Abstract
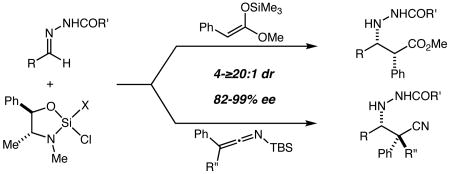
Mannich reactions with chiral silicon Lewis acid-activated acylhydrazones and α-aryl silyl ketene acetals and α-aryl, α-alkyl silyl ketene imines proceed efficiently and with good to excellent levels of both diastereoselectivity and enantioselectivity. The reactions provide access to α-aryl,β-hydrazido esters and α-aryl,α-alkyl,β-hydrazido nitriles, which are valuable analogs of β-amino acids.
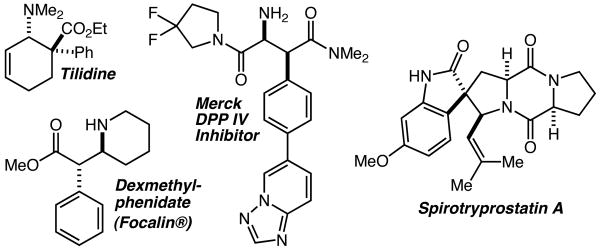
The Mannich reaction – broadly defined as the addition of an enol, enolate, or enolate equivalent to an imine – provides access to β-amino acids and related structures, and in recent years has been the subject of an extraordinary amount of effort directed towards the development of efficient and highly enantioselective variants.1 While impressive successes have been recorded using several different approaches, the substrate scope remains limited in notable ways. For example, there are relatively few reports that describe highly diastereoselective and/or enantioselective Mannich reactions of α-aryl substituted enolates (or enolate equivalents).2 This relative paucity of general methods is particularly noteworthy as an examination of the literature reveals many examples of bioactive compounds and natural products with an α-aryl,β-aminocarboxyl substructure (Figure 1). This subset of Mannich reactions is thus one of fundamental synthetic importance for which there are few practical, general, and highly enantioselective solutions. Herein we report highly enantioselective Mannich reactions with α-aryl silyl ketene acetals and with α-aryl,α-alkyl silyl ketene imines that allow direct access to structures such as those depicted in Figure 1 using convenient, inexpensive, and scalable procedures.
Figure 1.
Examples of bioactive compounds possessing an α-aryl,β-aminocarbonyl substructure.
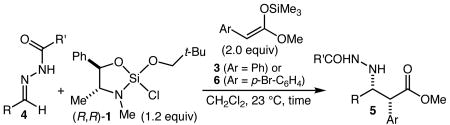
We recently reported neo-pentoxychlorosilane 1 and its use in highly enantioselective Mannich reactions of aliphatic ketone-derived acylhydrazones with silyl ketene acetal (SKA) 2 (Scheme 1),3,4 and this seemed a reasonable starting point for the present investigation. Indeed, it was quickly found that SKA 3 (prepared and employed as 13:1 Z:E mixture) reacts smoothly with the silane 1/hydrazone 4a complex to give syn-Mannich product 5a. Optimization was straightforward and by performing the reaction in CH2Cl2 at ambient temperature for 4 hours, 5a could be isolated in 77% yield as a single diastereomer in 97% ee.
Scheme 1.
Summarized in Table 1 is a brief survey of the scope of the reaction with respect to the hydrazone substrate. Optimization focused on the nature of the group (R′) on the hydrazone as we have found that this can have a significant effect on reaction performance. The use of both aromatic and aliphatic aldehyde-derived hydrazones resulted in excellent levels of enantioselectivity (entries 1-4), albeit with only moderate diastereoselectivity for unhindered aliphatic substrates (entries 2 and 3). As shown in entry 5, however, this moderate diastereoselectivity may be significantly improved simply by performing the reaction in PhCF3. Although the reaction is slower and required a higher silane loading, the product 5b was isolated with 13:1 dr and 94% ee. Glyoxylate-derived hydrazone 4e (R = CO2i-Pr, R′ = p-MeOC6H4) was employed as well (entry 6), and although the enantiopurity of the product (5e) was somewhat lower with this substrate, this reaction nevertheless provides a useful and direct entry into systems such as the Merck DPP IV inhibitor5,6 (see Figure 1). Finally, the use of a substituted aryl group (p-bromophenyl) on the SKA (6, prepared and employed as a 6:1 Z:E mixture) was demonstrated with hydrazone 4b, which led to the isolation of 5f (R = PhCH2CH2, R′ = Ph, Ar = p-Br-C6H4) in 79% yield (4:1 dr) and 95% ee (entry 7).
