Abstract
Leptomeningeal carcinomatosis (LC) is a serious complication found in approximately 1% to 8% of patients with solid cancer and carries substantial rates of morbidity and mortality. Up to 48% of patients may present with LC before the presence of systemic cancer is known. We present the case of a patient who presented with symptoms of cauda equina syndrome and for whom subsequent investigations revealed intrathecal metastases and locally advanced lung cancer without respiratory symptoms or brain or bone metastases. The case emphasizes the need for thorough investigation in the presence of the triad: back pain, weakness in the lower extremities, and urinary urgency/incontinence. Cauda equina syndrome due to intrathecal metastases should always be considered. Spine MRI with contrast is the most informative investigative study for these patients, and myelography remains an important diagnostic method.
Introduction
Leptomeningeal carcinomatosis (LC), the multifocal seeding of the leptomeninges by malignant cells, is a serious complication found in approximately 1% to 8% of patients with solid cancer and carries substantial rates of morbidity and mortality.1 The most frequent sources of such metastases are the lungs (30%–70%), breasts (10%–30%), gastrointestinal tract (2%–20%), and malignant melanomas (2%–15%). However, in 2% to 4% of cases, the primary tumor is not identified.1,2 Small-cell lung cancers spread to the leptomeninges in 9% to 25% of cases, melanomas in 23%, and breast cancers in 5%.1
Intradural extramedullary (intrathecal) metastases—a subtype of LC—constitute approximately 4% of spinal metastases.3,4 They most commonly represent tertiary drop metastases from concomitant intracranial secondary lesions that become entangled within the nerve roots of the cauda equina.3 They may occur either as the presenting sign or as a late complication. Without appropriate therapy, the outlook is poor, and untreated patients are unlikely to survive more than four to six weeks.
We present the case of a patient who presented with symptoms of cauda equine syndrome and for whom subsequent investigations revealed intrathecal metastases and locally advanced lung cancer without respiratory symptoms or brain or bone metastases.
Case Report
A 53-year-old Caucasian man presented with back pain and progressive weakness in the legs that he said had begun six to eight weeks earlier. He was unable to walk for the preceding two weeks and had to use a walker. He reported that his right leg felt dead to touch and that his left leg was numb. His feet flapped down when he attempted to walk, and he had had several falls. He is an electrician with a 40 pack-years (20 cigarettes per day, per year) smoking history, diabetes mellitus, and depression. The patient's father died at age 69 because of lung cancer, and his mother had diabetes and died at age 77. He said that he had lost 18 pounds over the last two months because of poor appetite. Recently he noticed swelling of the lower right side of his neck. He denied any seizures. He reported urgency in urination but not urinary incontinence. Physical examination revealed a confluent, 3-cm, irregular, nodular, minimally tender mass in the lower right side of his neck suggesting adenopathy. Motor examination revealed paresis of both of his feet, with 0–1/5 strength, and his thigh muscles had a strength of 4–4 ± 5. When he was admitted, his white blood cell count was 8.35 × 109 cells/L, his hemoglobin was 13.9 g/dL, his hematocrit was 40.2%, and his platelet count was 371 × 103μL .
