Abstract
Combination of chemotherapeutic agents and angiogenesis inhibitors is now commonly employed in the clinic to treat cancer. Here, we used angiostatic agents anginex and 0118, in combination with the chemotherapeutic irofulven, to treat human ovarian tumor xenografts in mice. General linear mixed models were used to statistically analyze tumor growth curves. Overall, combination of a low, non-toxic dose of irofulven with either angiogenesis inhibitor was more effective at inhibiting tumor growth than any of the single-agent therapies. For example, the anginex/irofulven and 0118/irofulven combinations inhibited tumor growth relative to controls by 92% (p<0.0001) and 96% (p<0.0001), respectively, with the 0118/irofulven combinations yielding 100% complete responses. This study suggests that combination therapy of 0118 or anginex and irofulven may be highly effective in the clinical setting.
Keywords: anginex, irofulven, galectin-1, angiogenesis, chemotherapy
Introduction
Recently, it was demonstrated in the clinic that chemotherapy is effective and well tolerated when used in combination with the antiangiogenic agent Avastin (Genentech) [1]. For some time, the inhibition of angiogenesis in tumors has been viewed as an attractive mechanism to control cancer. Consequently, antiangiogenic compounds, like Avastin and VEGF-Trap (Regeneron-Aventis), have demonstrated considerable potential as anti-cancer agents in the clinic, especially when administered in combination with conventional treatment [2, 3].
However, tumor angiogenesis is a highly complex biological process, and blocking only one step of this process may merely force a growing tumor to circumvent the particular pathway being inhibited. For example, agents that block specific angiogenic ligands, e.g. VEGF, or their receptor-mediated signaling pathway(s) only block one of the angiogenic factors secreted by tumors, leaving open the possibility that a tumor can adapt and switch to other angiogenic stimulators, e.g. from VEGF to FGF, in a second wave of VEGF independent angiogenesis [4, 5]. Besides switching to another growth factor, tumors cells have also been described to overcome anti-growth factor treatment by simply producing more of the growth factor being targeted, resulting in a more aggressive tumor [6]. Therefore, the need for a diversity of antiangiogenic strategies is apparent [7], and moreover the most promising anti-angiogenics, at least in theory, are those that act directly on ECs to inhibit tumor angiogenesis. This approach is less prone to drug resistance and can be more therapeutically effective against a broad spectrum of tumors [8].
Tumor vascular targeting agent (VTA) anginex, a designer peptide 33mer, has been shown recently to target galectin-1 (gal-1) [9], a novel target in antiangiogenesis research. Gal-1 belongs to a phylogenetically conserved family of carbohydrate binding lectins (galectins) that share a conserved carbohydrate recognition domain [10–12]. Gal-1 binds β-galactoside groups on various cell surface receptors, is highly upregulated in tumor-activated endothelial cells, and is crucial for activated endothelial cells to adhere to, and to migrate on, the extracellular matrix [9, 13–15]. Differential stromal elevation of gal-1 over the tumor parenchyma has been reported in several cancers including cancer of the breast [16], colon [17], prostate [18, 19] and ovaries [20]. On the molecular level, interaction between anginex and galectin-1 inhibits tumor-activated endothelial cell proliferation via anoikis [21] and attenuates tumor angiogenesis and tumor growth [22–24]. By weakly binding to ‘carrier protein’ plasma fibronectin [25], anginex is transported through the cardiovascular system to the tumor where the peptide strongly binds gal-1 (Kd = 90 nM) [9]. We have previously demonstrated that this peptide can synergistically enhance the effects of radiotherapy of several solid tumor types, presumably due to the anti-angiogenic and tumor vascular damaging effects induced by anginex treatment [26], as well as the induction of vascular normalization and reoxygenation of tumor tissue before radiation exposure [27].
Having established anginex as a potent antiangiogenic and antitumor agent, we used the folded structure of anginex [28, 29] to design a small library of calix[4]arene-scaffold protein surface topomimetics that embodied the molecular dimensions, surface topology, and overall chemical composition of the peptide. From screening of this library, we demonstrated that topomimetic 0118 was also a potent angiogenesis inhibitor in vitro, as well as in vivo [30].
The chemotherapeutic agent irofulven (MGI-114; 6-hydroxymethyl-acylfulvene) is a member of a semi-synthetic class of compounds called acylfulvenes, derived from illudin S, a toxin from the Omphalotus illudens mushroom [31]. Irofulven has shown to be a highly effective chemotherapeutic agent against a variety of cancers pre-clinically and clinically, either as monotherapy or in combination with other chemotherapeutic agents [32]. Similar to other alkylating agents, dose-limiting toxicities such as myelosuppression, grade 4 neutropenia, and thrombocytopenia, are seen when high doses of irofulven are used in patients [33, 34].
In this report, we first establish the efficacy of irofulven against human ovarian carcinoma in the MA148 xenograft mouse model [30, 35] and compare it to another alkylating compound and first-line standard chemotherapeutic agent, carboplatin [36, 37]. We then assess the ability of combination treatment with irofulven and anginex (or 0118) to inhibit the growth of MA148 tumors in the same mouse model.
Materials and Methods
Cell Culture
Human umbilical vein derived EC (HUVEC) and MA148, a human epithelial ovarian carcinoma cell line were kindly provided by Prof. Dr. Ramakrishnan (University of Minnesota) [35] and cultured as described earlier [26, 27, 30].
Proliferation assay
HUVEC were seeded in a 96-well culture plate coated with 0.2% gelatin for 2 hours at 20 °C (Sigma-Aldrich; St. Louis, MO). MA148 cells were seeded in non-coated 96-well plates (Corning; Lowell, MA). All cell types were seeded at a concentration of 3,000 cells per well and allowed to adhere for at least 3 hours at 37 °C in 5% CO2/95% air before treatments were initiated. The cells were then exposed to complete medium with 20 ng/ml basic Fibroblast Growth Factor (bFGF; Sigma-Aldrich; St. Louis, MO), with or without various concentrations of anginex for 72 hours or as indicated otherwise. Cell counting kit (CCK-8; Dojindo; Gaithersburg, MD) was used to assess cell proliferation rates relative to untreated cells, as described earlier [30, 38]. All measurements were done in triplicate, and the experiments were done at least three times.
