Abstract
Reactive oxygen species (ROS) have been implicated in direct killing of pathogens, increased tissue damage, and regulation of immune signaling pathways in mammalian cells. Available research suggests that analogous phenomena affect the establishment of Plasmodium infection in Anopheles mosquitoes. We have previously shown that provision of human insulin in a blood meal leads to increased ROS levels in Anopheles stephensi. Here, we demonstrate that provision of human insulin significantly increased parasite development in the same mosquito host in a manner that was not consistent with ROS-induced parasite killing or parasite escape through damaged tissue. Rather, our studies demonstrate that ROS are important mediators of both the mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling branches of the mosquito insulin signaling cascade. Further, ROS alone can directly activate these signaling pathways and this activation is growth factor specific. Our data, therefore, highlight a novel role for ROS as signaling mediators in the mosquito innate immune response to Plasmodium parasites. Antioxid. Redox Signal. 14, 943–955.
Introduction
Plasmodium falciparum infection is responsible for over 1 million deaths annually. The absence of an effective vaccine along with increasing drug-resistant parasites and pesticide-resistant mosquito vectors has resulted in a surge of malaria cases in recent years, which highlights the need for novel control strategies (40). One novel strategy is based on the genetic modification of mosquitoes so that they are unable to transmit malaria. Currently, only a few genetically engineered mosquito lines have been produced that are refractory to malaria parasites, and none are close to being field-tested (6, 28). This is due, in part, to our inability to identify effective gene targets for transformation that render the mosquito resistant to malaria infection without reducing mosquito fitness. Plasmodium parasites undergo a series of complex developmental transformations inside Anopheles mosquitoes during which they experience significant losses (39), due in part to the mosquito innate immune response. The greatest reduction in parasite numbers generally occurs as ookinetes cross the midgut epithelium to form oocysts (44). During this stage of infection, parasites are eliminated by a combination of anti-microbial peptides, nitric oxide, and complement-like factors (5). Thus, mosquito immunity can directly impact parasite transmission and provides an excellent target for genetic manipulation.
In the course of blood meal digestion, the mosquito midgut epithelium is exposed to a variety of parasite-derived and human blood-derived factors, such as human transforming growth factor (TGF)-beta1 and insulin, which can affect mosquito physiology and malaria parasite development (14, 24, 42). The signaling cascades that regulate these responses, including the mitogen-activated protein kinase (MAPK)-dependent cascades in general and the insulin/IGF-1 signaling (IIS; 25) cascade in particular, are highly conserved. The IIS cascade consists of two main signaling branches, an MAPK-dependent pathway and phosphatidylinositol 3-kinase (PI3K)/Akt-dependent pathway, both of which have been shown to regulate a variety of cellular functions, including innate immunity (Fig. 8; 25). We previously demonstrated that both branches of the IIS cascade in the mosquito midgut can be activated by human insulin ingested in a blood meal. In particular, both extracellular signal-regulated kinase (ERK) and Akt phosphorylation are increased in the mosquito midgut in response to ingested human insulin (14). Most recently, we demonstrated that expression of constitutively active Akt in the midguts of genetically engineered Anopheles stephensi can completely inhibit P. falciparum infection in homozygous transgenic mosquitoes (6). While Akt is a central regulator of IIS, the downstream mechanisms that underlie Akt-mediated refractoriness are likely to be complex and networked with multiple signaling pathways.
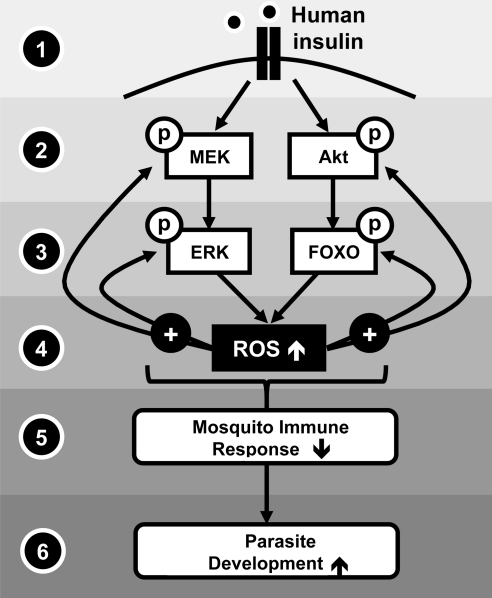
FIG. 8.
Proposed model of ROS-mediated insulin signaling in mosquitoes. (1) Human insulin signals in the mosquito midgut, (2) inducing the phosphorylation of MEK and Akt. (3) Activated MEK and Akt phosphorylate downstream effectors such as ERK and FOXO. (4) This signaling leads to increased ROS, which can positively feed back into the insulin/IGF-1 signaling pathway, increasing the phosphorylation of downstream effectors such as ERK and FOXO. (5) Ultimately, insulin/IGF-1 signaling, acting in part through increased ROS levels, leads to a decrease in the mosquito immune response and (6) a subsequent increase in parasite development in the mosquito midgut epithelium.
Provision of human insulin in the blood meal also significantly decreases superoxide dismutase (SOD) activity in A. stephensi and reduces mosquito lifespan (14), presumably due to increased levels of reactive oxygen species (ROS). Increased ROS can profoundly alter epithelial protein structure and function and such changes in the mosquito midgut could have deleterious consequences for the vector. In particular, numerous studies in mammals have linked ROS to the disruption of epithelial tight junctions and to increased permeability or leakiness of epithelial barriers to a variety of pathogens and toxins (34). In mosquitoes, the midgut epithelium serves both a physiological role in the absorption of nutrients and an immunological role as a barrier against pathogens. Greater levels of insulin-induced ROS could result in the loss of midgut barrier and integrity, allowing pathogens such as malaria parasites to establish infection more easily.
Although excessive ROS levels can be damaging to host cells, they can also be detrimental to infectious pathogens such as malaria parasites (17, 26, 29, 33). In particular, differences in systemic levels of ROS can result in differences in the mosquito immune response to Plasmodium parasites (17) and that provision of enzyme inhibitors or antioxidants in an infectious blood meal can enhance parasite development (26, 29). High levels of ROS can be detrimental to the host and invading organisms, whereas moderate levels of ROS can be beneficial to a variety of cell signaling processes (1, 20, 27, 43). For example, ROS can prime Drosophila hematopoietic cells for differentiation (31) and, from more recent work, may be directly involved in the catalytic cross-linking of proteins in a protective mucin layer in the midgut of the African malaria mosquito Anopheles gambiae (19). Further, an extensive body of mammalian research has described a critical role for ROS in facilitating normal signaling by insulin and a variety of other hormones and growth factors (8). For example, hydrogen peroxide (H2O2) has been shown to be a potent activator of both ERK and PI3K signaling pathways in mammalian cells (1, 43). Numerous studies have also shown that TGF-beta1 stimulation increases the synthesis of ROS, which can activate signaling by the full complement of MAPKs—ERK, p38, and JNK—in a variety of mammalian cell types (13, 15). Further, the signaling effects of ROS are moderated by antioxidants, including glutathione peroxidase, SODs, catalase, and the peroxiredoxins (12). Collectively, these data suggest that ROS may be functioning similarly in the mosquito in the regulation of MAPK signaling in general and in the IIS cascade in particular.
