Abstract
Abdominal aortic aneurysm (AAA) disease is a prevalent and highly morbid condition among older people in the US. There are currently no proven methods for reducing or eliminating enlargement in smaller preclinical aneurysms. Given their relatively slow increase in diameter (typically <0.4 cm/year), these smaller aneurysms offer a valuable window into the underlying pathophysiology of AAA disease. Through a Vascular Remodeling Specialized Center of Clinically Oriented Research program funded by the National Institutes of Health, we have established, in conjunction with Northern California Kaiser Permanente, a multidisciplinary research effort to efficiently identify and handicap suppressive therapeutic strategies for early AAA disease.
Background
Abdominal aortic aneurysm (AAA) disease is a prevalent and highly morbid condition among older people in the US. The aorta normally elongates and dilates with age, but when the diameter distal to the renal arteries exceeds 3 cm, it is considered aneurysmal. In the US, AAA disease affects approximately 6% of men and 1% of women older than 60 years, and more than 30,000 people die because of ruptured aneurysms or complications related to surgical repair of the aorta each year.1 Today AAAs are most commonly identified as incidental findings during abdominal imaging examinations. Small AAAs generally enlarge at a predictable rate,2 so patients in whom AAA has just been diagnosed usually enter a “watchful waiting” period before surgical intervention. There are no proven medical therapies for patients with early disease. The lack of nonsurgical therapies to prevent progression of early-stage disease and the absence of validated biomarkers or imaging parameters to monitor disease advancement present major clinical challenges in the management of small aneurysm disease.3–5 We aim to answer these clinical challenges through our study: AAA Disease: Mechanism, Stratification and Treatment, recently funded by the National Heart, Lung, and Blood Institute (NHLBI).
The pathophysiology of AAA disease is defined by transmural inflammation, smooth muscle cell apoptosis, and impaired extracellular remodeling. These events lead to progressive aortic enlargement and eventual rupture. The risk of rupture and sudden death is most closely related to aneurysm diameter. Surgical intervention through open or endovascular repair is presently the only effective method of treatment. At 5.5 cm in men, the risk of surgical repair is outweighed by the risk of aneurysm rupture or aneurysm-related death. For women, the guidelines are less well defined—repair is generally recommended at a diameter between 4.5 and 5.0 cm.6 There are currently no proven methods for reducing or eliminating enlargement in smaller preclinical aneurysms. Given their relatively slow increase in diameter (typically <0.4 cm/year), these smaller aneurysms offer a valuable window into the underlying pathophysiology of AAA disease.
Although AAA was once a relatively obscure and uncommon premortal diagnosis, AAA awareness among patients and physicians has increased significantly since 2004, meaning that patients who are otherwise quite healthy are becoming aware of their early AAA disease at relatively younger ages than ever before. In 2004 the Wall Street Journal received a Pulitzer Prize for its reporting on the growing health risks of AAA disease and its increasing impact on the aging baby-boom demographic.7 Shortly thereafter, the United States Preventative Services Task Force reported that screening reduced AAA-related mortality by 43% in men 65 to 75 years of age.8 Additionally, in January 2007, the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Amendment went into effect. It provides a one-time ultrasound screening examination for AAA disease to qualifying Medicare beneficiaries as part of their “Welcome to Medicare” physical examination when they reach age 65 years (for more information, see www.vascularweb.org/patients/medicarescreening/index.html). Ninety percent of AAAs detected through screening programs fall below the 5.5- or 4.5-cm threshold currently recommended for elective surgery eligibility for men and women, respectively. In the absence of clearly defined treatment guidelines for small AAA, the obvious question for many of these patients and their physicians is “What's next?”