MA148 Ovarian Carcinoma Mouse Model
Female athymic nude mice (nu/nu, 5–6 weeks old) were purchased from the National Cancer Institute and allowed to acclimate to local conditions for at least 1 week. Animals were given water and standard chow ad libitum and were kept on a 12-h light/dark cycle. Experiments were approved by the University of Minnesota Research Animal Resources ethical committee. Exponentially growing human ovarian MA148 epithelial carcinoma cells were cultured, harvested, suspended in serum free RPMI (2.0 x 107 cells/ml), and inoculated subcutaneously (s.c.) into the right flank of the mouse, as described previously [24, 35, 38]. Studies were carried out in a therapeutic intervention model with established tumors to test the capacity of treatment to inhibit tumor growth. Tumors were allowed to grow to the size of approximately 100 mm3 prior to randomization and initiation of treatment. Irofulven (provided by MGI Pharma, Bloomington, MN) and carboplatin (Sigma-Aldrich; St. Louis, MO) were given once every three days for 4 injections (q3dx4) intraperitoneal (IP), and anginex and 0118 were given subcutaneously via implanted Alzet osmotic mini-pumps (Durect; Cupertino, CA) for 28 days at an efficacious dose of 10 mg/Kg/day, as described earlier [35, 39]. Briefly, when the tumors reached a size of approximately (100 mm3), Alzet pumps were aseptically filled with the specific solutions, and implanted subcutaneously into the left lateral flank (opposite to the tumor) of the mice under anesthesia (ketamine 100 mg/kg and xylazine 3 mg/kg), and the incision was closed by wound clips. All procedures were performed aseptically and conform manufacturer’s guidelines.
Tumor volume was determined by measuring the size of the tumors on the flanks of the mice. The diameters of tumors were measured using calipers (Scienceware; Pequannock, NJ), and the volume was calculated using the equation to determine the volume of a spheroid: (a2 × b × π)/6, where a is the width of the tumor, and b is the length of the tumor. A complete response was defined as tumor regression to less than 15 mm3 (3 mm in diameter), and a partial response was defined as a smaller tumor than at the start of treatment. Stable disease was defined as no increase in tumor volume, i.e. maintained the tumor volume as at the start of treatment (100 mm3).
As an indirect measurement of general toxicity, body weights of mice were monitored twice weekly, using a digital balance (Ohaus; Florham, NJ).
Immunohistochemistry
Untreated MA148 tumors were embedded in tissue freezing medium (Miles Inc.; Elkart, IN) and snap frozen in liquid nitrogen and subsequently cut in 5 μm sections. Preparation and procedures for the frozen tumor sections were performed as described earlier with some slight modifications [23, 30]. Briefly, sections were fixed for 10 minutes in acetone. Subsequently, incubating for 30 minutes with PBS/5%BSA/3%FBS saturated aspecific binding. After washing with PBS the tissue was incubated with either anti-galectin antibody (1:50), a kind gift by Prof. Dr. L. Baum (University of California, Los Angeles)[9], fluorescein isothiocyanate (FITC)-conjugated monoclonal anti-α-smooth muscle actin antibody (1:50; Sigma-Aldrich; St. Louis, MO) to stain for pericytes (tumor stroma) or with phycoerythrin (PE)-conjugated monoclonal antibody to mouse CD-31 (1:50, PECAM-1; Pharmingen; San Diego, CA) and or combinations as indicated. After one hour incubation the slides were washed thoroughly with PBS and the secondary antibody anti-rabbit IgG phycoerythrin (PE) for galectin-1 detection was added (1:50; Sigma-Aldrich; St. Louis, MO) [9]. One hour later, after a thorough wash images of the sections were acquired on Olympus BX-60 microscope at 200x magnification and digitally analyzed and differentially quantified by morphometric analysis [27].
Statistical Analysis
Tumor volume was analyzed using general linear mixed models [40]. During the process of model selection, the likelihood ratio test was used to determine random effects and variance-covariance structures. The final general linear mixed model of tumor volume in the original scale contained interaction terms of treatment with both time and time2, while that of tumor volume in a natural log scale, i.e. ln(tumor volume +1), contained the interaction of treatment with time only. The variance-covariance structure of random effects was unstructured, whereas that of random errors was first-order auto-regression.
Using derived parameters, model-based tumor growth curves for raw data were drawn, and the integral from day 1 to day 26 for each curve was estimated from the area under the curve. This integral provided estimates of percentage inhibition in tumor growth for treatment versus control groups over the time course of the study, rather than just on a single day.
The Student’s t test was used where indicated to determine the validity of the differences between control and treatment data sets. A p value of 0.05 or less was considered significant.
Synergy determination
To determine whether or not and the degree to which the combination of irofulven and anginex or 0118 had synergistic effects on tumor growth inhibition, the following formula was applied [26, 35]: {(tumor growth inhibition from irofulven) x (tumor growth inhibition from anginex or 0118)}/observed tumor growth inhibition from combination treatment. A ratio of >1 indicates a synergistic (greater than additive) effect. Tumor growth inhibition was calculated by dividing the average tumor volume of the experimental group by the average tumor volume of the control tumors on a certain day.
Results
MA148 tumor growth inhibition by irofulven as a single agent
Since the effect of irofulven on human ovarian MA148 tumor cells and endothelial cells (EC) was unknown, we first determined the cytotoxicity of irofulven in vitro against these cell types. Irofulven potently inhibited MA148 tumor cell proliferation, showing an IC50 of 50 nM (Figure 1A). On the other hand, irofulven was 10-fold less potent against human EC (HUVEC), exhibiting an IC50 of 500 nM (Figure 1A).
Figure 1.