In this study, we found that the provision of human insulin in an infectious blood meal to the Indian malaria mosquito A. stephensi significantly increased development of P. falciparum and that addition of the antioxidant Mn(III) tetrakis(4-benzoic acid) porphyrin chloride (MnTBAP) significantly decreased parasite numbers via an effect that was not attributable to oxidative damage to the midgut. In the absence of an ROS-damaged midgut, we hypothesized that insulin-induced ROS may be altering immune signaling to regulate P. falciparum development. In support of this hypothesis, we demonstrate here that ROS can induce activation of mitogen activated protein kinase kinase (MEK), ERK, and p38, known immune modulatory MAPKs, as well as PI3K/Akt signaling in A. stephensi cells. In addition, inhibition of insulin-induced ROS resulted in decreased phosphorylation of downstream effectors of the IIS. By comparison, ROS scavenging had little to no effect on TGF-beta1-dependent MAPK activation. Our data highlight an ROS-dependent signaling specificity in A. stephensi that extends our appreciation of this biomedically important species for the study of innate immune cell signaling pathways that have applications for novel strategies for malaria control.
Materials and Methods
Reagents
Human insulin was purchased from Sigma-Aldrich and recombinant TGF-beta1 was purchased from R&D Systems. Monoclonal anti-diphosphorylated ERK1/2 was obtained from Sigma-Aldrich and polyclonal anti-ERK1/2 antibodies were purchased from Cell Signaling Technology. Anti-phospho-p38 MAPK antibody was obtained from Cayman Chemical, and anti-GAPDH antibody from Abcam. Anti-phospho-forkhead box O1 (FOXO) antibody and anti-phospho-p70S6K were purchased from Millipore.
Horseradish peroxidase-conjugated polyclonal rabbit anti-mouse IgG was purchased from Sigma-Aldrich. Horseradish peroxidase-conjugated goat anti-rabbit F(ab’)2 fragment and peroxidase-conjugated goat anti-rabbit IgG (H+L) were purchased from Biosource International and Pierce, respectively. The BCA assay kit and SuperSignal West Pico chemiluminescent detection kit were purchased from Pierce. RPMI 1640 with HEPES was purchased from Gibco/Invitrogen. All other chemicals and reagents were obtained from Sigma-Aldrich or Fisher Scientific. Human serum and red blood cells (RBCs) were obtained from Interstate Blood Bank. MnTBAP was purchased from EMD Chemicals.
Mosquito cell culture, mosquito rearing, and experimental treatments
Immortalized, A. stephensi embryo-derived (ASE), larva-derived MSQ43, and embryo-derived A. gambiae 4a3B cell lines (gift from Hans-Michael Muller, EMBL) were maintained as previously described (42). For in vivo studies, A. stephensi Liston (Indian wild-type strain) were reared and maintained at 27°C and 75% humidity. All mosquito rearing and feeding protocols were approved and in accordance with regulatory guidelines and standards set by the Institutional Animal Care and Use Committee of the University of California, Davis. For experimental treatments, laboratory-reared 3–5-day-old female mosquitoes were maintained on water for 24–48 h and then allowed to feed for 30 min on reconstituted human blood meals provided through a Hemotek Insect Feeding System (Discovery Workshops). Artificial blood meals contained washed human RBCs and saline (10 mmol l−1 NaHCO3, 15 mmol l−1 NaCl, and 1 mmol l−1 ATP, pH 7.0) with or without human insulin at 170 pM and with or without MnTBAP at 5 μM. Normal human blood insulin levels range from 17 pM at fasting to 0.59 nM without fasting, indicating that 170 pM insulin could be ingested by feeding mosquitoes (7). For western blot analyses, midguts were dissected from 60 mosquitoes in each treatment group and processed as previously described (42). Control mosquitoes were provided artificial blood meals supplemented with an equivalent volume of diluent phosphate-buffered saline (PBS).
Western blotting
The details of our western blotting protocols have been previously described (42). Protein lysates from cells or mosquito midguts were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis polyacrylamide gels, transferred to nitrocellulose membranes (BioRad), and probed for proteins of interest with various antibodies.
Quantitative reverse transcriptase–polymerase chain reaction
To detect A. stephensi nitric oxide synthase (NOS) gene expression, total RNAs were isolated from cultured cells using Trizol reagent (Invitrogen) at 6–48 h post-treatment. Samples were analyzed by quantitative reverse transcriptase–polymerase chain reaction using an ABI Prism 7300 Sequence Detection System (Applied Biosystems). NOS expression levels were normalized against S7 ribosomal protein gene expression levels and are represented as fold induction over control. Primers, probes, and amplification conditions were described previously (42). Technical replicates consisting of triplicate reactions with 100 ng template RNA and no template controls were analyzed simultaneously to assess amplification efficiency and lack of genomic DNA cross-contamination, respectively. Biological replicates from unique and separate groups of mosquito cells were used for statistical analysis.
2-cys peroxiredoxin overexpression
ASE cells were transfected with pcDNA3.1/V5-His vector (Invitrogen) expressing full-length A. stephensi 2-cys peroxiredoxin (AsPrx) referred to as “TLP-58” (33). Overexpressed AsPrx protein in mosquito cells was detected by immunoblotting with anti-V5 antibody as previously described in (33). The copper-inducible pMT/V5-His, vector (Invitrogen) referred to as “TLP-55” was used as the transfection control since in the absence of copper induction no expression of AsPrx is observed (33). ASE cells were plated in six-well plates and were transfected with plasmid using Effectene Transfection Reagent (Qiagen) according to the manufacturer's protocol. After 48 h, cells were replated in 12-well plates overnight and were treated with varying concentrations of insulin, TGF-beta1, or H2O2 for 5 min. After incubation, cell lysates were collected and processed for western blotting analysis.
Malaria parasite culture and mosquito infection
For mosquito infection with malaria parasites, one hundred twenty 3–5-day-old female A. stephensi were maintained on water pads for 24 h before blood feeding. Mosquitoes were allowed to feed on P. falciparum NF54-infected RBCs for 30 min. After 10 days, midguts from 50 mosquitoes with fully developed eggs (to confirm complete engorgement) from each group were dissected in PBS and stained with 0.1% mercurochrome for direct counting of P. falciparum oocysts. Means of oocysts per midgut in each treatment group were calculated from all dissected mosquitoes, including zeros for mosquitoes that contained no oocysts.
