Abstract
Objective
Arginine used for nitric oxide formation can be from intracellular stores or transported into cells. The study evaluated the rapidity and primary site of NO and vascular resistance responses to arginine at near physiological concentrations (100–400 μM).
Methods
Arginine was applied to a single arteriole through a micropipette to determine the fastest possible responses. For vascular blood flow and [NO] responses, arginine was added to the bathing media.
Results
Dilation of single arterioles to arginine began in 10–15 seconds and application over the entire vasculature increased [NO] in ~60–90 seconds and flow increased within 120–300 seconds. Resting periarteriolar [NO] for arterioles was 493.6±30.5 nM and increased to 696.1±68.2 and 820.1±110.5 nM at 200 and 400 μM L-arginine. The blood flow increased 50% at 400–1200 μM L-arginine. The reducd arterial resistance during topical arginine was significantly greater than microvascular resistance at 100 and 200 μM arginine. All responses were blocked by L-NAME.
Conclusions
This study demonstrated arterial resistance responses are as or more responsive to arginine induced NO formation as arterioles at near physiological concentrations of arginine. The vascular NO and resistance responses occurred rapidly at L-arginine concentrations at and below 400 μM, which predict arginine transport processes were involved.
Keywords: Microelectrode NO, Resistance, Blood Flow
Introduction
Arginine used by endothelial nitric oxide synthase (eNOS) has two potential sources, intracellular stores and extracellular arginine provided by the amino acid transporters. Multiple arginine transporters have been found for endothelial cells, as reviewed by Mann et al. (45), including the highly expressed cationic amino acid transporter-1 colocalized with eNOS within caveolar regions of endothelial cells (46). As would be expected for a transport system, in vivo and in vitro vascular studies (9; 14; 18; 22; 37; 40; 54–56) have shown that extracellular L-arginine administration in excess of 1 mM caused either increased NO formation or vascular relaxation attributable to NO within minutes. However, given the very high arginine concentrations used in these studies, the physiological relevance of such high extracellular arginine concentrations is debatable. The most common arginine transporter for endothelial cells is cationic amino acid transporter 1 (CAT-1) and reviews by Closs et al. (17) and Mann et al. (45) summarized that the half maximal transport rates for arginine by endothelial cells are generally reported in the 100–160 μM range. Consequently, transport of arginine would be saturated at concentrations below 1 mM arginine. Furthermore, there is evidence that arginine concentrations of 1 mM and higher are capable of releasing histamine from mast cells (26; 51) and histamine is an established NO dependent vasodilator (1; 21). Fortunately, dietary supplementation to raise plasma l-arginine to 100–400 μM is known to cause vasodilation or facilitate endothelial dependent dilation in the humans (4; 38; 40; 49). In animal studies, Frame and colleagues (23; 24) found local application of 100 μM L-arginine caused very fast dilation of arterioles that was suppressed by inhibition of endothelial nitric oxide synthase (eNOS). Therefore, both clinical and animal studies indicate relatively low arginine concentrations are potentially useful to influence endothelial nitric oxide physiology.
We evaluated our hypotheses by monitoring in vivo production of NO by direct measurement with NO sensitive microelectrodes and vasodilation of microvessels and small arteries. The majority of these responses were evaluated during acute arginine exposure at near physiological concentrations (100–400), although concentrations up to 1200 μM were evaluated. The 100–400 μM range has been achieved by dietary supplementation and is known to favorably influence endothelial function in humans (4; 40; 49). Our first hypothesis is that for low concentrations of arginine to be physiologically relevant, the increased perivascular NO concentration and vasodilation must both occur rapidly. Rapid vascular responses would support a transport mechanism. To confirm the vascular responses are linked to NO production, both increased nitric oxide concentrations ([NO]) and vasodilation must be suppressed by L-nitroarginine-methyl ester (L-NAME). We chose L-NAME because it is known to not influence CAT-1 transport of arginine (5). The second hypothesis was that arginine in the physiological concentration range should cause meaningful increases in blood flow through dilation of both arterioles and small arteries. This hypothesis was evaluated because in conditions where the systemic arginine concentration is elevated, both resistance arteries and arterioles might contribute to increased blood flow. This potential interaction of macro- and microvessels has not been investigated. In the majority of vascular beds, including the small intestine (7; 29), resistance arteries control about 40% of the total resistance. Furthermore, intestinal resistance arteries strongly contribute to blood flow regulation through flow mediated responses (7) and their NO dependent regulation could be influenced by the availability of arginine. Therefore, the resistance arteries of the intestine were evaluated as a model of how supplemental low concentrations of arginine might influence a significant resistance region of the vasculature.
Methods
Animal Setup
The protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Indiana University Medical School. Male Sprague Dawley rats (Harlan, Indianapolis, IN) in the weight range of 300–400 grams were used for all studies. The rat was anesthetized with sodium thiopental (200 mg/kg, 50 mg/ml) given subcutaneously at four sites. The trachea was cannulated for positive pressure ventilation at 70 breathes per minute and sufficient tidal volume to cause 95–97% oxygen saturation of hemoglobin in the ear vasculature. The right femoral artery was cannulated to monitor the arterial blood pressure and give physiological saline (~0.5 ml/hr/100 gm). The animals were heated with an underbody temperature heating mat perfused with 35°C water. If underbody heating was not sufficient to maintain a core temperature (esophagus) of 37–37.5°C, the rat was covered with a polyethylene insulating blanket. The jejunal region of the small intestine was exteriorized through a midline incision and prepared for transillumination by slitting the bowel along the antimesenteric border with a small thermal cautery. Cut edges were secured with thread ties in a temperature (37.5 °C) controlled bath support. The mesenteric arteries preceding the intestinal microcirculation consistently have one side of the vessel that is only partially covered by adipocytes. This anatomy was exploited to allow access of the bath media chemicals to the small arteries. The “exposed” side of the arteries always faced the bath and the side substantially covered with adipocytes was placed down on the bath support. The bath volume over the tissue was 5 ml and perfused at ~5 ml/minute with bicarbonate buffered physiological saline equilibrated with 5% oxygen and carbon dioxide and balance nitrogen(6). The bath oxygen tension over the tissue was 40–50 mmHg. The incoming bath heater and tissue support were perfused with ~900 ml/min of heated distilled water to insure precise thermal stability necessary for NO sensitive microelectrodes.