Irofulven concentration response in vitro and dose response in vivo. (A) HUVEC and MA148 tumor cell proliferation in vitro as a function of irofulven concentration. (B) MA148 tumor growth inhibited dose-dependently by irofulven. (C) MA148 tumor weights at the end of the study. (D) Body weight gain retarded by irofulven treatment. Irofulven at the doses indicated was administered by IP injection on a qd3x4 schedule (n = 8 each group). Data points represent the means ± SEM on day 28 after the initiation of treatment. *p < 0.05. Student’s t test.
Next, we assessed the efficacy of irofulven as a single agent in the MA148 tumor model in mice. Since we did not know how MA148 tumors in this mouse model would respond to treatment with irofulven, we initially performed a limited dose response study at irofulven concentrations of 1.5, 3.0, and 4.5 mg/kg. Doses of irofulven were administered IP every three days for a total of four injections (q3dx4), and treatment was initiated when tumors were approximately 100 mm3. Irofulven inhibited tumor growth in a dose dependent manner, in terms of either topical size measured at the end of the study or tumor weights determined upon autopsy (Figure 1B and C).
In addition, we compared the efficacy of irofulven to carboplatin in the same study [41], and we observed that irofulven at a dose of 1.5 mg/kg was as effective as carboplatin at a dose of 32.5 mg/kg (Figure 1B and C). Moreover, at a dose of 4.5 mg/kg, irofulven caused tumors to regress with 62.5% (5/8) complete responses (defined as tumor regression to tumors less than 3 mm in diameter) and 37.5% (3/8) partial responses (defined as a smaller tumor than at the start of treatment; Table 1). At 3.0 mg/kg, there were 12.5% (1/8) complete responses, 25% (2/8) partial responses, and 12.5% (1/8) animals with stable disease (defined as no increase tumor volume during treatment). Animals treated with irofulven at 1.5 mg/kg, as well as those treated with carboplatin at 32.5 mg/kg, showed only 12.5% (1/8) animals with stable diseases (Table 1).
Table 1.
Tumor responses after different treatment regimens.
| Dose response Irofulven | Group size | Complete | Responders Partial | Stable | |||
|---|---|---|---|---|---|---|---|
| (n) | (n) | (%) | (n) | (%) | (n) | (%) | |
| Control | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Iro [1.5] | 8 | 0 | 0 | 0 | 0 | 1 | 12.5 |
| Iro [3.0] | 8 | 1 | 12.5 | 2 | 25 | 1 | 12.5 |
| Iro [4.5] | 8 | 5 | 62.5 | 3 | 37.5 | - | - |
| Carboplatin [32.5] | 8 | 0 | 0 | 0 | 0 | 1 | 12.5 |
| Combination Iro + Ax | Group size | Complete | Responders Partial | Stable | |||
| (n) | (n) | (%) | (n) | (%) | (n) | (%) | |
| Control | 18 | 0 | 0 | 0 | 0 | 1 | 5.6 |
| Iro [1.5] | 18 | 0 | 0 | 2 | 11.1 | 3 | 16.7 |
| Ax [10] | 10 | 2 | 20 | 0 | 0 | 0 | 0 |
| Iro + Ax | 10 | 2 | 20 | 3 | 30 | 1 | 10 |
| Combination Iro + 0118 | Group size | Complete | Responders Partial | Stable | |||
| (n) | (n) | (%) | (n) | (%) | (n) | (%) | |
| Control | 25 | 0 | 0 | 0 | 0 | 2 | 8 |
| Iro [1.5] | 18 | 0 | 0 | 2 | 11.1 | 3 | 16.7 |
| 0118 [10] | 13 | 3 | 23.1 | 1 | 7.7 | 4 | 30.8 |
| Iro + 0118 | 6 | 6 | 100 | - | - | - | - |
Even though irofulven was highly effective at inhibiting or regressing tumor growth, systemic toxicity was apparent as reduced body weight gain during the study. At irofulven doses of 3.0 and 4.5 mg/kg, as well as for carboplatin at 32.5 mg/kg, body weight gain was significantly (p < 0.05) reduced compared to that from mice in the control group (Figure 1D). At the irofulven dose of 1.5 mg/kg, the average body weight gain was not significantly affected.
Combination treatment of anginex and irofulven
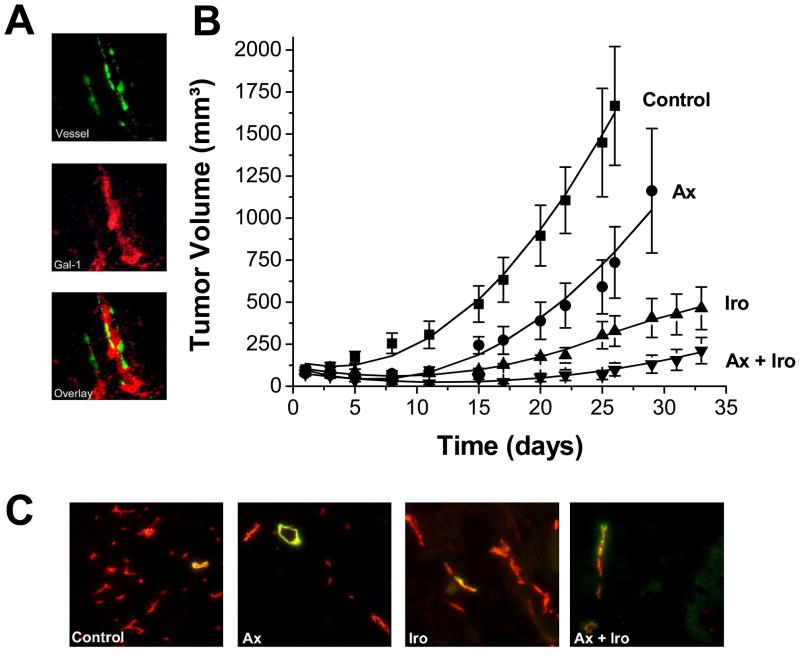
After determining that galectin-1 was expressed in the stroma of MA148 ovarian tumors from mice (Figure 2A), single agent and combination treatment studies were performed using anginex and irofulven. Tumor growth curves are shown in Figure 2B. On day 26, tumors of mice in the control and anginex-treated groups either reached sizes where animals had to be sacrificed and/or were collected for immunohistochemistry analysis. Tumor growth of mice in the other groups was monitored for another week, to day 33, as presented in Figure 2, and then tumor tissue was collected for analysis.