For mosquito infection, cultures of P. falciparum strain NF54 were grown in 10% heat-inactivated human serum and 6% washed human RBCs in RPMI 1640 with HEPES (Gibco) and hypoxanthine. At day 15, stage V gametocytes were evident and exflagellation rates were evaluated on the day of feeding. The 3–5-day-old A. stephensi were fed on mature gametocyte cultures diluted with human RBCs and heat-inactivated human serum with or without 170 pM human insulin and with or without 5 μM MnTBAP. All treatments were added to the diluted P. falciparum culture immediately before blood feeding. Protocols involving the culture and handling of P. falciparum for mosquito feeding were approved and in accordance with regulatory guidelines and standards set by the Biological Safety Administrative Advisory Committee of the University of California, Davis.
P. falciparum growth assays
Aliquots of P. falciparum NF54 culture were synchronized 48 h before the assay as previously described (21) and were then plated in 96-well flat-bottom plates in complete RPMI 1640 with HEPES, hypoxanthine, and 10% heat inactivated human serum. Parasites were treated with the equivalent volumes of PBS and human insulin at concentrations ranging from 170 pM to 17 μM or with PBS and MnTBAP at concentrations ranging from 50 nM to 50 μM for 48 h in a candle jar in a 37°C incubator. The assays were terminated by replacing the culture media with RPMI 1640 with 1% formalin. Erythrocytes were stained with 10 μg/ml of propidium iodide (Sigma) in PBS for 1 h at room temperature. Infected RBCs were counted with FACS Calibur flow cytometer, Becton Dickinson (BD Biosciences). Relative levels of parasite growth in response to treatment were normalized to PBS-treated controls, which were set to 100%.
Quantification of mosquito midgut protein carbonyl content
A total of 150 female A. stephensi (3–5 days old) from a single cohort were transferred into four, 1-gallon containers and were fed a single artificial blood meal supplemented with either 170 pM human insulin, 5 μM MnTBAP, insulin plus MnTBAP, or an equivalent volume of PBS as a control. All mosquitoes were allowed to feed for ∼1 h and were then provided with 10% sucrose-soaked cotton pads after blood feeding. Thirty mosquito midguts were dissected daily as described above and midgut protein carbonyl content (PCC) was determined by using the Oxyblot kit western blotting protocol (EMD chemicals) according to manufacturer's recommendations. Film exposures of PCC membranes and Coomassie-stained gels were scanned, and band densities were determined using a GS-800-calibrated densitometer. PCC levels were normalized to total protein levels in the same samples as determined by Coomassie staining.
Statistical analyses
Three tests for normality were used: Kolmogorov–Smirnov, D'Agostino–Pearson omnibus, and Shapiro–Wilk (Graphpad Prism 5.02). Data that were normally distributed were analyzed by analysis of variance for overall significance and Student–Neuman–Keuls for pairwise comparisons or by Student's t-test to assess differences between paired controls and treatments or between paired treatments. Data that were not normally distributed were analyzed using the Kruskal–Wallis test and Dunn's test for pairwise comparisons. The prevalence of parasite infection was analyzed using the chi-square test.
Results
Insulin-dependent ROS alter P. falciparum development in A. stephensi
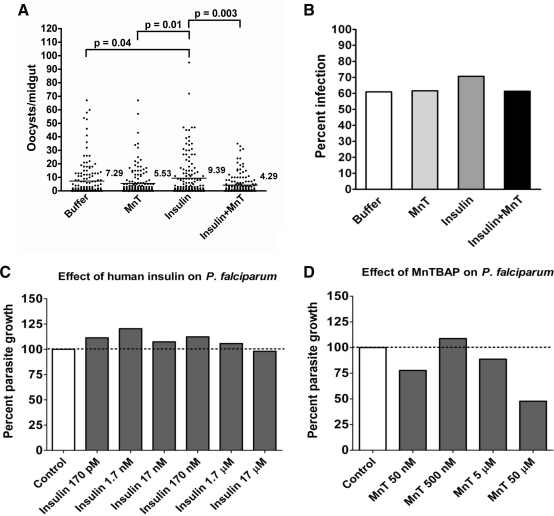
Our previous work showed that human insulin treatment increased H2O2 levels and decreased SOD activity in mosquito cells in vitro and in vivo (14). Because higher levels of ROS have been associated with decreased parasite development in mosquitoes (17, 26, 29, 33), we sought to determine the effects of insulin-dependent ROS production on P. falciparum oocyst development in A. stephensi. We expected that provision of human insulin in the blood meal would increase ROS production and decrease oocyst development. Further, we predicted that the addition of antioxidants such as MnTBAP, an SOD mimetic compound that has been utilized in mosquito studies previously (14, 38), should reverse the effects of insulin treatment on oocyst development. However, we found that P. falciparum oocyst numbers in insulin-fed mosquitoes were significantly higher than oocyst numbers in buffer-fed controls (Fig. 1A). Mosquitoes fed insulin in the presence of MnTBAP had significantly fewer parasites than did mosquitoes fed insulin alone. Additionally, mosquitoes fed MnTBAP alone had significantly decreased oocyst numbers relative to insulin-fed mosquitoes, but these levels were not significantly different from buffer-fed controls. Infection prevalence was not affected by treatment (Fig. 1B). One explanation for these results is that human insulin could enhance the growth of P. falciparum parasites directly in a manner analogous to human insulin/IGF-1 growth induction of Toxoplasma and Leishmania (10, 45). To test this possibility, we examined the effects of increasing concentrations of human insulin on the growth of synchronized asexual-stage P. falciparum parasites in vitro. At all concentrations, including those used for our in vitro assays (1.7 μM) and our in vivo assays (170 pM), human insulin had no significant effect on parasite growth (Fig. 1C). We also examined the effects of MnTBAP on P. falciparum growth and, although the highest concentration (50 μM) appeared to be toxic to parasites, the concentration used in our feeding studies (5 μM) had no significant effect on parasite growth (Fig. 1D). These results indicated that neither insulin nor MnTBAP had a direct positive effect on parasite growth in the mosquito.
FIG. 1.