Nitric Oxide Measurement
Carbon fiber microelectrodes (~ 7 μm, od) were sealed into borosilicate glass tubing (Frederick Hare, Bowdoinham, ME) during pulling of the electrode, filled with heat cured epoxy cement (Graphpoxy-PX, Dylon Industries, Cleveland, OH) that also conducted electricity to a copper wire advanced into the cement plug. The finished electrode was baked overnight at 100° C to harden the epoxy. This carbon fiber microelectrode is based on that of Freidemann et al (25) as well as design characteristics of a recessed tip microelectrode used by Buerk et al. (11). Carbon fiber microelectrodes do not require a specialized coating to be sensitive to nitric oxide (13; 25; 41), although appropriate coatings have been shown to increase the current generation for a given NO concentration (25; 43). Polarization voltage was maintained at +0.9 V. We only use microelectrodes that generated >2.5 picoamperes per 1000 nM of NO and most electrodes generated ~4 picoamperes per 1000 nM of NO. The increase in electrode current translated to a change in output voltage of a Keithley 6517A Electrometer of 250–400 mv/1000 nM NO in the 0–20 picoampere range. Microelectrodes that generated > 15 picoamperes in nitrogen equilibrated media at +0.9 V were excluded because they likely have a defect in the glass envelope and could respond to NO at sites other than the open tip.
The carbon in the electrode tip was electrolitically etched out to form a 3–5 μm recess after sharpening to a 10–12 μm outer diameter glass-carbon fiber tip. The recess was used to form a mechanically protected site for Nafion. Nafion was used as a membrane to exclude negatively charged inorganic and organic ions from inappropriately influencing the electrode(11; 25; 31; 44). To apply Nafion, the tip recess was electroplated with Nafion (Sigma Chemical) at +0.7 V for 20–30 minutes. We did not use heating to apply Nafion because the Nafion became very fragile when wet and peeled away during tissue penetration. Electroplating Nafion into the 3–5 μm tip recess provided a membrane covering that was visually intact after many tissue penetrations and basal electrode current remained nearly constant in the tissue bath after repeated tissue penetrations. Had the membrane been damaged or removed during tissue penetrations, basal current would have increased during the experiment.
Prior to penetration of the tissue to record the [NO], the microelectrode tip was placed about 200 μm above the tissue surface to obtain an electrical current reference equivalent to a “0” concentration of NO. There are only trivial differences in the microelectrode current in the distance range of 100–300 μm above the tissue. In recording [NO], the tip of the microelectrode was gently placed against the side of a vessel and moved as needed to maintain contact as the vessel dilated or contracted. In general, the micropipette was held in place about 10 minutes before making a perturbation to ensure that a stable [NO] was present. There is some slow fluctuation of the [NO] about the average concentration. After a perturbation that did not permanently alter the regulation of the vessel, the [NO] consistently returned to about the prior control [NO]. This can be seen for tracings (5 second interval data display) in Panels C–D of Figure 1.
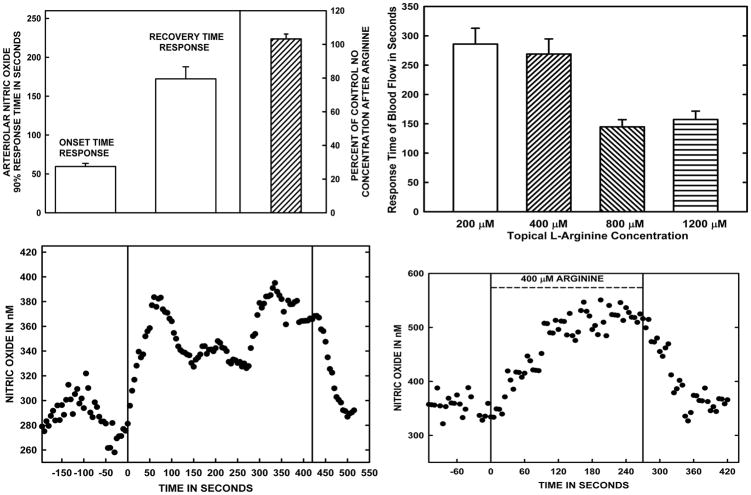
Figure 1.
Panel A. The averaged time to reach ~90% of the stable arteriolar wall [NO] response to 400 μM L-arginine added to the bath and the time to recover ~90% of the post-arginine resting [NO] after L-arginine solution was stopped are shown on the left side of the panel. On the right side of Panel A, the ratio of the post-arginine resting [NO] to that at control prior to arginine exposure is shown. The data in Panel A are based on studies of 9 arterioles in 9 rats. Panel B presents the time to stably increased blood flow for topical arginine concentrations of 200–1200 μM. These data are based on measurements in 12 rats. The flow responses represent combined resistance responses of small arteries in the mesentery as well as the microvasculature. Panels C and D are representative NO responses to the wash in and wash out of 400 μM L-arginine added to the bath. The overall data indicate a rapid rise in [NO] with arginine exposure, but a much slower decay with washout to near the original resting [NO].
Microvascular Pressure, Blood Flow, and Resistance
To measure the resistance of arterial and microvascular sections of the small intestine, the pressure drop from the aorta to the origin of the largest arterioles was needed: pressure in the portal venous system was assumed to be 6 mm Hg based on past experience (8). The pressure was measured with the servo-null technique (36)(Instrumentation for Physiology and Medicine, San Diego, CA) using glass microelectrodes sharpened to a ~ 10 μm outer diameter to penetrate the largest arterioles. The individual microelectrode-servo null combinations were calibrated at 0–100 mmHg prior to measurements. Blood flow was monitored with the optical Doppler flow velocimetry technique (10; 19) in which flow velocity in a large arteriole (50–70 μm) was measured along with inner vessel diameter during the protocols and simultaneously with the pressure recordings. During velocity measurements, the transillumination light intensity was increased to be sure that the velocity signal did not increase with light intensity. This precaution is necessary as the optical Doppler system is known to be sensitive to low light intensity(10; 19). The velocity system was calibrated against red blood cells dried onto the surface of a rotating clear plastic wheel at velocities both below, equal to, and far higher than encountered in the current study. Images of the vessels were displayed with a closed circuit video system (Hamamatsu Model XC-77, Tokyo, Japan) coupled with Metamorph image analysis software (Molecular Devices Corp., Sunnyvale, CA) calibrated with a stage micrometer marked in 10 and 100 μm units.
Amino Acid Exposure Methods
To evaluate global responses of arteries and arterioles to a given amino acid concentration, the amino acid was added to the bathing media by pumping very small amounts of concentrated (4 mM) amino acid-physiological saline solution into the known flow (5 ml/min) of bath media. This approach avoided appreciable changes in bath ionic composition or gas tension. There was a 12 second delay from the mixing point of bath media and amino acid media to the entrance of the tissue bath. This time was taken into account when “setting” the time of onset of amino acid exposure in the overall media. The chamber volume over the tissue was ~5 ml and the bath flow rate was 5 ml/min. The media flowed over the tissue and was immediately drained away, which allowed media changes in about one minute without temperature fluctuations that would compromise NO measurements. The time required for media exchange was verified by observing pumping of water soluble dye (Fast Green) rather than amino acid solutions.
