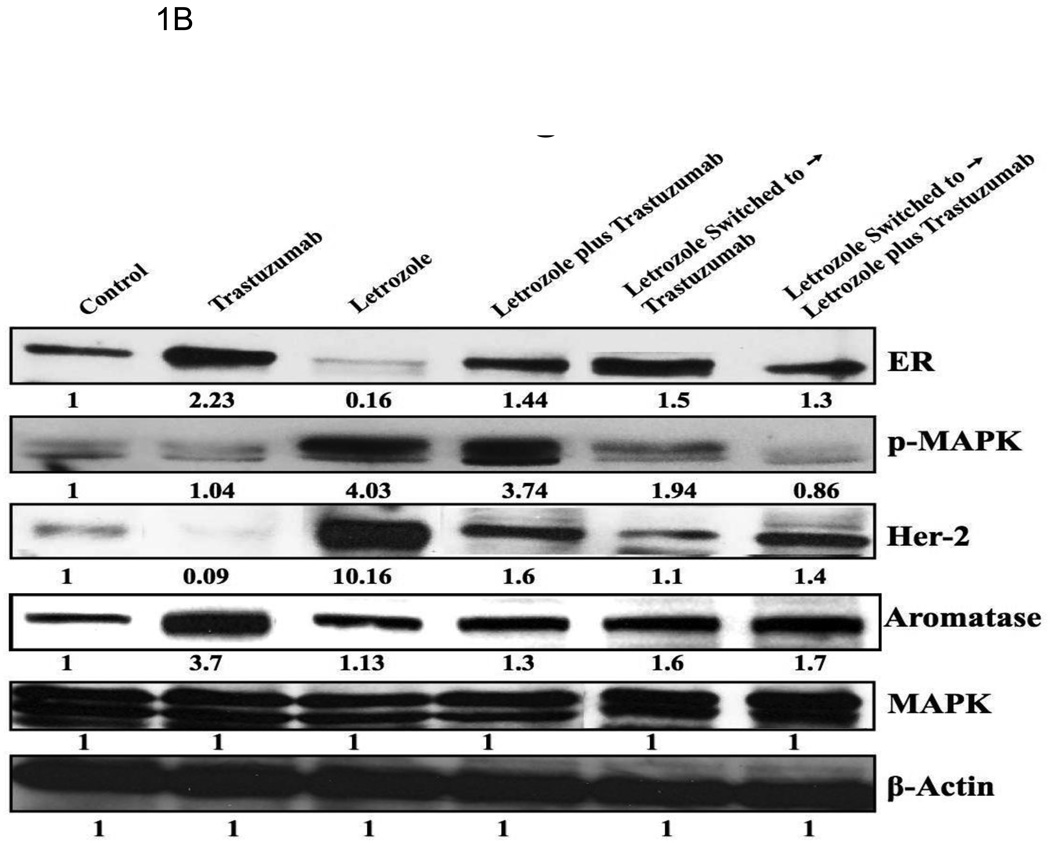
In previous studies we found decreased expression of ERα and increased expression of HER2 together with downstream proteins in the MAPK pathway in tumors resistant to letrozole after 56 weeks of treatment. Similar changes were also seen in LTLT cells isolated from the resistant tumors. When LTLT cells were incubated with a series of doses of MEK ½ inhibitor (UO126) and MAPK inhibitor (PD98059), there was a dose dependent inhibition of growth in LTLT cells. However, the inhibitors had no effect on the proliferation of parental MCF-7Ca cells. These findings provide evidence that the MAPK pathway has a functional role in enhancing LTLT cell proliferation. Furthermore, ER expression was restored to MCF-7Ca levels in LTLT cells treated with MEK inhibitor (PD98059) suggesting a return to hormone sensitivity could be achieved by inhibiting the MAPK pathway. As trastuzumab is used clinically to block the actions of HER-2, we investigated its effects on proliferation of LTLT cells [6]. There was a marked dose response inhibition of growth of LTLT cells as well as inhibition of pHER-2 and p-MAPK expression. This was accompanied by up regulation of ERα. Interestingly, pretreatment of LTLT cells with trastuzumab followed by 1h of E2 treatment induced ERα transcriptional activation to the same extent as in parental MCF-7Ca stimulated with E2 only. This was evident from the increase in recruitment of ERα to the pS2 promoter, a known ERα responsive gene, confirming HER2 regulation of ERα mediated transcription of downstream genes, such as pS2. Thus, HER-2 appears to be a negative regulator of ERα. ERα was also recruited to the aromatase 1.3/II promoter after treatment of LTLT cells with trastuzumab and E2 [6]. In addition, when ER negative HER2 positive SKBR3 cells were treated with trastuzumab, ERα was also induced. This finding provides further evidence of an interaction between ERα and HER2 and that HER2 regulation of ER is not limited only to MCF-7 cells. In the xenograft model, the addition of trastuzumab to letrozole treatment caused marked reduction of AI resistant tumor growth. As shown in Fig 1A tumor volumes remained only slightly above their starting volume even after 29 weeks of treatment. Analysis of the tumors from these animals indicated that the level of expression of ERα and aromatase was normalized to that of the control (MCF-7Ca) level. The level of p-MAPK was also similar to the control level but much reduced compared to that with letrozole treatment (Fig. 1B). These results demonstrate that combining treatments that block both the estrogen and growth factor signaling can restore sensitivity of tumors to letrozole treatment.