Provision of insulin significantly increased Plasmodium falciparum oocyst numbers, whereas the antioxidant MnTBAP reversed this effect. (A) Mosquitoes were fed with P. falciparum–infected red blood cells supplemented with PBS as a control (Buffer), 5 μM MnTBAP (MnT), 170 pM human insulin, or MnTBAP plus human insulin. Data from three independent experiments with separate cohorts of mosquitoes were analyzed for the main effects of experiment and treatment. No significant effects were noted for experiment, for treatment, or for the interaction of experiment and treatment, indicating that these data could be combined for analysis. Horizontal lines indicate the means for three combined sets of 50 mosquitoes (150 mosquitoes total) per treatment group. The data were not normally distributed and, therefore, analyzed using the Kruskal–Wallis test and Dunn's post-test. Significant differences between treatment groups are indicated. (B) Prevalence of infection (Anopheles stephensi with at least one P. falciparum oocyst) from three independent experiments shown as percentages of dissected mosquitoes. Data were analyzed by chi-square (α = 0.05) and no significant differences among treatments were observed. Human insulin (C) and MnTBAP (D) did not affect growth of asexual-stage P. falciparum. Replicate cultures of P. falciparum NF54 were incubated with increasing concentrations of human insulin or MnTBAP. Relative growth is compared to the PBS control, which is set at 100%. Data from three independent experiments were analyzed by analysis of variance and by Student–Neuman–Keuls (α = 0.05) for all pairwise comparisons. No significant differences among treatment groups and controls were observed. MnTBAP, Mn(III) tetrakis(4-benzoic acid) porphyrin chloride; PBS, phosphate-buffered saline.
Human insulin does not alter the levels of oxidative damage in the mosquito midgut
In previous studies, we demonstrated that blood meals supplemented with human insulin significantly reduced A. stephensi lifespan and that these effects were reversed by provision of the antioxidant MnTBAP (14), suggesting that ingested human insulin acts via the synthesis of ROS in mosquito midguts. Based on these findings and the increased parasite development observed in Figure 1A, we hypothesized that higher levels of insulin-induced ROS may lead to increased oxidative damage in the midgut that could facilitate the establishment of Plasmodium infection. Such a finding would be analogous to observations that increased damage of the human intestinal barrier has been correlated with increased bacterial translocation and disease progression in other infections, such as HIV (4).
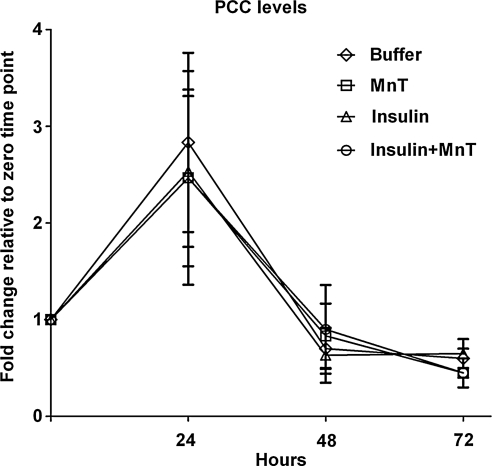
We quantified levels of oxidative damage in the midgut as PCC, an irreversible marker of oxidative protein modification. Midguts from A. stephensi were isolated at 0, 24, 48, and 72 h after a single blood meal supplemented with 170 pM human insulin, with 5 μM MnTBAP, with insulin plus MnTBAP, or with an equivalent volume of buffer as a control. All groups had increased PCC levels at 24 h after blood feeding (Fig. 2), an effect that was most likely due to the process of blood digestion, which is known to increase ROS levels (17). However, none of the treatments were significantly different from the buffer controls at any timepoint (Fig. 2). Further, any increase in PCC in the controls was not reduced by provision of the antioxidant MnTBAP (Fig. 2). As such it did not appear that insulin-induced ROS altered the midgut barrier to make it more permissive to invading parasites. It also did not appear that insulin-induced ROS were lethal to parasites, since we observed significantly higher oocyst development in the presence of insulin (Fig. 1A). Therefore, we hypothesized that the increased parasite development observed in Figure 1A in response to ingested human insulin resulted from the alteration of immune signaling pathways that indirectly regulate parasite development.
FIG. 2.
Insulin-induced ROS did not damage mosquito midgut proteins through 72 h after feeding. Mosquitoes were fed on blood supplemented with PBS, 5 μM MnTBAP (MnT), 170 pM human insulin, or MnTBAP plus human insulin. Midgut protein damage was quantified as total protein carbonyl content (PCC) in midgut tissue pooled from 30 mosquitoes at 0, 24, 48, and 72 h postblood feeding and normalized to total protein content in each sample. Fold changes in PCC levels relative to time 0 are shown. Data are represented as means ± SEMs from four independent experiments. No significant differences among treatment groups and controls were observed. ROS, reactive oxygen species. SEM, standard error of the mean.
ROS are important mediators of the IIS cascade
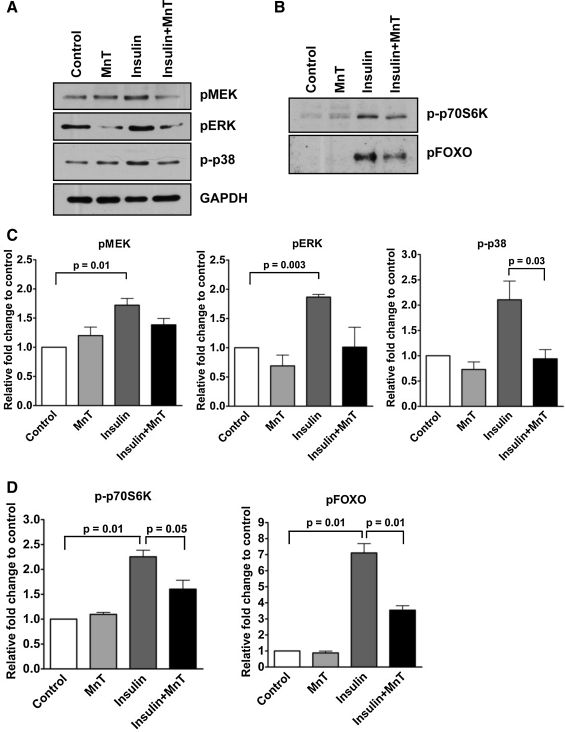
In our system, insulin-induced ROS did not appear to be directly deleterious to oocyst development, nor did they increase oxidative protein damage in the mosquito midgut. Therefore, we sought to determine whether insulin-induced ROS could modify immune signaling through the IIS pathway. Based on our previously published protocols (14), we treated A. stephensi cells with 1.7 μM human insulin in the presence or absence of 5 μM MnTBAP and examined the phosphorylation of downstream signaling proteins of the IIS pathway. Although immortalized mosquito cells provide a useful model for studying cell signaling, they are not identical to the highly specialized epithelial cells present in the midgut of mosquitoes and as such require the use of a higher concentration of human insulin. We found that in accordance with our previously published work (14, 24), insulin-treated mosquito cells had higher MEK, ERK, and p38 phosphorylation relative to PBS-treated controls (Fig. 3A, C). In addition to the effects on MEK, ERK, and p38, insulin significantly induced p70S6K and FOXO phosphorylation, downstream signaling targets of the PI3K/Akt signaling arm of the IIS pathway (Fig. 3B, D). When cells were pretreated with the antioxidant MnTBAP, phosphorylation of MEK, ERK, and p38 as well as FOXO and p70S6K was reduced relative to the controls (Fig. 3), suggesting that ROS participate in the IIS cascade in vitro.