The media containing amino acids could only reach the outer surface of the intestinal wall and was excluded from the mucosa in contact with the transillumination pedestal. The arterioles observed were about 20–30 μm beneath the surface of the bowel wall due to the overlying visceral smooth muscle layers of the bowel wall. The typical time for a vascular and [NO] response to develop was approximately one minute after the start of adding arginine to the bath. Full recovery of blood flow during washout required about 10 minutes, but most of the recovery will be shown to occur in slightly less than three minutes. Simultaneous measurements during this protocol included blood flow velocity, vessel diameter, and either [NO] or microvascular pressure. Resistance calculations shown in Figure 5 were made from simultaneous measurements of blood flow, arterial pressure, and the pressure in the largest arterioles as they enter the bowel wall.
Figure 5.
The upper panel presents the blood flow responses to 100–1200 μM topically applied L-arginine. These data represent steady state responses of the intestinal vasculature in 10 rats that were developed within 5 minutes of global application of arginine, as was shown in Figure 1, Panel B. To determine vascular responses to a neutral amino acid, 1200 μM L-alanine was suffused over the tissue, but no responses occurred. To maximally dilate the local vasculature and illustrate the very good vascular tone, 100 μM sodium nitroprusside was used to increase blood flow above ~250% of control. The flow results indicate 400 μM L-arginine was a near maximum effective dosage for blood flow responses. The relative vascular resistances of the arterial and microvascular regions of the small intestine at 100–400 μM were calculated from the changes in blood flow and intravascular blood pressures within the smallest arteries about to enter the bowel wall. The study revealed that the arterial component of the intestinal vasculature proved to be as responsive to exogenous arginine, on a relative basis, as the microvascular component of resistance. The relative resistance data are based on individual observations in 8 rats.
As will be shown in Results, the arterial response to arginine required about 5 minutes to be fully developed. However, arteriolar responses to arginine were nearly fully developed within one minute using the media change protocol just described. To determine just how rapidly arteriolar responses to exogenous arginine and lysine could occur, the desired concentration of an amino acid in buffered physiological solution was pumped onto the vessel wall from a micropipette. A WPI nanoliter pump was used to have essentially instant on or off perfusion through a 10–15 μm (od) sharpened glass micropipette. The flow of media was set at 10 nl/sec for <30 μm inner diameter arterioles and at 50 nl/sec for larger vessels. The micropipette tip was advanced through the tissue to nearly touch the outer wall of the vessel of interest. Perfusion of media without amino acid caused at most a slight and transient constriction for about 30 seconds. The diameter of the vessel of interest was recorded at 5 second intervals by the Metamorph software and stored as digital images for later analysis.
Statistics
Significant differences in responses relative to the control value were computed with Statistica (StatSoft, Tulsa, OK) using repeated measures one way analysis of variance with a least significant difference post-hoc test for simple control/response events and repeated two way analysis of variance with a least significant difference post-hoc test for conditions where arginine and L-NAME were used. Significant events were accepted at p≤0.05.
Results
Tests of interference by L-NAME and L-Arginine on NO microelectrode function
Even though NO sensitive microelectrodes were coated with Nafion to minimize electrochemical interference, there was a concern that L-arginine or L-nitro-methyl-ester arginine (L-NAME) might influence the microelectrode. We have previously shown that lysine at 200 μM did not change the NO sensitivity of the electrodes (61). To test for arginine effects, the response of the Nafion coated microelectrode to NO was measured in physiological saline and then in the same solution after addition of concentrated arginine solution to make a final arginine concentration of 400 μM. The saline NO calibration was 4.5±1.7 picoamps/1000 nM NO compared to 4.4±1.1 picoamps with 400 μM arginine for 6 randomly selected microelectrodes. The variability was due to inherent differences of sensitivity by individual microelectrodes. When individual electrodes were compared on the basis of NO sensitivity before and after addition of arginine, the ratio of sensitivities was 0.98±0.04. The same type of test was done for 1 mM L-NAME and the ratio of sensitivities was 0.96±0.07 for 4 electrodes chosen at random. During experiments, not a single electrode coated with Nafion responded to addition or removal of 400 μM arginine or 1 mM L-NAME while the microelectrode tip was in the tissue bath.
Response times of the vasculature to L-Arginine and post-stimulation effects of L-arginine
In pilot studies, we measured [NO] both at the vessel wall and at equivalent depths in the intestinal submucosa as far as possible from arterioles and venules to evaluate “tissue” NO responses. The intestinal wall contains a neural network that expresses nNOS, but these sites are away from most of the microvessels(12; 48). The major reservoir of NOS is reported in the form eNOS in the endothelial cells of the vessel walls(12; 48). The tissue parenchyma away from vessels generated an [NO] of less than 100 nM compared to ~300–500 nM for most arterioles. Unlike the large increases in [NO] on the vessel wall during exposure to L-arginine, tissue [NO] only increased slightly when vascular NO increased, such as during ≥400 μM L-arginine.
The panel A of Figure 1 presents the averaged time response of the perivascular [NO] to adding 400 μM L-arginine to the bath and panels C–D are representative tracings of the events used to obtain the data from individual vessels. The average time to reach ~90% of the stable arteriolar wall [NO] response to 400 μM L-arginine added to the bath was 59.5±3.9 seconds, and a time of 172.3±15.5 seconds was required to recover ~90% of the post-arginine resting [NO] after L-arginine solution was stopped (Fig. 1, Panel A). The 90% events were chosen because defining when a stable increase or decrease had occurred was difficult, but finding the time when 90% of the average change had occurred was straightforward. The right side panel A of Figure 1 also presents the ratio of the stable post-arginine resting [NO] relative to the resting [NO] prior to arginine exposure for 9 vessels in 9 rats. At that time of 5–10 minutes after arginine infusion stopped, the percent of original control [NO] was 103.2±2.9% and was not significantly different from the control [NO]. The overall recovery time and [NO] data are presented to demonstrate that arginine exposure for up to 20–30 minutes did not cause a sustained shift in the resting [NO], which was an important issue for later experiments using L-NAME both after and during ongoing exposure to arginine. Panel B of Figure 1 is a presentation of the times required for steady state increases in blood flow to occur at L-arginine concentrations of 200–1200 μM. The flow responses represent resistance changes by the microvessels at different depths in the intestinal wall and responses of the mesenteric arteries covered to a large extent by fatty tissues. Consequently, the flow response times likely reflect a large component due to diffusion time rather than vascular reaction times. The time to steady state blood flow responses required about 300 seconds to be fully developed at arginine concentrations of 200 and 400 μM. Much faster responses occurred in 120–150 seconds at 800 and 1200 μM L-arginine.