Figure 1. Effect of trastuzumab and letrozole alone or in combination.
A: The growth of MCF-7Ca tumor xenografts: Trastuzumab (5mg/kg/week) did not inhibit the growth of MCF-7Ca tumors. The difference in the exponential parameter governing growth rate of control versus trastuzumab treatment was 0.02 ± 0.14, which was not statistically significant (p = 0.86); trastuzumab versus trastuzumab plus letrozole was 0.49; p = 0.0001; trastuzumab versus letrozole was 0.32, p = 0.0009; letrozole versus letrozole switched to letrozole plus trastuzumab was 0.21 ± 0.08, p = 0.008; letrozole plus trastuzumab versus letrozole switched to letrozole plus trastuzumab was 0.39 ± 0.09, p<0.0001; letrozole switched to trastuzumab versus letrozole switched to letrozole plus trastuzumab was 0.2 ± 0.08, p = 0.011, over weeks 15–28. B: Protein expression of ERα, Her-2, MAPK and CYP-19 in MCF-7Ca tumor xenografts: Expression of proteins was examined using Western imunoblotting as described in Materials and Methods. Blot shows ERα at 66 kDa, Her-2 at 185 kDa, p-MAPK and MAPK at 42–44 kDa and CYP-19 at 55 kDa, β-actin at 45 kDa. The blots show a single representative of three independent experiments. The blots were stripped and reprobed for β-actin to verify equal loading.
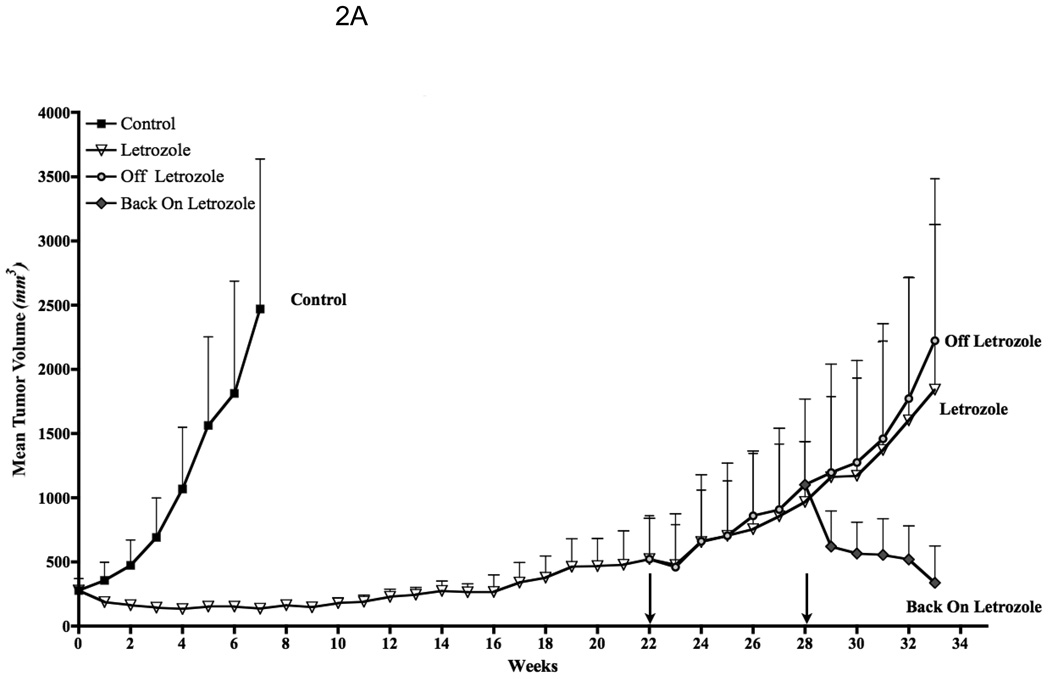
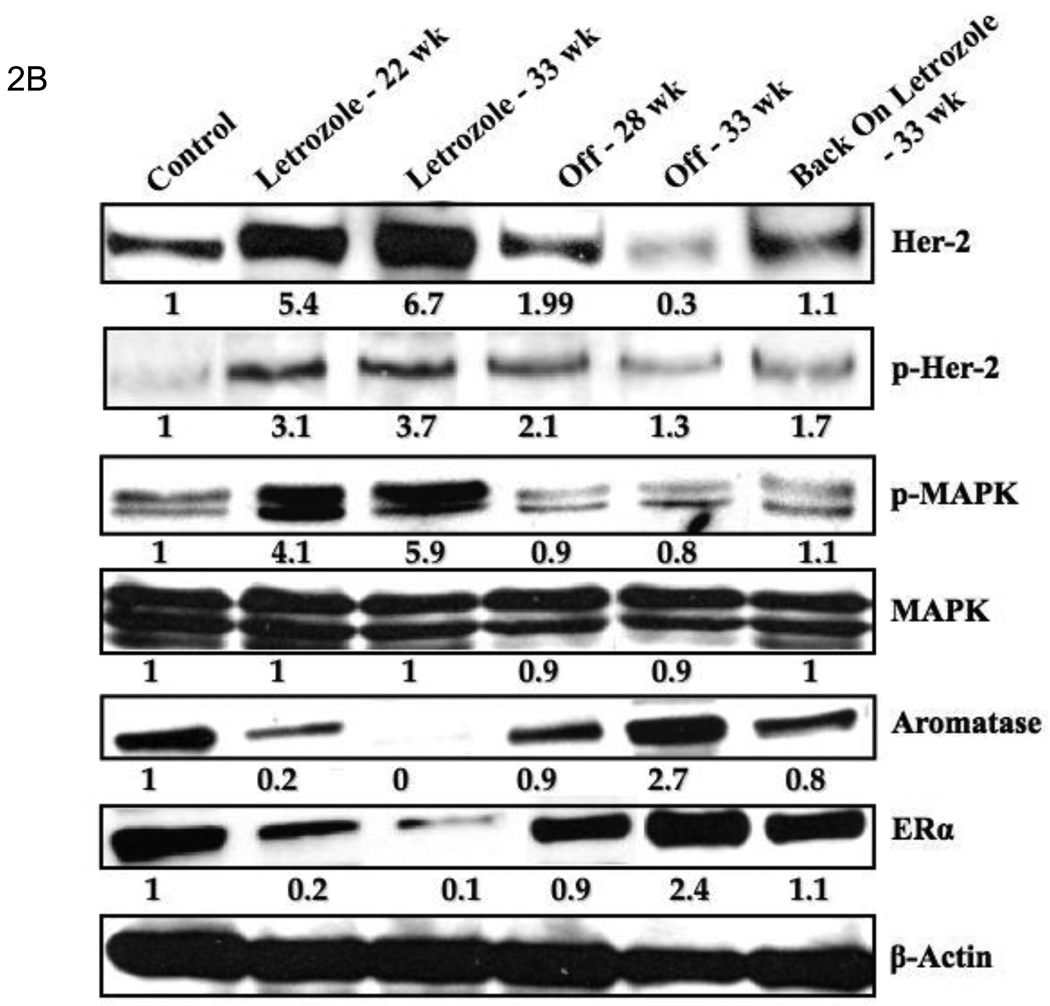
Evidence that tumors adapt to the pressure of treatment was seen when letrozole administration was stopped after tumors had become resistant to its effects [11]. After 6 week, a second course of letrozole treatment was administered. This resulted in secession of tumor growth for an additional 16 weeks. The duration of this response was almost as long as with the initial course of treatment (Fig. 2A). Tumor analysis revealed that levels of p-HER2, p-MAPK expression decreased and aromatase and ERα increased compared to letrozole resistant tumors and were similar to levels in tumors of the control estrogen sensitive MCF-7Ca tumors (Fig. 2). This finding suggests that the tumors had reverted to their original phenotype of estrogen dependent growth.