Current Research
The NHLBI initiated a AAA-specific research effort in 1999, funding 11 collaborative R0-1 research grants in seven different institutions, including Stanford University.9 Although initially limited to basic cellular, animal modeling, and genetic linkage studies, these programs proved quite productive in defining the mechanisms responsible for AAA pathogenesis and outlining potential translational research strategies.10–15 This was followed in 2004 by an NHLBI Request for Applications (RFA) for Specialized Centers of Clinically Oriented Research (SCCOR) programs in vascular injury, repair, and remodeling, which specifically solicited proposals to move AAA research squarely into a clinical framework. With the help of resources obtained through the Vascular Remodeling SCCOR, we have established, in conjunction with Kaiser Permanente Northern California (KPNC), a multidisciplinary research effort to efficiently identify and handicap suppressive therapeutic strategies for early AAA disease. Our program, AAA Disease: Mechanism, Stratification and Treatment, includes 28 investigators or coinvestigators, including Bradley B Hill, MD, Chief of Vascular Surgery at the Santa Clara Medical Center, and statisticians, study coordinators, engineers, physicians, exercise physiologists, technicians, and medical science graduate students. Together, our multidisciplinary team will develop an integrated approach to analysis, stratification, and treatment of AAA disease.
Our program includes three related projects and four support cores. The first project uses proteomic tools to improve diagnosis of early AAA disease, to identify disease-specific predictors of expansion, and to monitor response to potential medical therapies. The second project analyzes the influence of hemodynamic variability on AAA pathophysiology and disease progression. The third project examines the relationship between physical activity and AAA disease risk, including both the analysis of lifetime physical activity vis-à-vis aortic diameter and a randomized clinical trial testing the ability of supervised exercise training to modify AAA disease progression. The support cores provide histology, bioimaging, and clinical skills development training, as well as administrative support. The exercise trial described above anchors and connects the projects and supportive core units of this comprehensive translational research program.
Substantial evidence links sedentary existence and resulting proinflammatory aortic conditions to the pathogenesis of AAA disease.16,17
Substantial evidence links sedentary existence and resulting proinflammatory aortic conditions to the pathogenesis of AAA disease.16,17 For this cross-sectional correlation study to determine whether lifetime physical activity and measured exercise capacity represent independent risk factors for AAA disease, 1400 participants will be recruited. In addition, a subset of 340 study subjects with small AAAs will participate in a prospective, randomized, controlled longitudinal trial of exercise to suppress small AAA progression. The impact of exercise training will be monitored by ultrasound imaging surveillance and surrogate serum markers.
Defining the exact nature of those surrogate serum markers is the purpose of the proteomic project. In view of similar observations of patients with atherosclerotic occlusive disease, it is likely that AAAs produce unique signature profiles of proteins that include aspects of inflammation, apoptosis (programmed cell death), extracellular matrix breakdown, and thrombosis. In this project, transcriptional profiling of human AAA tissue, database mining for patterns of protein expression and serum multimarker assessment of experimental models will be used to develop proteomic profiles (simultaneous expression of disease-specific mediator proteins in serum) of AAA disease. Our expectation is that these proteomic profiles would not only provide insight into the AAA disease process but also enable serologic monitoring of aneurysm expansion and response to novel therapies.18,19
Aneurysm expansion and rupture are dynamic processes driven by luminal pressure, wall shear, and strain forces. The hemodynamic project will employ novel imaging and computer modeling techniques to quantify the biomechanical forces acting in AAAs during resting and exercise conditions. After refining our ability to quantify abdominal aortic blood flow at rest and during exercise, we will develop and validate computational methods to model blood flow, pressure, and wall motion in patient-specific reproductions of the abdominal aorta20 (Figure 1). This project will image and model blood flow in approximately 100 patients from the sustained exercise or usual activity arms of the clinical trial.
Figure 1.