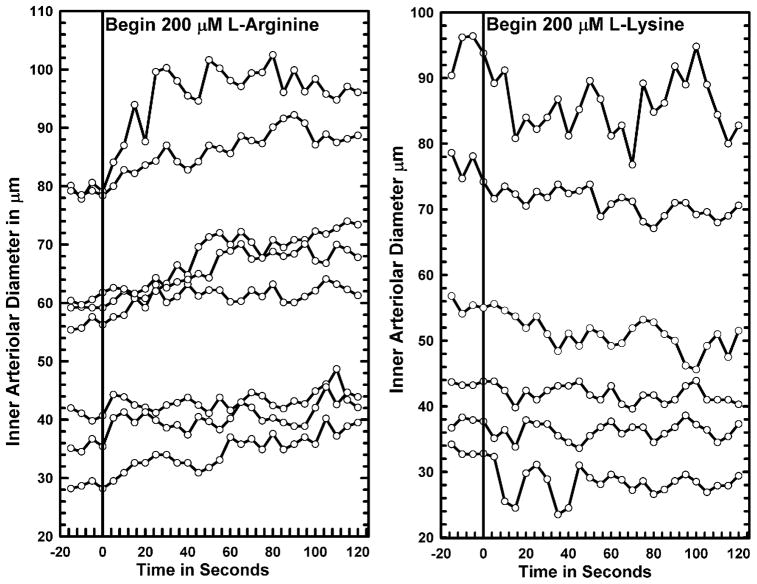
Having established that the onset of arteriolar [NO] responses to arginine likely were faster than we could change the entire bathing media, we used a microperfusion technique similar to that of Frame et al. (23; 24) to initiate the fastest possible vascular response to arginine. Examples of the diameter responses to L-arginine and L-lysine are shown in Figure 2. L-lysine competes with arginine for transport and is the preferred amino acid of the cationic transporters (2; 15). We have recently shown that topical 200 μM L-lysine effectively and quickly lowered perivascular [NO] and strongly suppressed increased [NO] and vasodilation to increased flow shear, locally reduced oxygen tension and NaCl hyperosmolarity, all of which are highly NO dependent in the small intestine vasculature (7; 9; 47; 59; 60). The dilatory response to arginine and constrictor response to lysine began consistently within 15 seconds and generally within 10 seconds and reached a stable diameter in 45–60 seconds. Sustained perfusion for 5–10 minutes maintained the arginine induced dilation or lysine associated constriction. We attempted NO measurement during the point source release of both arginine and lysine. We consistently found that the increase or decrease in [NO] was highly localized to the site of release and very difficult to follow as the vessel responded. As Frame et al. (23; 24) found, the vessels dilated or constricted over hundreds of microns distance by a vessel wall conducted mechanism. At distal sites along the arterioles, the [NO] invariably decreased with dilation and constriction presumably because shear stress decreased with both situations.
Figure 2.
Responses of large to small intestinal arterioles to locally applied 400 μM arginine or 200 μM lysine to individual microvessels from a pump perfused micropipette (10 nl/sec for <30 μm inner diameter arterioles and at 50 nl/sec for larger vessels) nearly touching the vessel wall. The purpose of these studies was to determine how rapidly vasodilation to arginine or constriction to lysine could be achieved. Vasodilation to arginine and constriction to lysine could often be detected at 5 seconds after release began and consistently occurred by 15 seconds of exposure. These results are based on observations from 14 vessels in 10 rats (8 vessels arginine; 6 vessels lysine).
Nitric Oxide and Vasodilatory Responses to L-Arginine and blockade by L-NAME
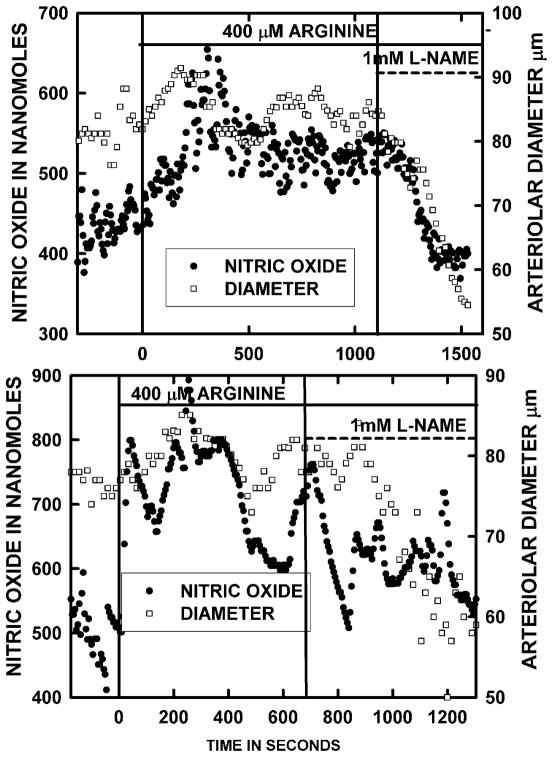
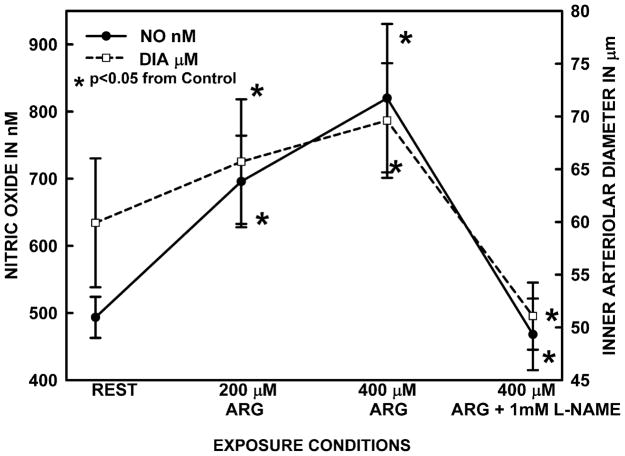
Figure 3 presents two representative experiments for both diameter and [NO] time events for bath addition of 400 μM arginine followed by L-NAME exposure as 400 μM arginine continued. The minimum time of arginine exposure was 10 minutes and often extended to 20 minutes or longer if the increases in [NO] and diameter developed a cycling pattern about a new increased mean. Although the increase in both [NO] did occur rapidly as described in Figure 1, the vessels invariably had some degree of vasomotion about the elevated [NO] and diameter. Figure 4 presents the averaged diameter and [NO] for 6 arterioles of 6 rats using this protocol. The average resting [NO] shown in Figure 4 was 493.6±30.5 nM and 200 and 400 μM L-arginine increased the concentration to 696.1±68.2 and 820.1±110.5 nM, respectively. We did use 100 μM L-arginine in pilot studies and found the [NO] and diameter responses were small and variable. L-NAME in the presence of 400 μM L-arginine reduced the [NO] to 468.2±53.4 nM, which was similar to the resting concentration and about half the concentration during 400 μM L-arginine. In addition, the L-NAME suppressed the dilation caused by L-arginine. When arginine infusion was stopped with ongoing infusion of L-NAME, intestinal wall motility became too rapid to reliably measure [NO] for 30–45 minutes. In a past studies, the [NO] was decreased ~ 40%(14) by 1 mM L-NAME and a similar amount by both L-N-nitroarginine (7) and embolization of an arteriole to stun the endothelial cells (47).
Figure 3.