Figure 2. Effect of stopping letrozole treatment on the growth of resistant tumors.
A: Discontinuous treatment prolongs responsiveness of MCF-7Ca xenografts to letrozole: The tumors of MCF-7Ca cells were grown as described in Materials and Methods. Letrozole treatment was stopped after 22 weeks and started again at week 28. Columns, mean tumor volume; bars, SE. B: Protein expression profile of the tumors of MCF-7Ca cells treated with letrozole: Expression of proteins was examined using Western immunoblotting as described in Materials and Methods. Lane 1, androstenedione-treated controls; lane 2, letrozole-treated tumors at week 22; lane 3, letrozole-treated tumors at week 33; lane 4, tumor from the “off” letrozole treatment group at week 28; lane 5, “off” tumors at week 33; and lane 6, tumor from the “back on” group at week 33. Blot shows Her-2 and phosphorylated Her-2 at 185 kDa, phosphorylated MAPK and MAPK at 42 to 44 kDa, aromatase at 55 kDa, ERα at 66 kDa, and β-actin at 45 kDa. The blots were stripped and reprobed for β-actin to verify equal loading. The blots show a single representative of three independent experiments.
Although our studies indicate that ERα is negatively regulated by HER-2, other mechanism may lead to suppression of the estrogen receptor in breast cancer. Epigenetic silencing has been shown to repress genes and may include excessive deacetylation of histones by abnormal histone deacetylase activity in tumors. This results in closed chromatin that prevents DNA transcription. Histone deacetylase inhibitors (HDACI) have been shown to re-express ERα in ER negative breast cancer cells [12]. Treatment of ER negative/HER-2 negative MDA-MB-231 cells with HDACI MS-275 was found to cause expression of ERα and also aromatase as well as the androgen receptor [13]. Based on these findings, we then treated groups of ovariectomized mice bearing tumors of MDA-MB-231 cells with MS-275, letrozole or the combination of both drugs. Letrozole and MS-275 had only marginal effects on tumor growth compared to vehicle treated control tumors. However, the combination of MS-275 and letrozole caused marked suppression of tumor growth. Thus, after 53 days of treatment, the volume of these tumors was below the volume measured when treatment was started. It was evident that aromatase was expressed in the tumors and estrogen was synthesized as the estrogen sensitive uterine weight of the mice treated with MS-275 was significantly increased (p<0.001) compared to uteri of the vehicle treated (ovariectomized) control mice. Furthermore uteri of mice treated with letrozole and letrozole plus MS275 were significantly decreased compared to those of the MS-275 treated animals [13].
In conclusion, our studies in the xenograft model of postmenopausal breast cancer, suggest that mechanisms of resistance to AIs may include activation of tyrosine kinase receptor pathways such as HER-2 and IGFR [14] that lead to suppression of the ERα. Although these may be reversible changes once treatment is stopped [11], other mechanisms such as result from abnormal histone deacetylase activity may also lead to ERα silencing. Inhibitors that target HER-2 and HDAC can restore the ER and aromatase rendering the tumors sensitive to the stimulating effects of estrogen which can be once again blocked by AIs. This strategy could improve treatment options for the breast cancer patient by extending the benefits of aromatase inhibitors and delay the need for chemotherapy.
Acknowledgements
This work was supported by grant CA-62483 to Dr. Brodie from the National Cancer Institute, National Institute of Health.