Figure 2.
Combination treatment of irofulven and anginex. (A) Gal-1 expression (red) is noted in the stroma (green) of blood vessels in MA148 tumors (magnification 200x). (B) A non-toxic dose of irofulven is combined with anginex resulting in tumor growth reduction in the MA148 human ovarian tumor xenograft mouse model. Administration was via IP injection for irofulven (1.5 mg/kg; qd3x4) and continuous infusion for anginex for 28 days (10 mg/kg; SC osmotic mini pumps). Actual data points are shown ± SEM. The solid line through each set of data points results from curve fitting using a linear mixed-effects growth curve model with treatment-specific coefficients for linear and quadratic terms of time (see Methods Section). (C) Typical co-localization staining for pericytes (green) and microvessel density (red) for control, anginex, irofulven, and combination treated tumors. Images represent the means for the amount of staining (Table 2). Original magnification is 200X.
Initially, we compared the inhibition of tumor growth on day 26 (the last day when all four groups can be compared), as well as by using the area under the model-based tumor growth curve (see Methods Section) for each group from day 1 to 26. On day 26, anginex and irofulven as single-agent therapies, inhibited tumor growth by approximately 56% and 80%, respectively, whereas combination of the two resulted in tumor growth inhibition of 91% compared to controls (Figure 2). Using the area under the curve approach, percent inhibition was essentially the same: anginex (58%), irofulven (78%), and combination (92%). Moreover, mice treated with a combination of irofulven and anginex demonstrated 20% (2/10) complete responses and 30% (3/10) partial responses, with 10% (1/10) animals with stable disease. In contrast, the group treated with irofulven (1.5 mg/kg) alone had no complete responses and 11% (2/18) partial responses, with 17% (3/18) animals with stable disease, and the anginex treated group showed 20% (2/10) complete responses (Table 1).
Analysis of raw tumor volume data (Figure 2) using general linear mixed models [40] showed that while the linear time trends of tumor volume for the four groups (control, anginex, irofulven, and the combination) did not differ significantly (F=1.33, p=0.2680), the quadratic time trends did (F=8.85, p<0.0001). Compared to the combination group, both control group (t=4.44, p<0.0001) and anginex group (t=2.44, p=0.0182) displayed a significantly different quadratic pattern in time, whereas the irofulven group (t=0.75, p=0.4560) did not. The general linear mixed model for the ln(tumor volume +1) also demonstrated that tumors in the control and anginex groups grew faster and became larger than those in the irofulven and combination groups. Compared to the combination group, both control (t=3.64, p=0.0005) and anginex (t=2.18, p=0.0325) groups showed significantly different linear time trends (i.e. tumor growth rates), whereas the irofulven group (t=0.90, p=0.3703) did not. On day 26, the least squares means of ln(tumor volume +1) of the four groups were: control (7.07) > anginex (5.60) > irofulven (5.13) > combination (3.81). The combination group had a significantly lower least squares mean ln(tumor volume +1) than control (t=5.91, p<0.0001) and anginex (t=2.91, p=0.0252) groups.
Immunohistochemistry (quantified by morphometric analysis [27]) indicated that microvessel density (MVD) was reduced by treatment with either anginex or irofulven (Table 2). Aside from vessel density (including number, size and length of vessels), this digital approach discriminates among vessel branch points, end points, and vessel length, and allows for quantification of these architectural parameters [27]. Single agent therapies significantly (p < 0.05) reduced MVD and changed vessel architecture, as well as increasing pericyte coverage compared to controls (Table 2 and Figure 2C). Combination treatment also reduced MVD significantly (p < 0.05) compared to controls and compared to either anginex or irofulven stand alone treatments (p < 0.03).
Table 2.
Histological analysis of microvessel density.a
| Vessel Densityb | End Pointsc | Branch Pointsd | Vessel Lengthe | ||||
|---|---|---|---|---|---|---|---|
| Control | 8877 ± 476 | 49.4 ± 3.5 | 3.7 ± 0.6 | 9.9 ± 0.6 | |||
| Iro [1.5] | 6215 ± 419* | 29.1 ± 3.0* | 2.0 ± 0.5* | 6.2 ± 0.5* | |||
| Ax [10] | 5039 ± 249* | 28.8 ± 2.7* | 2.1 ± 0.4* | 5.3 ± 0.4* | |||
| Iro + Ax | 4047 ± 179*# | 17.5 ± 1.6*# | 0.6 ± 0.1*# | 3.5 ± 0.2*# | |||
| Vessel Densityb | End Pointsc | Branch Pointsd | Vessel Lengthe | ||||
| Control | 8877 ± 476 | 49.4 ± 3.5 | 3.7 ± 0.6 | 9.9 ± 0.6 | |||
| Iro [1.5] | 6215 ± 419* | 29.1 ± 3.0* | 2.0 ± 0.5* | 6.2 ± 0.5* | |||
| 0118 [10] | 5136 ± 304* | 24.2 ± 1.8* | 1.8 ± 0.4* | 4.4 ± 0.4* | |||
| Iro + 0118 | 3138 ± 176*# | 11.7 ± 1.1*# | 0.3 ± 0.1*# | 2.2 ± 0.2*# | |||
At the end of the experiment similar size tumors without apparent widespread necrosis were excised and embedded in tissue freezing medium (Miles Inc.; Elkart, IN) and snap frozen in liquid nitrogen. Preparation and procedures are as described in the Methods section.
After binarization of the images from CD31-staining, microvessel density was estimated by scoring the total number of white pixels per field.
Mean number of vessel end points as determined after skeletonization of the images.
Mean number of vessel branch points/nodes per image.
Mean total vessel length per image. All results are expressed as mean pixel counts per image ± standard error from 25 images derived from 3 tumors per group.