Panels A and B present typical examples of [NO] and inner arteriolar diameter responses to the onset and sustained exposure to 400 μM arginine in the bath media. Once the responses to arginine were stable, 1 mM L-NAME was concurrently added to the bath media and the progressive suppression of responses was followed. Experiments of this type were the source for the data presented in Figure 4.
Figure 4.
The inner arteriolar diameter and vessel wall [NO] responses to 200 and 400 μM L-arginine and the suppression of these responses by L-NAME while 400 μM L-arginine continued to be present. Both 200 and 400 μM arginine elevated [NO] and a concomitant dilation occurred. L-NAME reduced increased [NO] and arteriolar diameter in the presence 400 μM L-arginine to values significantly below the original resting states. The results are based on 6 vessels from 6 rats.
Comparison of Arterial and Microvascular Resistance Responses to L-Arginine
During the preceding experiments, we noticed that not only did all sizes of arterioles dilate during topical arginine exposure, but the small arteries of the mesentery also were dilated. To evaluate arterial versus microvascular responses in relative terms, the resistances of both sections were measured from simultaneous blood flow and arterial pressure dissipation measurements. The blood flow to the entire intestinal wall, including muscular, submucosal, and mucosal layers, was measured in the largest arterioles and the data are shown in the upper panel of Figure 5. The intestinal vasculature was exposed to 100–1200 μM L-arginine with a recovery between each concentration. The data are presented in percent of control format because the randomly selected vessels for flow measurement in each animal perfused somewhat different amounts of intestinal tissue. Unlike the vasomotion that usually accompanied both [NO] and diameter responses of individual arterioles to arginine, flow was quite stable for a given status of arginine exposure. A significant increase in blood flow of 17% was found with 100 μM arginine and 400 μM arginine increased flow about 50%. L-arginine at 800 and 1200 μM did not significantly increase blood flow above that found with 400 μM. To determine if any amino acid would perhaps cause dilation, the L-alanine, a neutral amino acid that enters cells by a different carrier than L-arginine, was used. 1200 μM L-alanine for thirty minutes of exposure did not change the blood flow (101.8±3.7% of control). After all amino acids were tested, all vascular tone was abolished with100 μM sodium nitroprusside and blood flow increased to 265±16.1% of control.
The lower panel of Figure 5 presents percent of control resistance of arterial and microvascular regions during arginine exposure. Given that 400 μM L-arginine provided near maximal flow response, resistance calculations for arterial and microvascular responses were only measured for 100–400 μM L-arginine. The arterial resistance, which is composed of the mesenteric arteries and their major branches outside the intestinal wall, decreased significantly more at 100 and 200 μM arginine concentrations than did the microvascular resistance. These large vessels contributed 32.9% of the total intestinal vascular resistance based on the percentage of the total pressure dropped across the small arteries. During maximal dilation with 100 μM sodium nitroprusside, the resistances of the macro- and microvessel regions declined to 40.9±4.6 and 34.1±3.1 % of control.
Discussion
This study evaluated the hypothesis that for low concentrations of arginine to physiologically relevant, the increased in vivo perivascular NO concentration and vasodilation must both occur rapidly to reflect a transport mechanism and these responses must be blocked by suppression of eNOS by L-NAME. Physiological concentrations of arginine in the 100–400 μM range caused rapid dilation of both intestinal arterioles within one minute when applied through a topical bath and within 15 seconds when applied directly to individual small through large arterioles (Figs. 1–3). Furthermore, direct measurements of perivascular nitric oxide before and after blockade of eNOS with L-NAME indicated that the [NO] rapidly increased with topical exposure of L-arginine and both the dilation and increased [NO] were reversed by L-NAME during continued L-arginine exposure(Figs. 3 and 4). The rapid responses of both the [NO] and vasodilation to L-arginine were interpreted to indicate transport of the amino acid to NOS had immediate effects on generation of NO and vascular regulation. While arterioles dilated rapidly, the small arteries of the mesentery were much slower to respond, as shown by the increased time for total organ flow responses in Figure 1. This “slowness” may be diffusion limitations imposed by the overburden of mesenteric fatty tissue around the small arteries. However, the decrease in resistance by arterial vessels was actually larger than for microvessels at low arginine concentrations and similar at near maximal concentration, as shown in Figure 5, Panel B. The second hypothesis tested was that arginine in the physiological concentration range should cause meaningful increases in blood flow through dilation of both arterioles and small arteries. We found that a significant small increase in blood flow occurred at 100 μM topical L-arginine and for our preparation, near maximal responses of a ~50% increase in flow occurred at 400 μM topical L-arginine (Fig. 5). By comparison, Vukosavljevic et al. (56) found a similar increase in intestinal blood flow at ~2 millimolar L-arginine. This would support our findings that maximal flow responses to L-arginine occurred at much lower concentrations than previously suspected.
Most studies (9; 14; 18; 22; 37; 40; 54–56) of the acute effects of arginine on endothelial production of nitric oxide in vivo or in vitro have used topical concentrations more than ten times the plasma concentration of 50–75 μM (32; 33; 42; 53; 57; 58). Part of the rationale for using high concentrations of arginine for testing is that the intracellular L-arginine concentration range is 100 μM to 800 μM (2; 3; 28; 34) and even higher in freshly isolated endothelial cells (34). From the very high intracellular concentration, it would seem eNOS should, but does not, operate under a saturating intracellular arginine concentration. The basis for this comment is that the half saturating arginine concentration for eNOS is 1–10 μM (50; 52). Kurz and Harrison (39) have termed this situation as the arginine paradox. While studies using high concentrations of arginine do illustrate extracellular arginine can induce a nitric oxide related vasodilation in the in vivo microvasculature, such high concentrations of arginine are not realistic for long term clinical use of arginine and may be causing non-physiological effects of high concentrations of arginine, such as release of histamine from mast cells(26; 51). We chose to primarily use arginine concentrations at and below 400 μM that require transport by amino acid transporters (45) and not some indirect form of cell entry that is known to occur at mM concentrations (5; 16; 30). Our results for both increases in [NO], vasodilation, and blood flow are as large or larger than in earlier studies using much higher arginine concentrations(7; 56). The majority of in vivo studies of humans using physiologically attainable concentrations of 100–400 μM generated by dietary supplementation have shown enhanced microvascular perfusion and endothelial dependent dilation in the in vivo state(20) (18; 27; 35; 49) (4; 38; 40). The in vivo microvascular efficacy of low concentrations of arginine was shown in animal studies by Frame and colleagues (23; 24). They reported skeletal muscle arterioles of the rat rapidly dilated to local application of 100 μM L-arginine, which is just slightly higher or very near plasma concentrations of 50–100 μM generally reported for adult rats(32; 33; 42; 53; 57; 58) and the dilation was suppressed by inhibition of eNOS. In the current study with the small intestinal vasculature of rats, 200 and 400 μM L-arginine increased blood flow within three minutes and the increased [NO] and diameter of arterioles generally approached 90% of the new and larger steady state within about 60 seconds of adding 400 μM L-arginine to the topical bath (Fig. 1). A concentration dependent increase in organ blood flow was found for the 100–400 μM L-arginine concentration range with maximal responses beginning at about 400 μM (Figs. 5). The blood flow responses were not trivial because 400 μM L-arginine increased intestinal blood flow by 50%(Fig. 5), which is comparable to the increased intestinal blood flow of ~35% found by Vukosavljevic et al (56) using a bolus ejection of 100 millimolar arginine in a volume that would dilute to ~2 mM arginine in the ~50 ml tissue bath. Furthermore, 400 μM L-arginine in our experience increased the perivascular [NO] (Fig. 4) by about 400 nM, or nearly double the resting concentration. Virtually identical responses were found by Vukosavljevic et al (56) at 2 millimolar L-arginine and are comparable to our earlier observations of NO responses at 1 mM L-arginine (7). In both the current study and a more preliminary earlier study (7), 1 mM L-NAME during the ongoing exposure to L-arginine rapidly diminished the increased [NO] and vasodilation to below resting values within 5–10 minutes(Figs. 3 and 4). These observations indicate that in the near physiological range of arginine, most of the vasodilation was linked to increased NO generation rather than some non-specific effect of arginine on smooth muscle function.