Abbreviations used
- ER
Estrogen Receptor
- Δ4A
Androstenedione
- E2
Estradiol
- Her-2
Human Epidermal Growth factor Receptor- 2
- MAPK
Mitogen Activated Protein Kinase
- AIs
Aromatase Inhibitors
- AEs
Antiestrogens
- TRZ
Trastuzumab
- Let
Letrozole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brodie AM, Jelovac D, Macedo L, Sabnis G, Tilghman S, Goloubeva OG. Therapeutic Observations in MCF-7 Aromatase Xenografts. Clin. Cancer Res. 2005;11:884s–888s. [PubMed] [Google Scholar]
- 2.Long BJ, Jelovac D, Handratta V, Thiantanawat A, MacPherson N, Ragaz J, Goloubeva OG, Brodie AM. Therapeutic strategies using the aromatase inhibitor letrozole and tamoxifen in a breast cancer model. J. Natl. Cancer Inst. 2004;96(6):456–465. doi: 10.1093/jnci/djh076. [DOI] [PubMed] [Google Scholar]
- 3.Long BJ, Jelovac D, Thiantanawat A, Brodie AM. The effect of second-line antiestrogen therapy on breast tumor growth after first-line treatment with the aromatase inhibitor letrozole: long-term studies using the intratumoral aromatase postmenopausal breast cancer model. Clin. Cancer Res. 2002;8(7):2378–2388. [PubMed] [Google Scholar]
- 4.Jelovac D, Sabnis G, Long BJ, Macedo L, Goloubeva OG, Brodie AM. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res. 2005;65(12):5380–5389. doi: 10.1158/0008-5472.CAN-04-4502. [DOI] [PubMed] [Google Scholar]
- 5.Zhou D, Pompon D, Chen S. Stbale expression of human aromatase complementary DNA in mammalian cells: a useful system for aromatase inhibitor screening. Cancer Res. 1990;50(21):6949–6954. [PubMed] [Google Scholar]
- 6.Sabnis G, Schayowitz A, Goloubeva O, Macedo L, Brodie A. Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res. doi: 10.1158/0008-5472.CAN-08-0857. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue W, Wang J, Savinov A, Brodie A. Effect of aromatase inhibitors on the growth of mammary tumors in a nude mouse model. Cancer Res. 1995;55(14):3073–3077. [PubMed] [Google Scholar]
- 8.Long BJ, Tilghman SL, Yue W, Thiantanawat A, Grigoryev DN, Brodie AM. The steroidal antiestrogen ICI 182,780 is an inhibitor of cellular aromatase activity. J. Steroid. Biochem. Mol. Biol. 1998;67(4):293–304. doi: 10.1016/s0960-0760(98)00122-8. [DOI] [PubMed] [Google Scholar]
- 9.Kazi AA, Jones JM, Koos RD. Chromatin immunoprecipitaiton analysis of gene expression in the rat uterus in vivo: estrogen-induced recruitment of both estrogen receptor alpha and hypoxia-inducible factor 1 to the vascular endothelial growth factor promoter. Mol. Endocrinol. 2005;19(8):2006–2019. doi: 10.1210/me.2004-0388. [DOI] [PubMed] [Google Scholar]
- 10.Zhou C, Zhou D, Esteban J, Murai J, Siiteri PK, Wilczynski S, Chen S. Aromatase gene expression and its exon I usage in human breast tumors. Detection of aromatase messenger RNA by reverse transcription-polymerase chain reaction. J. Steroid. Biochem. Mol. Biol. 1996;59(2):163–171. doi: 10.1016/s0960-0760(96)00100-8. [DOI] [PubMed] [Google Scholar]
- 11.Sabnis GJ, Macedo LF, Goloubeva O, Schayowitz A, Brodie AM. Stopping treatment can reverse acquired resistance to letrozole. Cancer Res. 2008;68(12):4518–4524. doi: 10.1158/0008-5472.CAN-07-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001;61(19):7025–7029. [PubMed] [Google Scholar]
- 13.Sabnis G, Goloubeva O, Macedo L, Gilani R, Gediya L, Njar V, Brodie A. Comibination of HDAC inhibitor entinostat (SNDX-275) with letrozole provides control over increased growth in MDA-MB-231 xenograft models. Cancer Res. 2009;6128 Supplement 2:398s. [Google Scholar]
- 14.Macedo LF, Sabnis GJ, Goloubeva OG, Brodie A. Combination of anastrozole with fulvestrant in the intratumoral aromatase xenograft model. Cancer Res. 2008;68(9):4518–4524. doi: 10.1158/0008-5472.CAN-07-6807. [DOI] [PubMed] [Google Scholar]