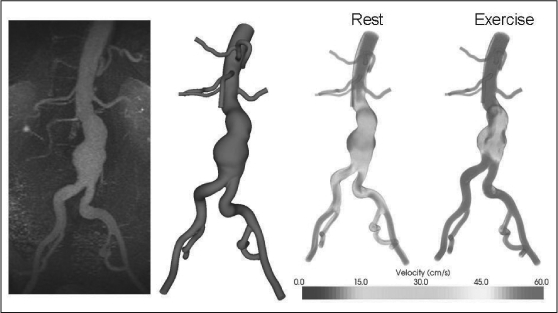
Custom software was used to convert magnetic resonance data (left) to a three-dimensional geometric model of the flow domain (center left). The three-dimensional model, in combination with patient-specific blood flow information, was used to simulate blood flow in an aneurysm during rest and exercise. During resting conditions, areas of low flow and flow stagnation exist within the aneurysm even at peak systole (center right); these regions are decreased during simulated exercise (right).
In addition to KPNC, Stanford University is collaborating with the Palo Alto Institute for Research and Education and the Veterans Affairs Palo Alto Health Care System (VAPAHCS) through consortium agreements on this study. The program was initiated in May 2006, with human subjects approval through Stanford University, VAPAHCS, KP, and the NHLBI Data Safety Monitoring Board finalized in November 2006. Recruitment began in late November 2006 for the prospective cross-sectional correlation study to determine whether lifetime physical activity and measured exercise capacity represent independent AAA risk factors. For this specific aim 1 (SA 1), a prospective cross-sectional study of lifetime physical activity as an independent risk factor for AAA disease, study subjects with small AAAs (<5.5 cm) complete the protocol shown in Table 1.
Table 1.
Protocol for specific aim 1 a
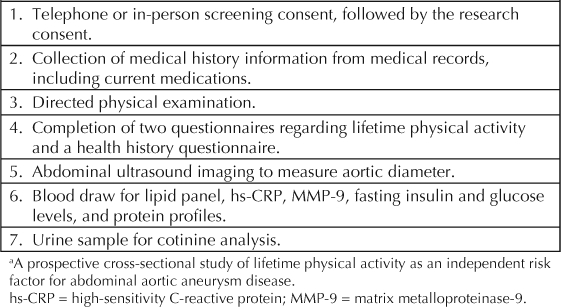
After initial screening, the participant completes these procedures during a half-day visit to the VAPAHCS exercise physiology laboratory. When this article was written, we had screened and enrolled 146 patients into this aim, at an average of 25 per month. Currently, there are no additional study requirements necessary for participation in SA 1; plans are underway, however, to modify the protocol to include one additional one-half day visit for ultrasound and serum marker testing at the end of the study.
A subset of patients with small aneurysms identified in SA 1, who qualify and are interested in participating, have the opportunity to enroll in the prospective evaluation of the ability of exercise training to reduce AAA progression (SA 2). Participation in SA 2 is clearly limited to highly motivated individuals with the highest level of commitment to the study. By the end of the second year of the study, we hope to randomize 170 patients to the exercise group and 170 to usual activity. Patients assigned to the exercise group participate in a three-year protocol outlined in Table 2.
Table 2.
Protocol for specific aim 2—exercise group a
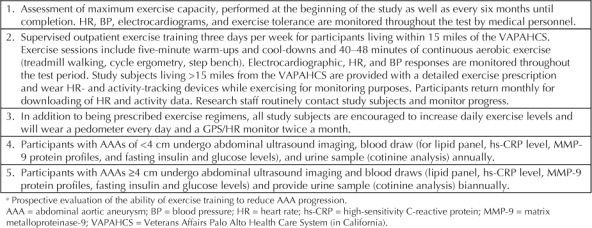
At the time of the baseline exercise test visit, study subjects are counseled regarding the identification of signs and symptoms of exertional intolerance and the need for medical attention. Patient progress, symptoms experienced, and compliance are monitored closely throughout the course of the study. Study subjects in the home program are also asked to return to the hospital monthly for maintenance counseling, to update questionnaires on physical activity patterns, and to download accelerometer (a device to measure displacement activity) data. Maximal exercise testing is repeated after the initial two months and at six-month intervals thereafter, providing ample opportunity to monitor training effects, ensure patients' medical stability, and update the exercise prescription (Figures 2 and 3).