FIG. 3.
ROS mediate insulin signaling in A. stephensi cells in vitro. (A) Pretreatment with the antioxidant MnTBAP reduced insulin-induced MAPK and PI3K/Akt signaling. ASE cells were treated with 5 μM MnTBAP (MnT) for 40 min before stimulation with 1.7 μM human insulin for 5 min. Cell lysates were analyzed by western blotting with anti-phospho-specific antibodies. The effects of MnTBAP pretreatment on MEK, ERK, and p38 phosphorylation (A, C), as well as FOXO and p70S6K phosphorylation (B, D) are shown. Fold changes in phospho-specific proteins in pairwise comparisons of treatments or of treatments and PBS-treated controls from three independent experiments were analyzed with Student's t-test (α = 0.05). GAPDH provided an assessment of protein loading and were used to normalize corresponding phospho-protein levels. Data are represented as means ± SEMs and significant differences are indicated. MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; ERK, extracellular signal-regulated kinase; FOXO, forkhead box O1; MEK, mitogen activated protein kinase kinase.
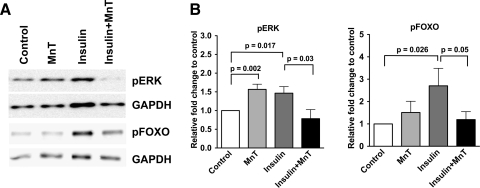
To confirm the participation of ROS in IIS regulation in vivo, A. stephensi mosquitoes were fed artificial blood meals containing a PBS control, a physiological dose of insulin (170 pM), insulin plus 5 μM MnTBAP, or MnTBAP alone. In accordance with our previously published data (14, 24) and in vitro results (Fig. 3), we found that ingested human insulin significantly increased the phosphorylation of both ERK and FOXO in mosquito midgut tissues at 30 min after bloodfeeding (Fig. 4). When mosquitoes were fed blood meals containing human insulin in the presence of MnTBAP, phosphorylation of both ERK and FOXO was significantly decreased relative to mosquitoes fed insulin alone (Fig. 4). Intriguingly, MnTBAP alone significantly increased pERK relative to control levels, suggesting that basal levels of ROS keep activation of this heavily networked signaling protein in check. In general, these data indicate that ROS are important mediators of both the MAPK and PI3K/Akt branches of the IIS cascade in the mosquito midgut epithelium, a key interface between malaria parasites and the mosquito immune response.
FIG. 4.
ROS mediate insulin signaling in the A. stephensi midgut epithelium in vivo. Mosquitoes were fed blood meals supplemented with PBS as a control, 170 pM human insulin, 5 μM MnTBAP, or insulin plus MnTBAP. (A) Representative western blots of pERK and pFOXO. (B) Fold changes in phospho-specific proteins in pairwise comparisons of treatments or of treatments and controls from three independent experiments were analyzed with Student's t-test (α = 0.05). GAPDH levels provided an assessment of protein loading and were used to normalize corresponding phospho-protein levels. Data are represented as means ± SEMs and significant differences are indicated.
ROS alone can activate MAPK and PI3K/Akt signaling pathways in Anopheles cells
While the effects of MnTBAP indirectly implicated ROS in IIS in A. stephensi (Figs. 3 and 4), it remained unclear whether ROS could directly participate in MAPK and PI3K/Akt signaling. To determine whether ROS had a direct effect on cell signaling in A. stephensi and A. gambiae, cells from both species were treated with increasing doses of H2O2 and activation of downstream signaling proteins was assessed by western blotting. Although H2O2 is the most stable ROS commonly used to study cell signaling (41), our time–course analyses revealed that maximum ERK phosphorylation occurred at 5 min and then gradually declined after H2O2 treatment (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/ars), suggesting that signaling by H2O2 peaks very rapidly in mosquito cells.
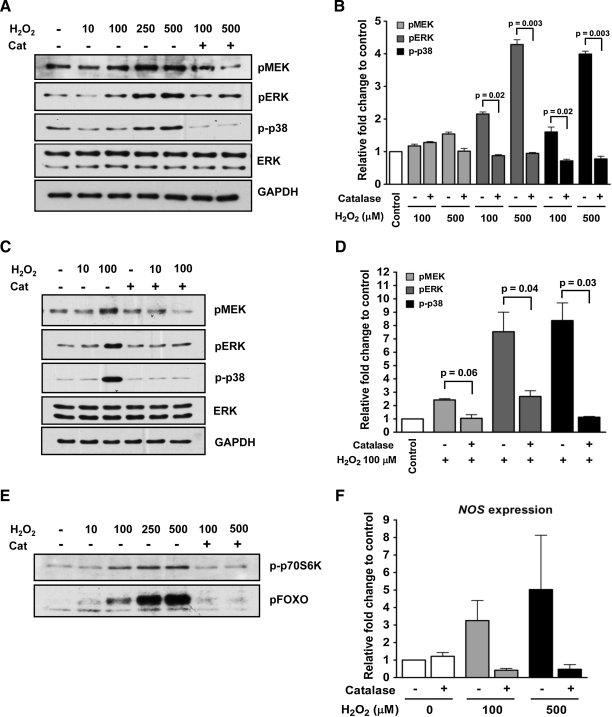
We found that H2O2 dose dependently induced MEK, ERK, and p38 phosphorylation in two A. stephensi cell lines (Fig. 5) and in A. gambiae cells (Supplementary Fig. S2). Notably, pretreatment with the antioxidant enzyme catalase significantly reduced H2O2-induced ERK and p38 phosphorylation in A. stephensi ASE cells (Fig. 5A, B) and MSQ43 cells (Fig. 5C, D), and showed a similar pattern in A. gambiae 4a3B cells (Supplementary Fig. S2). Although MEK phosphorylation levels did not change significantly after catalase treatment, we have previously shown that small alterations in phosphorylated MEK levels, such as those observed here, can lead to significant differences in ERK activation and are, therefore, biologically significant (42). In a manner similar to MAPK activation, H2O2 also dose dependently induced FOXO and p70S6K phosphorylation (Fig. 5E and Supplementary Fig. S3) and this increase was reduced by catalase pretreatment (Fig. 5E and Supplementary Fig. S3). Collectively, these data demonstrated that ROS alone can activate both the MAPK and PI3K/Akt signaling pathways, suggesting that ROS play a critical role in mosquito cell signaling.
FIG. 5.