Magnetic resonance imaging (MRI) without contrast of the lumbar spine revealed mild degenerative changes (Figure 1). After a one-week interval because of urinary urgency, lumbar myelography was done. This demonstrated numerous abnormal nodular densities within the thecal sac that appeared to be abutting from the nerve roots of the cauda equina (Figure 2). These extended at least from the level of T12 down to the level of L5. A relative paucity of contrast below the level of L5 was worrisome because it indicated the possibility of additional soft-tissue lesions filling or compressing the thecal sac. Because of these extensive abnormal findings, a complete computed tomography (CT) lumbar myelogram (with contrast) was obtained. This showed numerous nodular lesions within the thecal sac that were arising from the nerve roots of the cauda equina. These appeared increasingly enlarged along the lower lumbar canal and into the upper sacral canal, where the thecal sac was almost completely filled with soft-tissue masses. There were abnormal soft-tissue mass lesions involving both adrenal glands. On thoracic CT myelography, there was developing nodularity on the surface of the thoracic cord starting at the level of T11, and by the level of T12–L1, several nodular lesions abutted from the nerve roots of the conus. On cervical CT myelography, only abnormal cervical lymph nodes (lymphadenopathy) were seen. MRI of the lumbar spine with contrast clearly showed extensive intrathecal metastases (Figure 3). CT of the thorax with contrast revealed a very large mediastinal mass and a right hilar mass (Figure 4). At the level of the right hilum, this mass measured approximately 6.4 × 4.2 cm. There was irregularity on the anterior aspect of the right mainstem bronchus and occlusion of the right upper lobe bronchus with right upper lobe emphysematous changes, consistent with bronchial airway involvement. There were also multiple enlarged mediastinal lymph nodes, consistent with metastases. The large right paratracheal mass produced significant narrowing of the mid-superior vena cava (SVC), raising the possibility of incipient SVC occlusion. Brain CT showed no masses or abnormal enhancements. There were no significant findings on whole-body bone scan. Chest radiographs showed nothing remarkable. Bronchoscopy was done, and examination of the biopsied tissue demonstrated small-cell carcinoma. The patient had placement of an Ommaya reservoir for intrathecal chemotherapy. The reservoir was used four times for the administration of methotrexate. This therapy in combination with spinal radiotherapy did not produce significant improvement in his lower extremities. The patient presented to the Emergency Department two months later with headache and 103°F fever. The Ommaya reservoir was tapped, and culturing of the cerebrospinal fluid (CSF) revealed gram-positive cocci of the Staphylococcus species. CT of the brain with contrast showed vasogenic edema in the right frontal lobe but did not reveal any evidence of abscess. The Ommaya reservoir was removed. After the second round of systemic chemotherapy (with carboplatin and etoposide) CT of the thorax showed partial response, with >50% reduction in the size of the lung tumor. Ten months after the initial presentation, the patient is alive and receiving chemotherapy.
Figure 1.
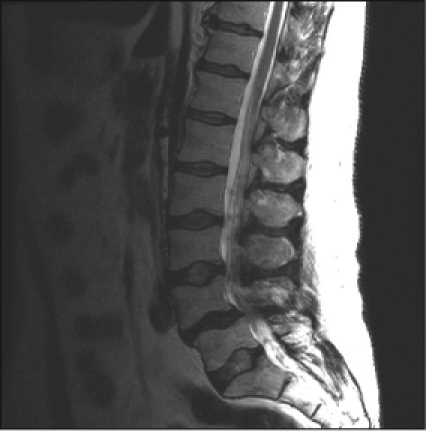
Lumbar magnetic resonance imaging without contrast showing mild degenerative changes.
Figure 2.
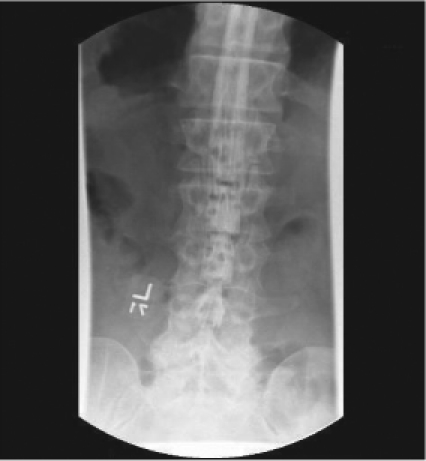
Lumbar myelography showing numerous abnormal nodular densities within the thecal sac abutting from the nerve roots of the cauda equina.
Figure 3.
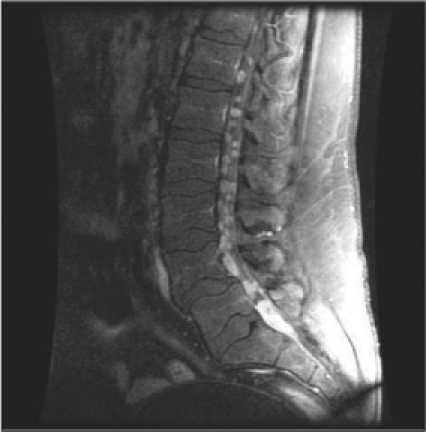
Lumbar magnetic resonance imaging with contrast showing multiple round nodular intrathecal densities throughout the lumbar dural sac, the largest, 1.4 × 1.0 × 3.2 cm, at L5–S1 level.
Figure 4.