Figure 2.

A study participant, supervised by a staff member, is exercising on an airdyne bicycle, which allows participants to get both an upper- and lower-body workout.
Figure 3.
A study participant exercises on a treadmill at the lab in the Veterans Affairs Palo Alto Hospital.
Patients randomly assigned to the usual activity group complete the protocol in Table 3 over a three-year period. Forty-two patients have enrolled in SA 2. As noted above, participation is limited to highly motivated study subjects who are committed to completing the entire period of the study. One patient remarked that “the opportunity to participate in an exercise program has been delightful and having the results contribute to useful medical knowledge is a bonus. I am an 80-year-old widower who had been degenerating physically from simple lack of ambition and motivation. This opportunity to learn proper exercise and enjoy some social life with fellow participants has been enjoyable.” This patient represents one of the motivated individuals who has successfully integrated this study into his routine and benefited greatly.
Table 3.
Protocol for specific aim 2—usual activity group a

Recruitment for both aims is ongoing through vascular and primary care clinics throughout the consortium hospitals, including all 14 KPNC facilities. We have also recently added significant resources to our KP affiliation by upgrading and expanding our contract with the KP Division of Research (DOR) in Oakland, CA. DOR personnel, led by Alan Go, MD, and Carlos Iribarren, MD, MPH, PhD, are collaborating on the scientific direction of the study and helping catalyze its successful completion.
I am an 80-year-old widower who had been degenerating physically from simple lack of ambition and motivation. This opportunity to learn proper exercise and enjoy some social life with fellow participants has been enjoyable.
Several thousand KPNC patients are already aware that they have small AAAs and will ultimately need surgical repair. Many, if not most, of those patients may be interested in learning more about their disease through participation in this important study, which is called Abdominal Aortic Aneurysms: Simple Treatment or Prevention (AAA: STOP). Should the opportunity arise, please consider mentioning AAA: STOP to your patients who have small aneurysms. Study personnel from Stanford are available to provide on-site education programs about AAA disease and current management options, as well as study-related information. Study contact information and additional AAA resource materials for health care providers are available on our Web site (http://aaastop.stanford.edu) or through the Vascular SCCOR offices in the Division of Vascular Surgery at Stanford University: Call 650-498-6039 or e-mail juliejohnsonwhite@stanford.edu. Patients who are interested in study participation are encouraged to visit our study Web site.
Research Outcomes
Ultimately, we hope that when patients in Northern California ask the question “What's next?” the answer will be an introduction to AAA: STOP by their primary care physician or vascular surgeon. In the near future, we anticipate that the answer will include a specific treatment recommendation enabled by the natural history information collected from this important study.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.


Acknowledgments
Katharine O'Moore-Klopf of KOK Edit provided editorial assistance.
References
- Kent KC, Zwolak RM, Jaff MR, et al. Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine & Biology Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg. 2004 Jan;39(1):267–9. doi: 10.1016/j.jvs.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Brady AR, Thompson SG, Greenhalgh RM, Powell JT, UK Small Aneurysm Trial Participants Cardiovascular risk factors and abdominal aortic aneurysm expansion: only smoking counts. Abstract. Br J Surg. 2003;90:492–3. [Google Scholar]
- Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006 Dec;26(12):2605–13. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- Golledge J, Powell JT. Medical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2007 Sep;34(3):267–73. doi: 10.1016/j.ejvs.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Powell JT, Brady AR. Detection, management, and prospects for the medical management of small abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004 Feb;24(2):241–5. doi: 10.1161/01.ATV.0000106016.13624.4a. [DOI] [PubMed] [Google Scholar]
- Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation. 2007 Jun 5;115(22):2865–9. doi: 10.1161/CIRCULATIONAHA.106.671859. [DOI] [PubMed] [Google Scholar]
- Helliker K, Burton TM.Medical ignorance contributes to toll from aortic illness: many doctors don't realize aneurysms are treatable; a paucity of specialists. The Wall Street Journal [serial on the Internet] 2003 Nov 4. [cited 2007 Sep 12]. Available from: www.pulitzer.org/year/2004/explanatory-reporting/works/story7.html.