ROS directly activated MAPK and PI3K/Akt signaling pathways in A. stephensi cells in vitro. Exogenous H2O2 induced MEK, ERK, and p38 phosphorylation in A. stephensi ASE cells (A, B) and MSQ43 cells (C, D). For catalase treatment, cells were pretreated with 200 units/ml of catalase for 40 min before treatment with increased doses of H2O2. Blots are representative of three independent experiments. Fold changes of pMEK, pERK, and p-p38 treated with 100 and 500 μM H2O2 in ASE cells (B) and 100 μM H2O2 in MSQ43 cells (D) are represented relative to paired PBS controls. Pairwise comparisons of treatments were analyzed by Student's t-test (α = 0.05). Data are represented as means ± SEMs from three independent experiments and significant differences within treatment pairs are indicated. (E) Increasing doses of H2O2 activated FOXO and p70S6K phosphorylation in ASE cells and pretreatment with catalase at 200 units/ml for 40 min before H2O2 treatment reduced this phosphorylation. Blots are representative of three independent experiments. Fold changes of pFOXO and p-p70S6K are shown in Supplementary Figure S3. (F) NOS gene expression was analyzed in ASE cells by quantitative reverse transcriptase–polymerase chain reaction at 24 h post-treatment. Fold inductions of NOS relative to paired controls from three independent experiments, represented as means ± SEMs, were analyzed by Student's t-test (α = 0.05). No significant differences within treatment pairs were observed. NOS, nitric oxide synthase; H2O2, hydrogen peroxide.
In previous studies, we showed that insulin-induced activation of MAPK, but not PI3K/Akt signaling, was associated with increased expression of A. stephensi NOS (24), an effector gene that contributes to the control of malaria parasite development (16, 18, 32). We have shown here that ROS alone can activate the MAPK pathway. Therefore, we sought to determine whether H2O2 could mediate the expression of NOS in Anopheles cells. We found that H2O2 dose dependently induced NOS expression in A. stephensi cells, with the highest induction occurring at 500 μM H2O2 although these trends were not significant (Fig. 5F). H2O2 also induced NOS expression in A. gambiae cells (Supplementary Fig. S4). Catalase pretreatment reduced H2O2-dependent NOS expression in cells of both mosquito species (Fig. 5F and Supplementary Fig. S4), suggesting that ROS can regulate anti-parasite immune responses as well as cell signaling in mosquito cells.
ROS are necessary for insulin signaling, but not TGF-beta1 signaling, in mosquito cells
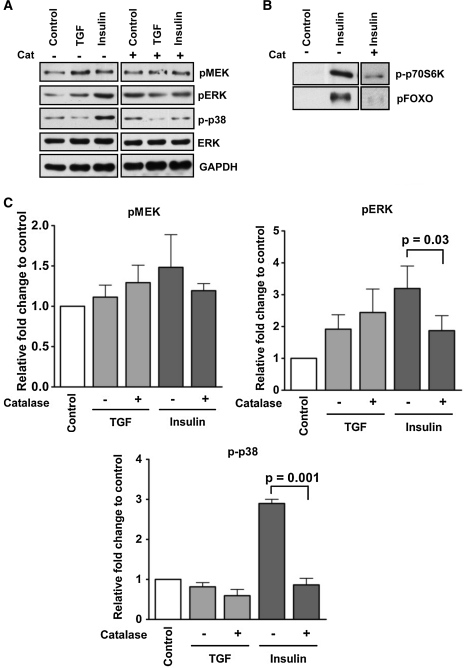
ROS have been shown to be necessary and important mediators of both the IIS and TGF-beta1 signaling pathways in a variety of organisms. We have previously shown that both human TGF-beta1 and human insulin can activate the MAPK pathway and induce NOS gene expression in mosquito cells (14, 24, 42). Therefore, we presumed that TGF-beta1 signaling would provide an excellent counterpart to IIS to assess the roles of ROS in these pathways. We found that pretreatment of cells with catalase reduced insulin-induced activation of MEK, ERK, and p38 phosphorylation but had no impact on TGF-beta1 signaling (Fig. 6A, C, and Supplementary Fig. S5), suggesting a specific role for ROS in insulin signaling. Catalase pretreatment also reduced insulin-induced activation of FOXO and p70S6K phosphorylation (Fig. 6B and Supplementary Fig. S6). Our previous observations that ROS levels are increased in insulin-treated mosquito cells (14) are consistent with the function of ROS in IIS. In contrast to insulin, increasing doses of TGF-beta1 did not induce significant ROS production in A. stephensi or in A. gambiae cells (data not shown), consistent with that the fact that ROS are not involved in TGF-beta1 signaling (Fig. 6 and Supplementary Fig. S5). Taken together, our findings indicated a specific role for ROS in insulin signaling, but not in TGF-beta1 signaling, in mosquito cells.
FIG. 6.
ROS mediated the signaling response of A. stephensi cells in vitro to human insulin but not to human TGF-beta1. (A) ASE cells were stimulated with 6000 pg/ml human TGF-beta1 or 1.7 μM human insulin for 5 min. To determine the effect of ROS on signaling protein activation, cells were pretreated with 200 units/ml catalase for 40 min before stimulation with each treatment. Blots are representative of three independent experiments. (B) ASE cells were treated with 200 units/ml catalase for 40 min before treatment with 1.7 μM of human insulin. Representative blots of FOXO and p70S6K phosphorylation from two experiments are shown. Fold changes of pFOXO and p-p70S6K are shown in Supplementary Figure S6. (C) Relative fold changes of pMEK, pERK, and p-p38 were normalized to total ERK or GAPDH and are shown relative to paired PBS controls. Pairwise comparisons of treatments were analyzed by Student's t-test (α = 0.05). Data are represented as means ± SEMs from three independent experiments. Significant differences within treatment pairs are indicated. TGF, transforming growth factor.