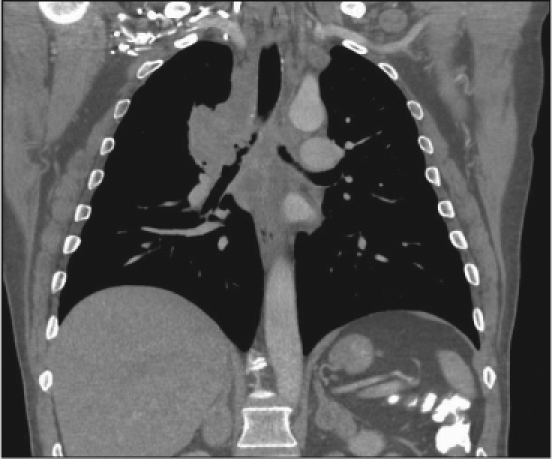
Computed tomography of the thorax showing a large mass, 6.4 × 4.2 cm, at the level of the right hilum; irregularity of the right mainstem bronchus, occlusion of the right upper lobe bronchus and multiple enlarged mediastinal lymph nodes.
Discussion
Leptomeninges consist of the arachnoid and the pia mater; the space between the two contains the CSF. The pathophysiologic mechanism of intrathecal metastases is thought to involve CSF spread.3 When tumor cells enter the CSF (either by direct extension, as in primary brain tumors, or by hematogenous dissemination, as in metastases and leukemia), they are transported throughout the nervous system by CSF flow, causing either multifocal seeding of the leptomeninges or diffuse infiltration in a sheetlike fashion along the surface of the brain and spinal cord (leukemic meningitis). Patients with brain metstases located in the posterior fossa are at a higher risk of developing LC compared with patients with cerebral brain metastases.5 Our case was unusual for the absence of evident brain metastases on MRI. However, in a series reported by Chow and McCutcheon,6 in which only intrathecal metastases of non-neurogenic origin were examined, 80% of patients also harbored intracranial metastatic foci, also suggesting drop metastases as the most common mechanism of spread. In that series, the most frequent histologic subtype was adenocarcinoma, and the most frequent primary source was the lung. Yet, in the Pavlidis review,7 breast cancer was found to be the most common solid tumor complicated by LC.
Up to 48% of patients may present with LC before the presence of systemic cancer is known.8 In a series of 126 patients with LC from the Mayo Clinic,9 84% were found to have advanced malignancy at the time of diagnosis. Patients with small cell lung carcinoma have a two-year cumulative incidence of LC of 10%, with a prevalence of 2%.5 A typical clinical syndrome is caused by intrathecal metastases—the cauda equina syndrome—which is characterized by low back pain, unilateral or usually bilateral sciatica, saddle sensory disturbances, bladder and bowel dysfunction, and variable lower extremity motor and sensory loss.4,10 Pain is the initial symptom in 90% to 95% of patients. This pain is usually local and associated with tenderness elicited by palpation over the spinous process at the level of involvement. On occasions, a component of radicular pain, radiating in the distribution of the nerve root at the involved level, may accompany neck or back pain. Pain of a severe, burning, dysesthetic nature is often associated with intrathecal metastatic lesions,4 whereas pain that is aggravated by movement and alleviated by immobility should raise suspicion of spinal instability and pathologic fracture-dislocation.11 In general, myofascial, discogenic, and spondylotic pain are more common in the cervical and lumbar spine and are relieved with rest. On the contrary, pain induced by spinal metastasis most commonly localizes to the thoracic segments and is often worse with rest and at night.10 Local back or neck pain can be present for a significant duration before a correct diagnosis is reached; the median time to diagnosis is two months.10
Neurologic compromise in the form of weakness, sensory loss, and sphincter disturbance usually occurs after the onset of pain. At the time of diagnosis, sensory or motor deficits are present in 38% to 76% of patients, and 50% of patients are nonambulatory (secondary to pain and/or neurologic deficit).10,12
Sphincter disturbance is also common, with 37% of patients in one series13 requiring placement of a urinary catheter. Urinary urgency/incontinence, is a symptom of hyperreflexic bladder due to irritation of the autonomic nerves by infiltrating metastases. Unfortunately, the correct diagnosis of intrathecal metastases is often not made until signs and symptoms of spinal cord compromise have manifested and disease is advanced.
Our patient noticed a recent swelling in the right lower part of his neck. CT of the thorax with contrast showed SVC compression and collateral circulation. The SVC, which drains into the right atrium of the heart, can become compressed by a tumor or enlarged lymph nodes inside the thorax. SVC syndrome is usually a sign of locally advanced bronchogenic carcinoma or the result of nonHodgkin lymphoma.14 Enlarged lymph nodes compress the vein and forces the blood into collateral circulation. The 24-month survival rate is 9% in patients without SVC syndrome and 3% in those with the syndrome.15
Treatment of patients with symptomatic secondary spinal lesions is aimed at relieving the pain and preserving/restoring neurologic function. Cure is not a realistic target in patients with metastatic spinal tumors, but palliation is a reasonable expectation. These modest goals contribute immeasurably to the quality of life and decrease the burden of care. Life expectancy is often relatively short. Pavlidis7 stated that even with treatment, the median survival for these patients is about eight weeks. By the time of preparation of this paper, the patient has survived ten months, but the prognosis is still poor.