All results are expressed as mean pixel counts per image ± standard error from 25 images derived from 3 tumors per group.
p < 0.05 Experimental group vs. Control;
p < 0.03 Combination vs. either single agent; Student’s t-test.
Combination treatment of topomimetic 0118 and irofulven
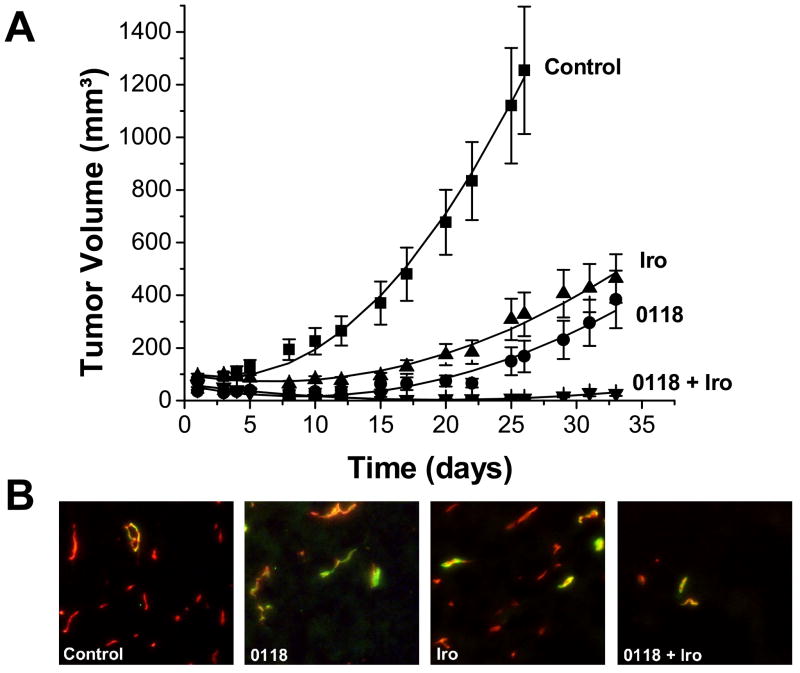
In the second comparative study (0118 and irofulven), we found that topomimetic 0118 was more effective than parent anginex, either as a single agent or in combination with irofulven (Figure 3). On day 26, irofulven and 0118 as single agents inhibited tumor growth by 74% and 78%, respectively, whereas combination of the two inhibited tumor growth by 99%. Using the area under the curve approach (see Methods Section), the inhibitory effects were essentially the same for irofulven (70%) and the combination of irofulven and 0118 (96%), and distinctly larger for treatment with single agent 0118 (87%) [n.b. single agent anginex was 58%, see above]. Moreover, combination therapy yielded 100% (6/6) complete responses. With single agent therapies, irofulven and 0118 had 11% (2/18) and 8% (1/13) partial responses, respectively, and 17% (3/18) and 31% (4/13) animals with stable disease, respectively, whereas only 0118 showed complete responses (3/13 or 23%; Table 1).
Figure 3.
Combination treatment of irofulven and 0118. (A) A non-toxic dose of irofulven was combined with 0118, resulting in tumor growth reduction in the MA148 human ovarian tumor xenograft mouse model. Administration was by IP injection for irofulven (1.5 mg/kg; qd3x4) and continuous infusion for 0118 (SC osmotic mini pumps; 10 mg/kg). Actual data points are shown ± SEM. The solid line through each set of data points results from curve fitting using a linear mixed-effects growth curve model with treatment-specific coefficients for linear and quadratic terms of time (see Methods Section). (B) Typical co-localization staining for pericytes (green) and microvessel density (red) for control, 0118, irofulven and combination treated tumors. Images represent the means for the amount of staining (Table 2). Original magnification is 200X.
Analysis of raw tumor volume data (Figure 3) using general linear mixed models showed once again that while the linear time trends of tumor volume for the four groups (control, 0118, irofulven, and the combination) did not differ significantly (F=0.57, p=0.6377), the quadratic time trends did (F=9.15, p<0.0001). The slopes of tumor growth curves of the control group were steep, while those of the single-agent 0118 group, single-agent irofulven group, and combination group (0118 and irofulven) were flatter. Compared to the combination group, the control group (t=3.69, p=0.0005) had a significantly different quadratic pattern in time, whereas the 0118 (t=0.69, p=0.4958) and irofulven (t=0.79, p=0.4306) groups did not. Furthermore, the general linear mixed model for the ln(tumor volume +1) demonstrated that tumors in the control group grew rapidly, tumors in the 0118 and irofulven groups grew slower, and tumors in the combination group actually decreased in size over time. Compared to the combination group, the control (t=5.77, p<0.0001), 0118 (t=3.68, p=0.0004) and irofulven (t=3.39, p=0.0011) groups all demonstrated significantly different linear time trends (i.e. tumor growth rates). The linear decreasing time trend for the combination group (0118 and irofulven) reflected tumor growth regression during the initial period of treatment. On day 26, the least squares means of ln(tumor volume +1) for the four groups were: control (6.49) > irofulven (5.33) > 0118 (4.39) > combination (2.67). The combination group had a significantly lower least squares mean ln(tumor volume +1) compared to any of the other three groups: control (t=7.71, p<0.0001), irofulven (t=5.30, p<0.0001), and 0118 (t=3.25, p<0.0123).
Immunohistochemical analysis indicated that MVD was reduced by treatment with 0118 or irofulven (Table 2). These single agent therapies significantly (p < 0.05) reduced MVD and changed vessel architecture, and also increased pericyte coverage compared to controls (Table 2 and Figure 3C). Combination treatment inhibited MVD significantly (p < 0.05) compared to controls, as well as compared to either 0118 or irofulven (p < 0.03).