Table 1.
Highly enantioselective Mannich reactions with α-aryl SKAs 3 and 6.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | R | R′ | SKA | time (h) | product | yield (%) | dr | ee (%) |
| 1 | Ph | p-CF3C6H4 | 3 | 4 | 5a | 77 | >20:1 | 97 |
| 2 | PhCH2CH2 | Ph | 3 | 1 | 5b | 89 | 5:1 | 95 |
| 3 | i-PrCH2 | Ph | 3 | 2 | 5c | 86 | 4:1 | 95 |
| 4a | i-Pr | p-CF3C6H4 | 3 | 48 | 5d | 70 | 9:1 | 99 |
| 5b | PhCH2CH2 | Ph | 3 | 20 | 5b | 74 | 13:1 | 94 |
| 6c | CO2i-Pr | p-MeOC6H4 | 3 | 2.5 | 5e | 91 | 5:1 | 82 |
| 7 | PhCH2CH2 | Ph | 6 | 1 | 5f | 79 | 4:1 | 95 |
This reaction was run at 0 °C.
This reaction was run with 1.5 equiv of (R,R)-1 and 3 equiv of SKA 3 in PhCF3 at 0 °C.
This reaction was run with 2.4 equiv of SKA 3 in PhCF3.
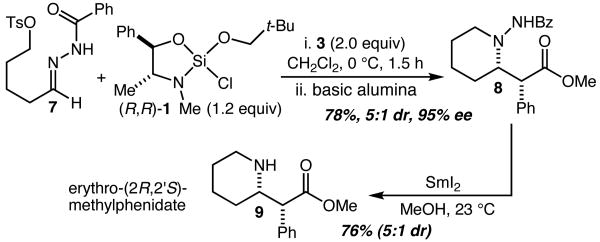
As an additional demonstration of the power of this method to allow direct and efficient access to medicinally relevant structures, hydrazone 7 was prepared and subjected to the reaction conditions described in Table 1, above (Scheme 2). Prior to isolation, the unpurified Mannich product was treated with basic alumina resulting in smooth cyclization to give 8 in 78% overall yield (5:1 dr) and 95% ee. Reductive cleavage of the N-N bond was accomplished with SmI2,7 and led to the isolation of erythro-(2R,2′S)-methylphenidate 9 as the major product of a 5:1 mixture of diastereomers in 76% yield.
Scheme 2.
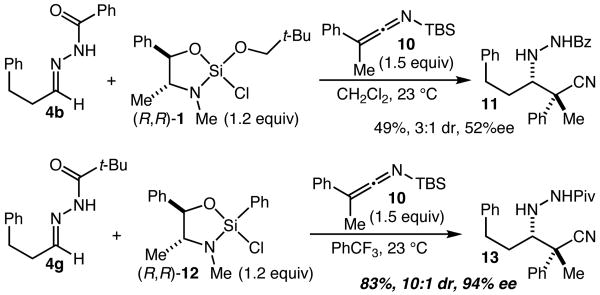
Structures such as tilidine8 and spirotryprostatin A9 (Figure 1), and more generally the challenge of establishing quaternary carbon stereocenters in the context of complex β-amino acid derivatives led us to examine α-aryl-α-alkyl enolate equivalents.2i While a variety of α,α-disubstituted SKAs were found to be unreactive towards silane-hydrazone complexes, we were delighted to find that silyl ketene imine (SKI) 1010,11 smoothly added to the complex formed from hydrazone 4b and silane 1 to give 11 (R = Ph) in 49% yield (3:1 dr, 52% ee) (Scheme 3). Previously described (and commercially available) phenylsilane 1212 was found to give improved stereoselectivity, and, when paired with the bulkier pivaloylhydrazone 4g in PhCF3 as the solvent, gave 13 (R = t-Bu) in 83% yield (10:1 dr) and 94% ee.
Scheme 3.