Conclusion
Although LC is not unusual in patients with small-cell carcinoma of the lung, this case report points to very important moments. It demonstrates the need to be thorough in the investigation of the triad: back pain, weakness in the lower extremities, and urinary urgency/incontinence. It should be axiomatic that cauda equina syndrome in a patient with a recent history of weight loss is due to spinal metastases until proven otherwise. Spine MRI with contrast is the most informative investigative study for these patients, and myelography remains an important diagnostic method.
Up to 48% of patients may present with LC before the presence of systemic cancer is known.8
Disclosure Statement
The author(s) have no conflicts of interest to disclose.




Acknowledgments
Katharine O'Moore-Klopf of KOK Edit provided editorial assistance.
References
- Sewell RA, Recht LD.Leptomeningeal carcinomatosis [monograph on the Internet] New York: eMedicine; 2005 Oct. [last updated 2007 Aug 30] [cited 2008 Jan 2]. Available from: www.emedicine.com/neuro/topic188.htm. [Google Scholar]
- Wagner AL.Leptomeningeal carcinomatosis [monograph on the Internet] New York: eMedicine; 2007 Feb. [last updated 2007 Feb 1] [cited 2008 Jan 2]. Available from: www.emedicine.com/radio/topic390.htm. [Google Scholar]
- Schick U, Marquardt G, Lorenz R. Intradural and extradural metastases. Neurosurg Rev. 2001 Mar;24(1):1–8. doi: 10.1007/pl00011959. [DOI] [PubMed] [Google Scholar]
- Perrin RG, Livingston KE, Aarabi B. Intradural extra-medullary spinal metastasis. A report of 10 cases. J Neurosurg. 1982 Jun;56(6):835–7. doi: 10.3171/jns.1982.56.6.0835. [DOI] [PubMed] [Google Scholar]
- Seute T, Leffers P, ten Velde GP, Twijnstra A. Leptomeningeal metastases from small cell lung carcinoma. Cancer. 2005 Oct 15;104(8):1700–5. doi: 10.1002/cncr.21322. [DOI] [PubMed] [Google Scholar]
- Chow TS, McCutcheon IE. The surgical treatment of metastatic spinal tumors within the intradural extra-medullary compartment. J Neurosurg. 1996 Aug;85(2):225–30. doi: 10.3171/jns.1996.85.2.0225. [DOI] [PubMed] [Google Scholar]
- Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(Suppl 4):iv285–91. doi: 10.1093/annonc/mdh941. [DOI] [PubMed] [Google Scholar]
- Greenberg MS.Handbook of Neurosurgery. 6th ed. New York: Thieme; 2006. [Google Scholar]
- Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996 Jul;53(7):626–32. doi: 10.1001/archneur.1996.00550070064013. [DOI] [PubMed] [Google Scholar]
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978 Jan;3(1):40–51. doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- Perrin RG, Livingston KE. Neurosurgical treatment of pathological fracture-dislocation of the spine. J Neurosurg. 1980 Mar;52(3):330–4. doi: 10.3171/jns.1980.52.3.0330. [DOI] [PubMed] [Google Scholar]
- Onimus M, Papin P, Gangloff S. Results of surgical treatment of spinal thoracic and lumbar metastases. Eur Spine J. 1996;5(6):407–11. doi: 10.1007/BF00301969. [DOI] [PubMed] [Google Scholar]
- Helweg-Larsen S. Clinical outcomes in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94:269–75. doi: 10.1111/j.1600-0404.1996.tb07064.x. [DOI] [PubMed] [Google Scholar]
- Yellin A, Rosen A, Reichert N, Lieberman Y. Superior vena cava syndrome: the myth—the facts. Am Rev Respir Dis. 1990;141(5):1114–8. doi: 10.1164/ajrccm/141.5_Pt_1.1114. [DOI] [PubMed] [Google Scholar]
- Urban T, Lebeau B, Chastang C, Leclerc P, Botto MJ, Sauvaget J. Superior vena cava syndrome in small-cell lung cancer Arch Intern Med 1993 Feb 8. 153 3?p )?> 384 7 [PubMed] [Google Scholar]