The past in vivo studies by both Frame and colleagues (23; 24) and Vukosavljevic et al. (56) have shown that arteriolar responses to L-arginine are fast, within tens of seconds. Our results in Figures 1 and 3 confirm the rapidity and stability of the NO response to arginine both in terms of vasodilation and increased generation of NO. In a prior study (54), we used an entirely different approach to study arginine effects by restricting transport of native arginine by providing additional L-lysine to compete for transport. Our results indicated lysine competition for transport quickly diminished NO and dilatory responses to increased flow, decreased oxygen tension, and NaCl hyperosmolarity, each of which is dependent on the NO mechanism (7; 9; 47; 59; 60). The evidence from past studies and the new data predict that ongoing transport of cationic amino acids (45) is used to support NO production and the availability of extracellular arginine can quickly and substantially impact NO generation by eNOS.
In the earlier studies of arginine effects (23:24:56), the period of exposure to arginine was of the order of 3–5 minutes. We questioned whether the prolonged exposure to arginine for 15–20 minutes and longer in many cases might cause a fast initial rise in [NO] followed by a gradual lowering of the response as eNOS regulatory feedback mechanisms were activated. As shown in Figure 3, exposure of arginine for 15–20 minutes was not associated with a significant decline in either [NO] or vasodilation. Sustained l-arginine exposure did increase vasomotion of the arteriole about a larger average diameter and a corresponding cycling of the [NO] about a higher average concentration (Fig. 3). For these time periods studied, it appears that excess arginine even at relatively low concentrations can force a sustained increase in NO production. What was particularly interesting is that even with prolonged arginine exposure, the vessels recovered most of their basal diameter and [NO] in about three minutes, as shown in Figures 1 and 3. The current observations of persistent elevation of [NO] and diameter during arginine exposure yet rapid recovery of response during arginine withdrawal indicate relatively small changes in extracellular arginine availability modulated NO production much more than is currently appreciated.
The intestinal preparation allowed us to evaluate to what extent the arterial and microvascular sections of the intestinal vasculature caused the reduction of resistance and the increase in blood flow during arginine exposure. This information is not currently available for any vascular bed. The data in Figure 5 indicate that mesenteric arteries reduced their resistance proportionately and significantly more than did the microvessels at 100 and 200 μM arginine. However, in following the blood flow responses, the arterioles had completed their responses while flow continued to increase for about 60–90 seconds (Fig. 1, Panel B). The delay in flow response is due to gradual dilation of the small arteries in the mesentery. Based on the fraction of arterial pressure dissipated, the small arteries regulated about one-third of the arterial pressure prior to the entry of the largest arterioles into the bowel wall(Results), which is in agreement with our earlier observations(6; 8). Therefore, dilation of the resistance arteries to arginine can and did make a significant contribution to increased blood flow. However, the arteriolar response is the dominant cause of decreased resistance and increased blood flow for the overall vasculature. Our measurement of microvascular resistance in Figure 5 included the nearly fixed resistance of the capillary bed and venular vasculature(6). Assuming capillary and venular resistance is about 30% of the microvascular resistance based on our past measurements(6; 29), the arterioles would have had to decrease their resistance by nearly twice that of arteries to achieve the reductions in total microvascular resistance shown in Figure 5 for each arginine concentration tested. Therefore, from a functional stand point, both arteries and arterioles were very responsive to arginine for the physiological concentration range tested, but arterioles were the dominant resistance site of the arginine induced NO response. However, the data and the associated conclusions on resistance reductions by arteries and microvessels in Figures 4 and 5 may somewhat underestimate vascular responses to arginine because topical arginine was used to avoid systemic vascular effects. Consequently, the endothelial cells are exposed to both the plasma concentration of arginine and the extravascular arginine concentration such that the net arginine concentration influencing the cells would be lower than the bath concentration. However, even with this caveat, the results did indicate a functionally important vasodilation of macro- and microscopic resistance vessels in response to excess arginine at realistic physiological concentrations.
Acknowledgments
Supported by NIH Grant HL-20605 and Ms. Pezzuto was supported by the Medical Student Summer Research Program at Indiana University Medical School.