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005 Feb 1;142(3):203–11. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- Wassef M, Baxter BT, Chisholm R, et al. Pathogenesis of abdominal aortic aneurysms: a multidisciplinary research program supported by the National Heart, Lung and Blood Institute. J Vasc Surg. 2001 Oct;34(4):730–8. doi: 10.1067/mva.2001.116966. [DOI] [PubMed] [Google Scholar]
- Nakahashi TK, Hoshina K, Karwowski JK, et al. Flow loading induces macrophage heme oxygenase 1 expression in experimental aneurysms. Arterioscler Thromb Vasc Biol. 2002 Dec 1;22(12):2017–22. doi: 10.1161/01.atv.0000042082.38014.ea. [DOI] [PubMed] [Google Scholar]
- Hoshina K, Sho E, Sho M, Nakahashi TK, Dalman RL. Wall shear stress and strain modulate experimental aneurysm cellularity. J Vasc Surg. 2003 May;37(5):1067–74. doi: 10.1016/s0741-5214(03)70052-4. [DOI] [PubMed] [Google Scholar]
- Sho E, Sho M, Hoshina K, Kimura H, Nakahashi TK, Dalman RL. Hemodynamic forces regulate mural macrophage infiltration in experimental aortic aneurysms. Exp Mol Pathol. 2004 Apr;76(2):108–16. doi: 10.1016/j.yexmp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Sho E, Chu J, Sho M, et al. Continuous retroperitoneal infusion improves doxycycline efficacy in experimental aortic aneurysms. J Vasc Surg. 2004 Jun;39(6):1312–21. doi: 10.1016/j.jvs.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Sho E, Sho M, Nanjo H, Kawamura K, Masuda H, Dalman RL. Hemodynamic regulation of CD34+ cell localization and differentiation in experimental aneurysms. Arterioscler Thromb Vasc Biol. 2004 Oct;24(10):1916–21. doi: 10.1161/01.ATV.0000142805.20398.74. [DOI] [PubMed] [Google Scholar]
- Sho E, Sho M, Nanjo H, Kawamura K, Masuda H, Dalman RL. Comparison of cell-type-specific vs transmural aortic gene expression in experimental aneurysms. J Vasc Surg. 2005 May;41(5):844–52. doi: 10.1016/j.jvs.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Myers J, Lee M. Fitness, physical activity, and inflammatory markers for cardiovascular disease. ACSM's Certified News [serial on the Internet] 2006 Apr–Jun;16(2):5–7. [cited 2007 Sep 12] Available from www.acsm.org/AM/Template.cfm?Section=ACSM_s_Certified_News&CONTENTID=4869&TEMPLATE=/CM/ContentDisplay.cfm. [Google Scholar]
- Myers J, Kaykha A, George S, et al. Fitness versus physical activity patterns in predicting mortality in men. Am J Med. 2004 Dec 15;117(12):912–8. doi: 10.1016/j.amjmed.2004.06.047. [DOI] [PubMed] [Google Scholar]
- Ardigo D, Assimes T, Fortmann SP, et al. Circulating chemokines accurately identify individuals with clinically significant atherosclerotic heart disease. Physiol Genomics. 2007 Nov 14;31(3):402–9. doi: 10.1152/physiolgenomics.00104.2007. [DOI] [PubMed] [Google Scholar]
- Tabibiazar R, Wagner RA, Ashley EA, et al. Signature patterns of gene expression in mouse atherosclerosis and their correlation to human coronary disease. Physiol Genomics. 2005 Jul 14;22(2):213–26. doi: 10.1152/physiolgenomics.00001.2005. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Draney MT. Experimental and computational methods in cardiovascular fluid mechanics. Annual Review of Fluid Mechanics. 2004 Jan;36:197–231. [Google Scholar]