Overexpression of AsPrx reduced insulin signaling and NOS expression
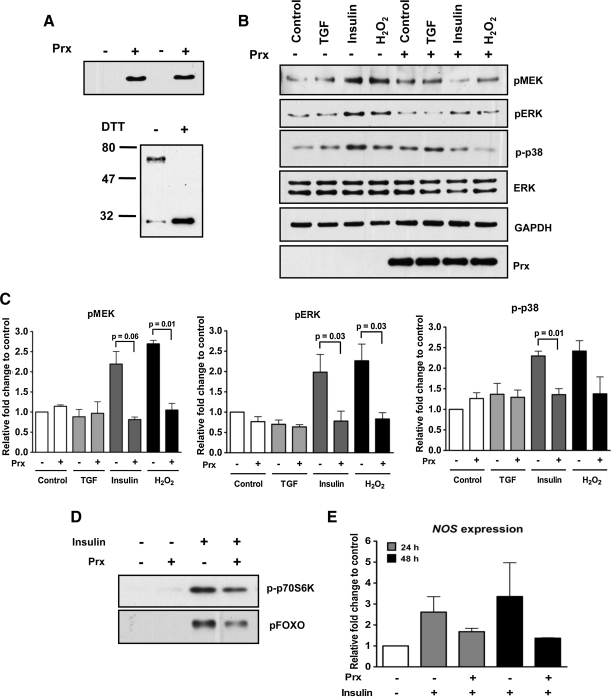
Thus far, we used exogenous MnTBAP and catalase to attribute a function for ROS in mosquito cell signaling. We previously showed that the endogenous AsPrx can protect mosquito cells from oxidative damage (33). Therefore, we sought to determine whether endogenous AsPrx could control ROS-mediated IIS in mosquito cells. To this end, we overexpressed AsPrx and analyzed the subsequent effects on cell signaling. The expression of full-length AsPrx protein was confirmed using antisera to the V5 tag protein (Fig. 7A). Disulfide bonding required for AsPrx homodimerization and, hence, catalytic activity was confirmed under reducing conditions (Fig. 7A). Transfected cells were stimulated with human TGF-beta1, human insulin, or H2O2. Similar to our results with MnTBAP and catalase, cells stimulated with insulin and H2O2, but not TGF-beta1 that also overexpressed AsPrx exhibited reduced activation of MEK, ERK, and p38 relative to cells lacking AsPrx (Fig. 7B, C). Cells overexpressing AsPrx also trended toward a reduction of insulin-induced phosphorylation of FOXO and p70S6K (Fig. 7D and Supplementary Fig. S7). Although not statistically significant, overexpression of AsPrx also reduced NOS expression by 1.6-fold at 24 h and by 1.4-fold at 48 h after insulin treatment (Fig. 7E). Taken together, these data suggested that insulin-induced ROS levels in mosquito cells can be regulated by endogenous antioxidants, such as AsPrx.
FIG. 7.
Overexpression of A. stephensi 2-cys peroxiredoxin (AsPrx) reduced the signaling response of A. stephensi cells in vitro to human insulin but not to human TGF-beta1. ASE cells were transfected with plasmid TLP-58 encoding a constitutively expressed AsPrx and were stimulated with 6000 pg/ml human TGF- beta1, 1.7 μM human insulin, or 500 μM H2O2 for 5 min (32). (A) Overexpressed 2-cys Prx was detected with anti-V5 antisera (upper panel) at 48 h after transfection. Disulfide bonding required for homodimerization and, hence, catalytic activity was confirmed (lower panel) under reducing conditions with dithiothreitol (DTT). (B) Phosphorylation of MEK, ERK, and p38 by insulin and H2O2, but not by TGF-beta1, was decreased in cells overexpressing AsPrx indicated as “Prx+.” In the absence of copper stimulation, no AsPrx was expressed in cells transfected with the copper-inducible plasmid TLP-55, so these controls are indicated as “Prx−.” Blots are representative of three independent experiments. (C) Fold changes of pMEK, pERK, and p-p38 were normalized to total ERK or GAPDH and are shown relative to paired PBS-treated control cells transfected with TLP-55. Pairwise comparisons of treatments were analyzed by Student's t-test (α = 0.05). Data are represented as means ± SEMs from three independent experiments. Significant differences within treatment pairs are indicated. (D) Overexpressed AsPrx reduced insulin induction of FOXO and p70S6K phosphorylation. Blots are representative of two independent experiments. Fold changes of pFOXO and p-p70S6K are shown in Supplementary Figure S7. (E) ASE cells were transfected with TLP-58 for 48 h, and then stimulated with 1.7 μM human insulin for 24 or 48 h before RNA isolation. NOS gene expression was analyzed with quantitative reverse transcriptase–polymerase chain reaction. Fold inductions of NOS relative to paired TLP-55 transfected controls are shown from three independent experiments, represented as means ± SEMs, and were analyzed by Student's t-test (α = 0.05). No significant differences within treatment pairs were observed.
Discussion
We have shown that ingested insulin is beneficial for P. falciparum oocyst development in A. stephensi and that this effect is mediated in part by ROS-dependent signaling. Further, this beneficial effect of insulin contrasts with the detrimental effects of Akt overexpression on P. falciparum oocyst development in A. stephensi (6), suggesting that the effects of insulin activation of IIS are complex and distinct from those mediated by overexpression of the single IIS protein Akt. The effects of insulin-induced ROS do not appear to be mediated by increased permeability of the midgut epithelial barrier as a result of ROS-induced damage but rather they are a consequence of ROS-dependent signaling in Anopheles mosquitoes. In vitro, the trend toward increased expression of the anti-parasite effector gene NOS at 24 h appeared to be partly dependent on insulin-induced ROS. In vivo, we have shown previously that induction of NOS expression by insulin in the A. stephensi midgut did not exceed control values until 36 h after feeding (24), a point at which P. falciparum oocyst formation is largely completed (3). Thus, the timing of signaling events observed here in vitro and in vivo would suggest that IIS-induced ROS regulate additional, earlier responses independent of NOS to govern oocyst development. Indeed, given the negative effects of ROS scavenging on P. falciparum oocyst development, we propose that early regulation by IIS-induced ROS may prime an anti-inflammatory state in which parasite development is favored.
In support of our observations and hypotheses, the IIS pathway and IIS-induced ROS-dependent signaling (2) have been shown to broadly regulate the innate immune responses of a variety of organisms. In particular, the balance of signaling among the two IIS branches can dictate the biological effects of IIS. The PI3K/Akt branch of the IIS pathway has been linked to anti-inflammatory responses in mammals (11). In contrast, the MAPK branch of the IIS pathway has been shown to have pro-inflammatory effects (11). Elevated insulin levels and IIS have been correlated with the release of pro-inflammatory cytokines and chemokines (37). It is possible, therefore, that the MAPK and PI3K/Akt branches of IIS have distinct and opposing effects on the mosquito innate immune response. Based on the above observations and our in vivo results (Fig. 4), we are currently analyzing the kinetics of IIS in our mosquito hosts to fully elucidate the resultant temporal and immune modulatory effects of human insulin on Plasmodium development.
We have demonstrated that the requirement for ROS is specific to IIS since scavenging of ROS had no effect on TGF-beta1-dependent MAPK activation (Fig. 6). In mammalian cells, ROS have been shown to play a critical role in TGF-beta1 signaling (13, 15). Although mosquitoes possess a complement of highly conserved components to support TGF-beta1 signaling (23), our data suggest that divergent, ROS-independent mechanisms regulate TGF-beta1 signaling in mosquito cells. Although the exact mechanism by which ROS regulate IIS in mosquito cells remains to be determined, multiple mechanisms for ROS facilitation of both the MAPK- and PI3K/Akt-dependent signaling branches of the IIS cascade have been identified in mammals. For example, ROS can directly alter the activity of PTEN, a known inhibitor of the IIS pathway (22). Alternatively, ROS can stimulate protein kinase C (PKC) activity through the oxidative modification of PKC regulatory domains (9). Several PKC isoforms have been shown to be involved in the activation of the IIS cascade in mammalian cells (30, 36). Mosquitoes possess many of the conserved components of the IIS cascade, including PTEN and PKC isoforms (35), so ROS-dependent IIS regulation may occur through these signaling proteins in mosquito cells.