Reference List
- 1.Bagi Z, Hamar P, Antus B, Rosivall L, Koller A. Chronic renal failure leads to reduced flow-dependent dilation in isolated rat skeletal muscle arterioles due to lack of NO mediation. Kidney Blood Press Res. 2003;26:19–26. doi: 10.1159/000069762. [DOI] [PubMed] [Google Scholar]
- 2.Baydoun AR, Emery PW, Pearson JD, Mann GE. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem Biophys Res Commun. 1990;173:940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- 3.Block ER, Herrera H, Couch M. Hypoxia inhibits L-arginine uptake by pulmonary artery endothelial cells. Am J Physiol. 1995;269:L574–L580. doi: 10.1152/ajplung.1995.269.5.L574. [DOI] [PubMed] [Google Scholar]
- 4.Bode-Boger SM, Muke J, Surdacki A, Brabant G, Boger RH, Frolich JC. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8:77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- 5.Bogle RG, Baydoun AR, Pearson JD, Mann GE. Regulation of L-arginine transport and nitric oxide release in superfused porcine aortic endothelial cells. J Physiol. 1996;490:229–241. doi: 10.1113/jphysiol.1996.sp021138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohlen HG. Determinants of resting and passive intestinal vascular pressures in rat and rabbit. Am J Physiol. 1987;253:G587–G595. doi: 10.1152/ajpgi.1987.253.5.G587. [DOI] [PubMed] [Google Scholar]
- 7.Bohlen HG. Mechanism of increased vessel wall nitric oxide concentrations during intestinal absorption. Am J Physiol. 1998;275:H542–H550. doi: 10.1152/ajpheart.1998.275.2.H542. [DOI] [PubMed] [Google Scholar]
- 8.Bohlen HG, Maass-Moreno R, Rothe CF. Hepatic venular pressures of rats, dogs, and rabbits. Am J Physiol. 1991;261:G539–G547. doi: 10.1152/ajpgi.1991.261.3.G539. [DOI] [PubMed] [Google Scholar]
- 9.Bohlen HG, Nase GP. Dependence of intestinal arteriolar regulation on flow-mediated nitric oxide formation. Am J Physiol Heart Circ Physiol. 2000;279:H2249–H2258. doi: 10.1152/ajpheart.2000.279.5.H2249. [DOI] [PubMed] [Google Scholar]
- 10.Borders JL, Granger HJ. An optical doppler intravital velocimeter. Microvasc Res. 1984;27:117–127. doi: 10.1016/0026-2862(84)90047-5. [DOI] [PubMed] [Google Scholar]
- 11.Buerk DG, Riva CE, Cranstoun SD. Nitric oxide has a vasodilatory role in cat optic nerve head during flicker stimuli. Microvas Res. 1996;52:13–26. doi: 10.1006/mvre.1996.0040. [DOI] [PubMed] [Google Scholar]
- 12.Chen YM, Qian ZM, Zhang J, Chang YZ, Duan XL. Distribution of constitutive nitric oxide synthase in the jejunum of adult rat. World J Gastroenterol. 2002;8:537–539. doi: 10.3748/wjg.v8.i3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christodoulou D, Kudo S, Cook JA, Krishna MC, Miles A, Grisham MB, Murugesan R, Ford PC, Wink DA. Electrochemical methods for detection of nitric oxide. Meth Enzym. 1996;268:69–83. doi: 10.1016/s0076-6879(96)68010-0. [DOI] [PubMed] [Google Scholar]
- 14.Chu S, Bohlen HG. High concentration of glucose inhibits glomerular endothelial eNOS through a PKC mechanism. Am J Physiol Renal Physiol. 2004;287:F384–F392. doi: 10.1152/ajprenal.00006.2004. [DOI] [PubMed] [Google Scholar]
- 15.Closs EI, Basha FZ, Habermeier A, Forstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 16.Closs EI, Mann GE. Identification of carrier systems in plasma membranes of mammalian cells involved in transport of L-arginine. Methods Enzymol. 1999;301:78–91. doi: 10.1016/s0076-6879(99)01071-x. [DOI] [PubMed] [Google Scholar]
- 17.Closs EI, Simon A, Vekony N, Rotmann A. Plasma membrane transporters for arginine. J Nutr. 2004;134:2752S–2759S. doi: 10.1093/jn/134.10.2752S. [DOI] [PubMed] [Google Scholar]
- 18.Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90:1248–1253. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis MJ. Determination of volumetric flow in capillary tubes using an optical Doppler velocimeter. Microvasc Res. 1987;34:223–230. doi: 10.1016/0026-2862(87)90055-0. [DOI] [PubMed] [Google Scholar]
- 20.Egashira K, Hirooka Y, Kuga T, Mohri M, Takashita A. Effects of L-arginine supplementation on endothelium-dependent coronary vasodilation in patients with angina pectoris and normal coronary arteriograms. Circulation. 1996;94:130–134. doi: 10.1161/01.cir.94.2.130. [DOI] [PubMed] [Google Scholar]
- 21.Erdei N, Toth A, Pasztor ET, Papp Z, Edes I, Koller A, Bagi Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol. 2006;291:H2107–H2115. doi: 10.1152/ajpheart.00389.2006. [DOI] [PubMed] [Google Scholar]
- 22.Fike CD, Kaplowitz MR, Rehorst-Paea LA, Nelin LD. L-Arginine increases nitric oxide production in isolated lungs of chronically hypoxic newborn pigs. J Appl Physiol. 2000;88:1797–1803. doi: 10.1152/jappl.2000.88.5.1797. [DOI] [PubMed] [Google Scholar]
- 23.Frame MD. Conducted signals within arteriolar networks initiated by bioactive amino acids. Am J Physiol. 1999;276:H1012–H1021. doi: 10.1152/ajpheart.1999.276.3.H1012. [DOI] [PubMed] [Google Scholar]
- 24.Frame MDS, Sarelius IH. L-Arginine-induced conducted signals alter upstream arteriolar responsivity to L-Arginine. Circ Res. 1995;77:695–701. doi: 10.1161/01.res.77.4.695. [DOI] [PubMed] [Google Scholar]
- 25.Friedemann MN, Robinson SW, Gerhardt GA. o-Phenylenediamine-modified carbon fiber electrodes for the detection of nitric oxide. Anal Chem. 1996;68:2621–2628. doi: 10.1021/ac960093w. [DOI] [PubMed] [Google Scholar]
- 26.Giraldelo CM, Zappellini A, Muscara MN, De LI, Hyslop S, Cirino G, Zatz R, De NG, Antunes E. Effect of arginine analogues on rat hind paw oedema and mast cell activation in vitro. Eur J Pharmacol. 1994;257:87–93. doi: 10.1016/0014-2999(94)90698-x. [DOI] [PubMed] [Google Scholar]
- 27.Gokce N. L-arginine and hypertension 1. J Nutr. 2004;134:2807S–2811S. doi: 10.1093/jn/134.10.2807S. [DOI] [PubMed] [Google Scholar]
- 28.Gold ME, Bush PA, Ignarro LJ. Depletion of arterial L-arginine causes reversible tolerance to endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989;164:714–721. doi: 10.1016/0006-291x(89)91518-0. [DOI] [PubMed] [Google Scholar]
- 29.Gore RW, Bohlen HG. Microvascular pressures in rat intestinal muscle and mucosal villi. Am J Physiol. 1977;233:H685–H693. doi: 10.1152/ajpheart.1977.233.6.H685. [DOI] [PubMed] [Google Scholar]
- 30.Greene B, Pacitti AJ, Souba WW. Characterization of L-arginine transport by pulmonary artery endothelial cells. Am J Physiol. 1993;264:L351–L356. doi: 10.1152/ajplung.1993.264.4.L351. [DOI] [PubMed] [Google Scholar]
- 31.Guo JP, Murohara T, Buerke M, Scalia R, Lefer AM. Direct measurement of nitric oxide release from vascular endothelial cells. J Appl Physiol. 1996;81:774–779. doi: 10.1152/jappl.1996.81.2.774. [DOI] [PubMed] [Google Scholar]