Discussion
Chemotherapeutic drugs are generally toxic due to their chemical nature and lack of specificity. In this regard, it is important to define their ‘therapeutic window’, i.e. the usually narrow dose range that is both safe and efficacious. Below this dose range, they exhibit minimal efficacy, and above this range, (acute) toxic side effects and long-term complications are observed. Like all alkylating chemotherapeutics, irofulven also exhibits these dose-limiting toxicities in the clinic [33]. This is consistent with our observation in the MA148 ovarian tumor model in mice, where we observed that higher and more efficacious doses of irofulven resulted in greater apparent toxicity as reflected by reduced weight gain over the course of treatment. We hypothesized that combination therapy using irofulven and vascular targeting agents, which are known to effect tumor physiology [27] and potentially improve chemotherapeutic efficacy, would result in less systemic toxicity and enhanced antitumor activity In fact, it is expected that angiogenesis inhibitors will be used in the clinic as combination treatments rather than as single agent therapies [7], as has been shown previously by others, pre-clinically [42, 43] and clinically [3].
Prior studies with irofulven, in other tumor models in mice, demonstrated good antitumor efficacy at a maximally tolerated dose of 3.5 mg/kg [44]. Here, we observed greater than additive effects using a lower dose of irofulven (1.5 mg/kg) in combination with anginex or its topomimetic 0118 [30]. The augmentation from the combination of these agents was hypothesized since irofulven is predominantly cytotoxic to MA148 cells (Figure 1A), whereas anginex is specific for activated EC and shows no effect on tumor cell lines, such as human ovarian MA148 [23], murine breast carcinoma SCK [26, 27], and human squamous cell carcinoma SCCVII [45]. The proliferation inhibition of MA148 cells by irofulven (IC50 of 50 nM), falls within the range of irofulven activity against other cancer cell types [46]. Moreover irofulven is about 10-fold less effective against human EC (IC50 of 500 nM), which in turn is consistent with previous reports showing that irofulven is more effective against carcinoma cell types, as opposed to normal cell types [46].
Since other alkylating agents, like carboplatin, are the most widely used drugs in the first-line treatment of ovarian cancer [36, 37], we compared irofulven to carboplatin (32.5 mg/kg) in the same study [41]. While the effects from this dose of carboplatin were essentially the same as reported earlier [35, 41], we observed that irofulven was more efficacious in this model, with doses below maximally tolerated doses producing similar antitumor activity.
Because higher doses of irofulven impaired body weight gain in mice (Figure 1D), we asked whether vascular targeting agents anginex or its topomimetic 0118 could be used in combination with a less toxic dose of irofulven to achieve an improved anti-tumor effect in vivo. Combination of irofulven and anginex resulted in tumor growth inhibition of 91% compared to controls, and was deemed to be synergistic (synergy ratio [26, 35] of 1.5; see Table 1 in the Supplemental Data Section). In addition, mice treated with this combination demonstrated 20% (2/10) complete responses and 30% (3/10) partial responses, with 10% (1/10) of animals with stable disease, an improved profile as compared to either single agent therapy at the doses tested (Table 1). Similar results were obtained previously when anginex was combined with carboplatin [35] or angiostatin [35], as well as with radiation [26, 27, 45]. Moreover, employing 0118 in combination with irofulven showed improved activity relative to the combination of anginex and irofulven. We consider this combination therapy to be synergistic, because analysis of the tumor curves yielded a synergy ratio [26, 35] of 6 (see Table 1 in Supplemental Data Section), with tumor growth inhibition of 99% (Figure 3) and 100% (6/6) complete responses. Immunohistochemical analysis showed that microvessel density (MVD) was reduced by the different treatment strategies and that the vessel architecture was affected as well (Table 2). Interestingly, the combination treatment strategies inhibited the MVD and architecture significantly more than the single agent therapies (Table 2). In addition, staining for pericytes (α-SMA) showed that the pericytes covered vessels increased upon treatment. This suggests that either the ‘mature’ tumor vessels that already had pericyte coverage, or those that could rapidly recruit it, were able to survive angiostatic treatment, and moreover that the treatments preferentially eliminated the smaller ‘immature’ vessels first.
The greater effects that we observed from combination of irofulven and antiangiogenics anginex/0118, apparently result from the different mechanisms of action from these agents. It has been suggested that irofulven functions by activating the ATM-CHK2 DNA damage-signaling pathway, and this CHK2 activation contributes to S phase cell cycle arrest [47], causing cytotoxic effect against a broad spectrum of tumors [46, 47], predominantly targeting the tumor parenchyma. On the other hand, antiangiogenic agents anginex and topomimetic 0118 target endothelial cells in the vessels of tumors. More precisely, anginex targets galectin-1 in the stroma of tumors [9], and we have shown here that galectin-1 is also up regulated in the stroma of MA148 tumors (Figure 2A). This is supported by earlier reports showing that galectin-1 is differential elevated in the stroma of ovarian cancer [20].
In summary, this study demonstrated that the chemotherapeutic agent irofulven can be effectively administered in combination with antiangiogenic agents anginex and 0118 in order to achieve tumor growth reduction without exacerbating systemic toxicity. This work will aid in the possible broader implementation of irofulven clinically and the use of these angiostatic agents in combination with chemotherapeutics in general.
Supplementary Material
Acknowledgments
This research was supported by research grants to KHM from the National Cancer Institute (NIH CA-096090) and from MGI Pharma, Inc., Bloomington, MN, and to RJG from the National Cancer Institute (NIH CA-107160).