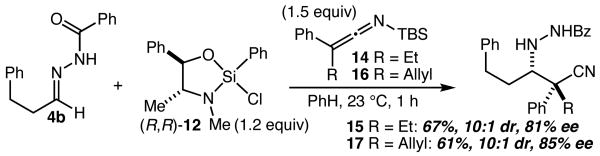
Attempts to expand the scope of this reaction with respect to the SKI structure revealed that this result was not general, as reactions with bulkier SKIs and pivaloylhydrazone 4g led to poorly efficient reactions. Good results nevertheless proved attainable by employing the less hindered benzoyl hydrazone 4b. As shown in Scheme 4, when the complex formed from 4b and 12 was treated with ethyl-substituted SKI 14 in benzene, 15 was obtained in 67% yield (10:1 dr) and 81% ee, while under the same conditions SKI 16 led to 17 in 84% yield (10:1 dr) and 85% ee. While more work will be necessary to develop this reaction into a method that more reliably provides higher levels of enantioselectivity with a broader substrate scope, the reactions described in Schemes 3 and 4 establish that α,α-disubstituted β-amino acid analogs can be effectively accessed using this approach.
Scheme 4.
We have developed a series of Mannich reactions involving the addition of α-aryl silyl ketene acetals and α-aryl,α-alkyl silyl ketene imines to chiral silicon Lewis acid-activated acylhydrazones. The reactions proceed efficiently and with good to excellent levels of both diastereoselectivity and enantioselectivity. We believe these reactions may find utility as a convenient entry into some relatively structurally and stereochemically complex α-aryl,β-amino acid analogs.
Supplementary Material
Acknowledgments
This work was supported by a grant (CHE-08-09659) from the National Science Foundation. G.T.N. is the recipient of a postdoctoral fellowship (F32GM080859) from the National Institute of General Medical Sciences. We thank Novartis for a graduate fellowship to J.M.B. We thank Prof. Ged Parkin and Mr. Wesley Sattler (Columbia University, Department of Chemistry) for an X-ray structure analysis (see Supporting Information), and the National Science Foundation (CHE-0619638) is thanked for acquisition of an x-ray diffractometer.
Footnotes
Supporting Information Available: Experimental procedures, characterization data, and stereochemical proofs. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Kobayashi S, Ishitani H. Chem Rev. 1999;99:1069. doi: 10.1021/cr980414z. [DOI] [PubMed] [Google Scholar]; (b) Liu M, Sibi MP. Tetrahedron. 2002;58:7991. [Google Scholar]; (c) Taggi AE, Hafez AM, Lectka T. Acc Chem Res. 2003;36:10. doi: 10.1021/ar020137p. [DOI] [PubMed] [Google Scholar]; (d) Marques MMB. Angew Chem Int Ed. 2006;45:348. doi: 10.1002/anie.200502630. [DOI] [PubMed] [Google Scholar]; (e) Friestad GK, Mathies AK. Tetrahedron. 2007;63:2541. doi: 10.1016/j.tet.2007.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ting A, Schaus SE. Eur J Org Chem. 2007:5797. [Google Scholar]; (g) Verkade JMM, van Hemert LJC, Quaedflieg PJLM, Rutjes PJT. Chem Soc Rev. 2008;37:29. doi: 10.1039/b713885g. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kunz H, Burgard A, Schanzenbach D. Angew Chem Int Ed Engl. 1997;36:386. [Google Scholar]; (b) Poulsen TB, Alemparte C, Saaby S, Bella M, Jørgensen KA. Angew Chem Int Ed. 2005;44:2896. doi: 10.1002/anie.200500144. [DOI] [PubMed] [Google Scholar]; (c) Guerrini A, Varchi G, Daniele R, Samorì C, Battaglia A. Tetrahedron. 2007;63:7949. [Google Scholar]; (d) Huang H, Guo X, Hu W. Angew Chem Int Ed. 2007;46:1337. doi: 10.1002/anie.200604389. [DOI] [PubMed] [Google Scholar]; (e) Hu W, Xu X, Zhou J, Liu WJ, Huang H, Hu J, Yang L, Gong LZ. J Am Chem Soc. 2008;130:7782. doi: 10.1021/ja801755z. [DOI] [PubMed] [Google Scholar]; (f) Tian X, Jiang K, Peng J, Du W, Chen YC. Org Lett. 2008;10:3583. doi: 10.1021/ol801351j. [DOI] [PubMed] [Google Scholar]; (g) Cheng L, Liu L, Jia H, Wang D, Chen YJ. J Org Chem. 2009;74:4650. doi: 10.1021/jo9006688. [DOI] [PubMed] [Google Scholar]; (h) Izumiseki A, Yoshida K, Yanagisawa A. Org Lett. 2009;11:5310. doi: 10.1021/ol9022613. [DOI] [PubMed] [Google Scholar]; (i) Yin L, Kanai M, Shibasaki M. J Am Chem Soc. 2009;131:9610. doi: 10.1021/ja9036675. [DOI] [PubMed] [Google Scholar]
- 3.Notte GT, Leighton JL. J Am Chem Soc. 2008;130:6676. doi: 10.1021/ja800830h. [DOI] [PubMed] [Google Scholar]
- 4.Silane 1 is isolated and employed as a 2.2:1 mixture of diastereomers. We have previously provided evidence that upon reaction with a hydrazone, the diastereomers converge upon a single complex prior to the C-C bond-forming event. See ref. 3.