- 32.Hardy TA, May JM. Coordinate regulation of L-arginine uptake and nitric oxide synthase activity in cultured endothelial cells. Free Radic Biol Med. 2002;32:122–131. doi: 10.1016/s0891-5849(01)00781-x. [DOI] [PubMed] [Google Scholar]
- 33.Hartman WJ, Seyoum E, Villalobos-Molina R, Joseph JA, Prior RL. Responses of circulating urea cycle and branched-chain amino acids to feeding in adult and aged Fischer-344 rats. Aging (Milano) 1997;9:198–206. doi: 10.1007/BF03340150. [DOI] [PubMed] [Google Scholar]
- 34.Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990;87:8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huynh NN, Chin-Dusting J. Amino acids, arginase and nitric oxide in vascular health 1. Clin Exp Pharmacol Physiol. 2006;33:1–8. doi: 10.1111/j.1440-1681.2006.04316.x. [DOI] [PubMed] [Google Scholar]
- 36.Intaglietta M, Parvula PR, Tompkins WR. Pressure measurement in the mammalian microvasculature. Microvasc Res. 1970;2:212–220. doi: 10.1016/0026-2862(70)90009-9. [DOI] [PubMed] [Google Scholar]
- 37.Jiang J, Valen G, Tokuno S, Thoren P, Pernow J. Endothelial dysfunction in atherosclerotic mice: improved relaxation by combined supplementation with L-arginine-tetrahydrobiopterin and enhanced vasoconstriction by endothelin. Br J Pharmacol. 2000;131:1255–1261. doi: 10.1038/sj.bjp.0703705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakoki M, Kim HS, Edgell CJ, Maeda N, Smithies O, Mattson DL. Amino acids as modulators of endothelium-derived nitric oxide 1. Am J Physiol Renal Physiol. 2006;291:F297–F304. doi: 10.1152/ajprenal.00417.2005. [DOI] [PubMed] [Google Scholar]
- 39.Kurz S, Harrison DG. Insulin and the arginine paradox. J Clin Invest. 1997;99:369–370. doi: 10.1172/JCI119166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lerman A, Burnett JC, Jr, Higano ST, McKinley LJ, Holmes DR., Jr Long-term L-Arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–2128. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Liu Q, Gupta E, Zorko N, Brownlee E, Zweier JL. Quantitative measurements of NO reaction kinetics with a Clark-type electrode. Nitric Oxide. 2005;13:68–77. doi: 10.1016/j.niox.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 42.MacKenzie A, Wadsworth RM. Extracellular L-arginine is required for optimal NO synthesis by eNOS and iNOS in the rat mesenteric artery wall. Br J Pharmacol. 2003;139:1487–1497. doi: 10.1038/sj.bjp.0705380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malinski T, Mesaros S, Tomboulian P. Nitric oxide measurement using electrochemical methods. Meth Enzym. 1996;268:58–69. doi: 10.1016/s0076-6879(96)68009-4. [DOI] [PubMed] [Google Scholar]
- 44.Malinski T, Taha Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature. 1992;358:676–678. doi: 10.1038/358676a0. [DOI] [PubMed] [Google Scholar]
- 45.Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- 46.McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J Biol Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 47.Nase GP, Tuttle J, Bohlen HG. Reduced perivascular PO2 increases nitric oxide release from endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285:H507–H515. doi: 10.1152/ajpheart.00759.2002. [DOI] [PubMed] [Google Scholar]
- 48.Nichols KSWKA. Nitric oxide synthase distribution in the rat intestine: a histochemical analysis. Gastroenterology. 1993;105:1651–1661. doi: 10.1016/0016-5085(93)91060-u. [DOI] [PubMed] [Google Scholar]
- 49.Palloshi A, Fragasso G, Piatti P, Monti LD, Setola E, Valsecchi G, Galluccio E, Chierchia SL, Margonato A. Effect of oral L-arginine on blood pressure and symptoms and endothelial function in patients with systemic hypertension, positive exercise tests, and normal coronary arteries. Am J Cardiol. 2004;93:933–935. doi: 10.1016/j.amjcard.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 50.Palmer RM, Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989;158:348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- 51.Peh KH, Moulson A, Wan BY, Assem EK, Pearce FL. Role of nitric oxide in histamine release from human basophils and rat peritoneal mast cells 3. Eur J Pharmacol. 2001;425:229–238. doi: 10.1016/s0014-2999(01)01205-5. [DOI] [PubMed] [Google Scholar]
- 52.Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prados P, Matsunaga H, Mori T, Santa T, Fukushima T, Homma H, Kasai C, Imai K. Changes of plasma L-arginine levels in spontaneously hypertensive rats under induced hypotension. Biomed Chromatogr. 1999;13:27–32. doi: 10.1002/(SICI)1099-0801(199902)13:1<27::AID-BMC807>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Schini VB, Vanhoutte PM. L-arginine evokes both endothelium-dependent and -independent relaxations in L-arginine-depleted aortas of the rat. Circ Res. 1991;68:209–216. doi: 10.1161/01.res.68.1.209. [DOI] [PubMed] [Google Scholar]
- 55.Sun D, Messina EJ, Koller A, Wolin MS, Kaley G. Endothelium-dependent dilation to L-arginine in isolated rat skeletal muscle arterioles. Am J Physiol. 1992;262:H1211–H1216. doi: 10.1152/ajpheart.1992.262.4.H1211. [DOI] [PubMed] [Google Scholar]
- 56.Vukosavljevic N, Jaron D, Barbee KA, Buerk DG. Quantifying the l-arginine paradox in vivo. Microvasc Res. 2006;71:48–54. doi: 10.1016/j.mvr.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Wagner L, Riggleman A, Erdely A, Couser W, Baylis C. Reduced nitric oxide synthase activity in rats with chronic renal disease due to glomerulonephritis 2. Kidney Int. 2002;62:532–536. doi: 10.1046/j.1523-1755.2002.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wakabayashi Y, Yamada E, Yoshida T, Takahashi H. Arginine becomes an essential amino acid after massive resection of rat small intestine 3. J Biol Chem. 1994;269:32667–32671. [PubMed] [Google Scholar]
- 59.Zani BG, Bohlen HG. Sodium channels are required during in vivo sodium chloride hyperosmolarity to stimulate an increase in intestinal endothelial nitric oxide production. Am J Physiol Heart Circ Physiol. 2004 doi: 10.1152/ajpheart.00644.2004. [DOI] [PubMed] [Google Scholar]
- 60.Zani BG, Bohlen HG. Sodium channels are required during in vivo sodium chloride hyperosmolarity to stimulate increase in intestinal endothelial nitric oxide production. Am J Physiol Heart Circ Physiol. 2005;288:H89–H95. doi: 10.1152/ajpheart.00644.2004. [DOI] [PubMed] [Google Scholar]
- 61.Zani BG, Bohlen HG. Transport of extracellular L-arginine via the cationic amino acid transporter is required during in vivo endothelial nitric oxide production. Am J Physiol Heart Circ Physiol. 2005;289:H1381–H1390. doi: 10.1152/ajpheart.01231.2004. [DOI] [PubMed] [Google Scholar]