Abbreviations
- EC
endothelial cell
- HUVEC
human umbilical vein EC
- PBS
phosphate buffered saline
- BSA
bovine serum albumin
- VTA
vascular targeting agents
- IP
intraperitoneal
- ln
natural logarithm
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willett CG, Boucher Y, Duda DG, di Tomaso E, Munn LL, Tong RT, Kozin SV, Petit L, Jain RK, Chung DC, Sahani DV, Kalva SP, Cohen KS, Scadden DT, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Shellito PC, Mino-Kenudson M, Lauwers GY. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 4.Viloria-Petit A, Crombet T, Jothy S, Hicklin D, Bohlen P, Schlaeppi JM, Rak J, Kerbel RS. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 2001;61:5090–5101. [PubMed] [Google Scholar]
- 5.Kerbel RS, Yu J, Tran J, Man S, Viloria-Petit A, Klement G, Coomber BL, Rak J. Possible mechanisms of acquired resistance to anti-angiogenic drugs: implications for the use of combination therapy approaches. Cancer Metastasis Rev. 2001;20:79–86. doi: 10.1023/a:1013172910858. [DOI] [PubMed] [Google Scholar]
- 6.Davidoff AM, Ng CY, Zhang Y, Streck CJ, Mabry SJ, Barton SH, Baudino T, Zhou J, Kerbel RS, Vanin EF, Nathwani AC. Careful decoy receptor titering is required to inhibit tumor angiogenesis while avoiding adversely altering VEGF bioavailability. Mol Ther. 2005;11:300–310. doi: 10.1016/j.ymthe.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Griffin RJ, Molema G, Dings RP. Angiogenesis treatment, new concepts on the horizon. Angiogenesis. 2006;9:67–72. doi: 10.1007/s10456-006-9031-3. [DOI] [PubMed] [Google Scholar]
- 8.Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52:237–268. [PubMed] [Google Scholar]
- 9.Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, Mayo KH, Poirier F, Griffioen AW. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci U S A. 2006;103:15975–15980. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 11.Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988;263:9557–9560. [PubMed] [Google Scholar]
- 12.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 13.Rabinovich GA. Galectin-1 as a potential cancer target. Br J Cancer. 2005;92:1188–1192. doi: 10.1038/sj.bjc.6602493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Brule F, Califice S, Castronovo V. Expression of galectins in cancer: a critical review. Glycoconj J. 2004;19:537–542. doi: 10.1023/B:GLYC.0000014083.48508.6a. [DOI] [PubMed] [Google Scholar]
- 15.Scott K, Weinberg C. Galectin-1: a bifunctional regulator of cellular proliferation. Glycoconj J. 2004;19:467–477. doi: 10.1023/B:GLYC.0000014076.43288.89. [DOI] [PubMed] [Google Scholar]
- 16.Gabius HJ, Brehler R, Schauer A, Cramer F. Localization of endogenous lectins in normal human breast, benign breast lesions and mammary carcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52:107–115. doi: 10.1007/BF02889955. [DOI] [PubMed] [Google Scholar]
- 17.Lotan R, Matsushita Y, Ohannesian D, Carralero D, Ota DM, Cleary KR, Nicolson GL, Irimura T. Lactose-binding lectin expression in human colorectal carcinomas. Relation to tumor progression. Carbohydr Res. 1991;213:47–57. doi: 10.1016/s0008-6215(00)90597-4. [DOI] [PubMed] [Google Scholar]
- 18.Clausse N, van den Brule F, Waltregny D, Garnier F, Castronovo V. Galectin-1 expression in prostate tumor-associated capillary endothelial cells is increased by prostate carcinoma cells and modulates heterotypic cell-cell adhesion. Angiogenesis. 1999;3:317–325. doi: 10.1023/a:1026584523789. [DOI] [PubMed] [Google Scholar]
- 19.van den Brule FA, Waltregny D, Castronovo V. Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. J Pathol. 2001;193:80–87. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH730>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Allen HJ, Sucato D, Woynarowska B, Gottstine S, Sharma A, Bernacki RJ. Role of galaptin in ovarian carcinoma adhesion to extracellular matrix in vitro. J Cell Biochem. 1990;43:43–57. doi: 10.1002/jcb.240430105. [DOI] [PubMed] [Google Scholar]
- 21.Griffioen AW, van der Schaft DW, Barendsz-Janson AF, Cox A, Struijker Boudier HA, Hillen HF, Mayo KH. Anginex, a designed peptide that inhibits angiogenesis. Biochem J. 2001;354:233–242. doi: 10.1042/0264-6021:3540233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Schaft DW, Dings RP, de Lussanet QG, van Eijk LI, Nap AW, Beets-Tan RG, Bouma-Ter Steege JC, Wagstaff J, Mayo KH, Griffioen AW. The designer anti-angiogenic peptide anginex targets tumor endothelial cells and inhibits tumor growth in animal models. Faseb J. 2002;16:1991–1993. doi: 10.1096/fj.02-0509fje. [DOI] [PubMed] [Google Scholar]
- 23.Dings RP, van der Schaft DW, Hargittai B, Haseman J, Griffioen AW, Mayo KH. Anti-tumor activity of the novel angiogenesis inhibitor anginex. Cancer Lett. 2003;194:55–66. doi: 10.1016/s0304-3835(03)00015-6. [DOI] [PubMed] [Google Scholar]
- 24.Brandwijk RJ, Dings RP, van der Linden E, Mayo KH, Thijssen VL, Griffioen AW. Anti-angiogenesis and anti-tumor activity of recombinant anginex. Biochem Biophys Res Commun. 2006;349:1073–1078. doi: 10.1016/j.bbrc.2006.08.154. [DOI] [PubMed] [Google Scholar]
- 25.Akerman ME, Pilch J, Peters D, Ruoslahti E. Angiostatic peptides use plasma fibronectin to home to angiogenic vasculature. Proc Natl Acad Sci U S A. 2005;102:2040–2045. doi: 10.1073/pnas.0409844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dings RP, Williams BW, Song CW, Griffioen AW, Mayo KH, Griffin RJ. Anginex synergizes with radiation therapy to inhibit tumor growth by radiosensitizing endothelial cells. Int J Cancer. 2005;115:312–319. doi: 10.1002/ijc.20850. [DOI] [PubMed] [Google Scholar]