- 5.Edmondson SD, Mastracchio A, Mathvink RJ, He J, Harper B, Park YJ, Beconi M, Di Salvo J, Eiermann GJ, He H, Leiting B, Leone JF, Levorse DA, Lyons K, Patel RA, Patel SB, Petrov A, Scapin G, Shang J, Sinha Roy R, Smith A, Wu JK, Xu S, Zhu B, Thornberry NA, Weber AE. J Med Chem. 2006;49:3614. doi: 10.1021/jm060015t. [DOI] [PubMed] [Google Scholar]
- 6.The Merck Research Laboratories Department of Process Research has also reported a Mannich reaction-based approach to the synthesis of these bioactive compounds. See: Janey JM, Hsiao Y, Armstrong JD., III J Org Chem. 2006;71:390. doi: 10.1021/jo0519458.
- 7.Burk MJ, Martinez JP, Feaster JE, Cosford N. Tetrahedron. 1994;50:4399. [Google Scholar]
- 8.(a) Satzinger G. Liebigs Ann Chem. 1969;728:64. doi: 10.1002/jlac.19697280111. [DOI] [PubMed] [Google Scholar]; (b) Satzinger G. Liebigs Ann Chem. 1972;758:43. [Google Scholar]
- 9.(a) Cui CB, Kakeya H, Osada H. J Antibiot. 1996;49:832. doi: 10.7164/antibiotics.49.832. [DOI] [PubMed] [Google Scholar]; (b) Cui CB, Kakeya H, Osada H. Tetrahedron. 1996;52:12651. [Google Scholar]
- 10.For early studies on the generation of silyl ketene imines and their use in reactions with electrophiles, see: Gornowicz GA, West R. J Am Chem Soc. 1971;93:1714.Watt DS. Synth Commun. 1974;4:127.Cazeau P, Llonch JP, Simonin-Dabescat F, Frainnet E. J Organomet Chem. 1976;105:145.Cazeau P, Llonch JP, Simonin-Dabescat F, Frainnet E. J Organomet Chem. 1976;105:157.Meier S, Würthwein EU. Chem Ber. 1990;123:2339.
- 11.For two recent examples of the use of silyl ketene imines in asymmetric catalytic reactions, see: Mermerian AH, Fu GC. Angew Chem Int Ed. 2005;44:949. doi: 10.1002/anie.200461886.Denmark SE, Wilson TW, Burk MT, Heemstra JR., Jr J Am Chem Soc. 2007;129:14864. doi: 10.1021/ja077134y.
- 12.(a) Berger R, Duff K, Leighton JL. J Am Chem Soc. 2004;126:5686. doi: 10.1021/ja0486418. [DOI] [PubMed] [Google Scholar]; (b) Shirakawa S, Berger R, Leighton JL. J Am Chem Soc. 2005;127:2858. doi: 10.1021/ja042522a. [DOI] [PubMed] [Google Scholar]; (c) Shirakawa S, Lombardi PJ, Leighton JL. J Am Chem Soc. 2005;127:9974. doi: 10.1021/ja052307+. [DOI] [PubMed] [Google Scholar]; (d) Bou-Hamdan FR, Leighton JL. Angew Chem Int Ed. 2009;48:2403. doi: 10.1002/anie.200806110. [DOI] [PubMed] [Google Scholar]; (e) Valdez SC, Leighton JL. J Am Chem Soc. 2009;131:14638. doi: 10.1021/ja9066354. [DOI] [PubMed] [Google Scholar]; (f) Tambar UK, Lee SK, Leighton JL. J Am Chem Soc. 2010;132:10248. doi: 10.1021/ja104480g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.