- 27.Dings RP, Loren M, Heun H, McNiel E, Griffioen AW, Mayo KH, Griffin RJ. Scheduling of radiation with angiogenesis inhibitors Anginex and Avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res. 2007;13:3395–3402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arroyo MM, Mayo KH. NMR solution structure of the angiostatic peptide anginex. Biochim Biophys Acta. 2007;1774:645–651. doi: 10.1016/j.bbapap.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dings RP, Arroyo MM, Lockwood NA, Van Eijk LI, Haseman JR, Griffioen AW, Mayo KH. Beta-sheet is the bioactive conformation of the anti-angiogenic anginex peptide. Biochem J. 2003;23:281–288. doi: 10.1042/BJ20030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dings RP, Chen X, Hellebrekers DM, van Eijk LI, Zhang Y, Hoye TR, Griffioen AW, Mayo KH. Design of nonpeptidic topomimetics of antiangiogenic proteins with antitumor activities. J Natl Cancer Inst. 2006;98:932–936. doi: 10.1093/jnci/djj247. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald JR, Muscoplat CC, Dexter DL, Mangold GL, Chen SF, Kelner MJ, McMorris TC, Von Hoff DD. Preclinical antitumor activity of 6-hydroxymethylacylfulvene, a semisynthetic derivative of the mushroom toxin illudin S. Cancer Res. 1997;57:279–283. [PubMed] [Google Scholar]
- 32.Baekelandt M. Irofulven (MGI Pharma) Curr Opin Investig Drugs. 2002;3:1517–1526. [PubMed] [Google Scholar]
- 33.Eckhardt SG, Baker SD, Britten CD, Hidalgo M, Siu L, Hammond LA, Villalona-Calero MA, Felton S, Drengler R, Kuhn JG, Clark GM, Smith SL, MacDonald JR, Smith C, Moczygemba J, Weitman S, Von Hoff DD, Rowinsky EK. Phase I and pharmacokinetic study of irofulven, a novel mushroom-derived cytotoxin, administered for five consecutive days every four weeks in patients with advanced solid malignancies. J Clin Oncol. 2000;18:4086–4097. doi: 10.1200/JCO.2000.18.24.4086. [DOI] [PubMed] [Google Scholar]
- 34.Alexandre J, Raymond E, Kaci MO, Brain EC, Lokiec F, Kahatt C, Faivre S, Yovine A, Goldwasser F, Smith SL, MacDonald JR, Misset JL, Cvitkovic E. Phase I and pharmacokinetic study of irofulven administered weekly or biweekly in advanced solid tumor patients. Clin Cancer Res. 2004;10:3377–3385. doi: 10.1158/1078-0432.CCR-03-0349. [DOI] [PubMed] [Google Scholar]
- 35.Dings RP, Yokoyama Y, Ramakrishnan S, Griffioen AW, Mayo KH. The designed angiostatic peptide anginex synergistically improves chemotherapy and antiangiogenesis therapy with angiostatin. Cancer Res. 2003;63:382–385. [PubMed] [Google Scholar]
- 36.Harries M, Kaye SB. Recent advances in the treatment of epithelial ovarian cancer. Expert Opin Investig Drugs. 2001;10:1715–1724. doi: 10.1517/13543784.10.9.1715. [DOI] [PubMed] [Google Scholar]
- 37.Tattersall MHN. Ovarian cancer chemotherapy: carboplatin as standard. The Lancet. 2002;360:500–501. doi: 10.1016/s0140-6736(02)09757-x. [DOI] [PubMed] [Google Scholar]
- 38.Mayo KH, Dings RP, Flader C, Nesmelova I, Hargittai B, van der Schaft DW, van Eijk LI, Walek D, Haseman J, Hoye TR, Griffioen AW. Design of a partial peptide mimetic of anginex with antiangiogenic and anticancer activity. J Biol Chem. 2003;278:45746–45752. doi: 10.1074/jbc.M308608200. [DOI] [PubMed] [Google Scholar]
- 39.Dings RP, Mayo KH. A Journey in Structure-Based Drug Discovery: From Designed Peptides to Protein Surface Topomimetics as Antibiotic and Antiangiogenic Agents. Acc Chem Res. 2007 Jul 28; doi: 10.1021/ar700086k. [DOI] [PubMed] [Google Scholar]
- 40.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Springer; New York: 2000. [Google Scholar]
- 41.Wild R, Dings RP, Subramanian I, Ramakrishnan S. Carboplatin selectively induces the VEGF stress response in endothelial cells: Potentiation of antitumor activity by combination treatment with antibody to VEGF. Int J Cancer. 2004;110:343–351. doi: 10.1002/ijc.20100. [DOI] [PubMed] [Google Scholar]
- 42.Teicher BA, Sotomayor EA, Huang ZD. Antiangiogenic agents potentiate cytotoxic cancer therapies against primary and metastatic disease. Cancer Res. 1992;52:6702–6704. [PubMed] [Google Scholar]
- 43.Teicher BA, Holden SA, Ara G, Northey D. Response of the FSaII fibrosarcoma to antiangiogenic modulators plus cytotoxic agents. Anticancer Res. 1993;13:2101–2106. [PubMed] [Google Scholar]
- 44.Britten CD, Hilsenbeck SG, Eckhardt SG, Marty J, Mangold G, MacDonald JR, Rowinsky EK, Von Hoff DD, Weitman S. Enhanced antitumor activity of 6-hydroxymethylacylfulvene in combination with irinotecan and 5-fluorouracil in the HT29 human colon tumor xenograft model. Cancer Res. 1999;59:1049–1053. [PubMed] [Google Scholar]
- 45.Amano M, Suzuki M, Andoh S, Monzen H, Terai K, Williams B, Song CW, Mayo KH, Hasegawa T, Dings RP, Griffin RJ. Antiangiogenesis therapy using a novel angiogenesis inhibitor, anginex, following radiation causes tumor growth delay. Int J Clin Oncol. 2007;12:42–47. doi: 10.1007/s10147-006-0625-y. [DOI] [PubMed] [Google Scholar]
- 46.Poindessous V, Koeppel F, Raymond E, Comisso M, Waters SJ, Larsen AK. Marked activity of irofulven toward human carcinoma cells: comparison with cisplatin and ecteinascidin. Clin Cancer Res. 2003;9:2817–2825. [PubMed] [Google Scholar]
- 47.Wang J, Wiltshire T, Wang Y, Mikell C, Burks J, Cunningham C, Van Laar ES, Waters SJ, Reed E, Wang W. ATM-dependent CHK2 activation induced by anticancer agent, irofulven. J Biol Chem. 2004;279:39584–39592. doi: 10.1074/jbc.M400015200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.