The mosquito midgut is a key interface between the vector, human host, and the malaria parasite and the sum of these interactions determines the ability of mosquitoes to transmit malaria. In this study we show that insulin-induced ROS are critical mediators of the MAPK- and PI3K/Akt-dependent signaling branches of the IIS cascade and of P. falciparum development in A. stephensi (Fig. 8). Identifying the mechanisms whereby IIS regulates innate immunity has obvious practical value for understanding of mosquito physiology, but this knowledge can also provide insights into the evolution of IIS regulation in higher organisms. Further, selective targeting of critical mediators of signaling pathways in the mosquito midgut can facilitate discovery of novel suites of anti-parasite effector genes that can be manipulated to develop mosquitoes that are refractory to malaria infection.
Supplementary Material
Abbreviations Used
- ASE
A. stephensi embryo-derived
- AsPrx
A. stephensi 2-cys peroxiredoxin
- DTT
dithiothreitol
- ERK
extracellular signal-regulated kinase
- FOXO
forkhead box O1
- H2O2
hydrogen peroxide
- IIS
insulin/IGF-1 signaling
- MAPK
mitogen-activated protein kinase
- MEK
mitogen activated protein kinase kinase
- MnTBAP
Mn(III) tetrakis(4-benzoic acid) porphyrin chloride
- NOS
nitric oxide synthase
- p38
P38 mitogen-activated protein kinase
- PBS
phosphate-buffered saline
- PCC
protein carbonyl content
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- RBCs
red blood cells
- ROS
reactive oxygen species
- SEM
standard error of the mean
- SOD
superoxide dismutase
- TGF-beta1
transforming growth factor-beta1
Acknowledgments
We would like to thank Dr. Edwin E. Lewis for assistance with statistical analyses and Hannah Smithers for critical review of the article. We would also like to thank Laura Dickson for her assistance in establishing the protocols for quantitation of protein oxidation. Funding for these studies was provided by NIH NIAID AI073745 and AI080799 and with facilities improvement support by NIH NCRR C06 RR-12088–01.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Baas AS. Berk BC. Differential activation of mitogen-activated protein kinases by H2O2 and O2 in vascular smooth muscle cells. Circ Res. 1995;77:29–36. doi: 10.1161/01.res.77.1.29. [DOI] [PubMed] [Google Scholar]
- 2.Bashan N. Kovsan J. Kachko I. Ovadia H. Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89:27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 3.Baton LA. Ranford-Cartwright LC. Plasmodium falciparum ookinete invasion of the midgut epithelium of Anopheles stephensi is consistent with the Time Bomb model. Parasitology. 2004;129:663–676. doi: 10.1017/s0031182004005979. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley JM. Price DA. Schacker TW. Asher TE. Silvestri G. Rao S. Kazzaz Z. Bornstein E. Lambotte O. Altmann D. Blazar BR. Rodriguez B. Teixeira-Johnson L. Landay A. Martin JN. Hecht FM. Picker LJ. Lederman MM. Deeks SG. Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 5.Cirimotich CM. Dong Y. Garver LS. Sim S. Dimopoulos G. Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol. 2010;34:387–395. doi: 10.1016/j.dci.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corby-Harris V. Drexler A. Watkins de Jong L. Antonova Y. Pakpour N. Ziegler R. Ramberg F. Lewis EE. Brown JM. Luckhart SL. Riehle MA. A novel strategy for controlling malaria transmission in the mosquito Anopheles stephensi. PLoS Pathog. 2010;6:e1001003. doi: 10.1371/journal.ppat.1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darby SM. Miller ML. Allen RO. LeBeau M. A mass spectrometric method for quantitation of intact insulin in blood samples. J Anal Toxicol. 2001;25:8–14. doi: 10.1093/jat/25.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein BJ. Mahadev K. Wu X. Zhu L. Motoshima H. Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal. 2005;7:1021–1031. doi: 10.1089/ars.2005.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopalakrishna R. Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 10.Goto H. Gomes CM. Corbett CE. Monteiro HP. Gidlund M. Insulin-like growth factor I is a growth-promoting factor for Leishmania promastigotes and amastigotes. Proc Natl Acad Sci USA. 1998;95:13211–13216. doi: 10.1073/pnas.95.22.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwasaki Y. Nishiyama M. Taguchi T. Asai M. Yoshida M. Kambayashi M. Terada Y. Hashimoto K. Insulin exhibits short-term anti-inflammatory but long-term proinflammatory effects in vitro. Mol Cell Endocrinol. 2009;298:25–32. doi: 10.1016/j.mce.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Janssen-Heininger YM. Mossman BT. Heintz NH. Forman HJ. Kalyanaraman B. Finkel T. Stamler JS. Rhee SG. van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Junn E. Lee KN. Ju HR. Han SH. Im JY. Kang HS. Lee TH. Bae YS. Ha KS. Lee ZW. Rhee SG. Choi I. Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF-beta 1-induced IL-6 expression. J Immunol. 2000;165:2190–2197. doi: 10.4049/jimmunol.165.4.2190. [DOI] [PubMed] [Google Scholar]
- 14.Kang MA. Mott TM. Tapley EC. Lewis EE. Luckhart S. Insulin regulates aging and oxidative stress in Anopheles stephensi. J Exp Biol. 2008;211:741–748. doi: 10.1242/jeb.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YK. Bae GU. Kang JK. Park JW. Lee EK. Lee HY. Choi WS. Lee HW. Han JW. Cooperation of H2O2-mediated ERK activation with Smad pathway in TGF-beta1 induction of p21WAF1/Cip1. Cell Signal. 2006;18:236–243. doi: 10.1016/j.cellsig.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S. Barillas-Mury C. Ookinete-induced midgut peroxidases detonate the time bomb in anopheline mosquitoes. Insect Biochem Mol Biol. 2005;35:721–727. doi: 10.1016/j.ibmb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S. Christophides GK. Cantera R. Charles B. Han YS. Meister S. Dimopoulos G. Kafatos FC. Barillas-Mury C. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci USA. 2003;100:14139–14144. doi: 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S. Gupta L. Han YS. Barillas-Mury C. Inducible peroxidases mediate nitration of Anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. J Biol Chem. 2004;279:53475–53482. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S. Molina-Cruz A. Gupta L. Rodrigues J. Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–1648. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon J. Lee SR. Yang KS. Ahn Y. Kim YJ. Stadtman ER. Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambros C. Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 22.Leslie NR. Bennett D. Lindsay YE. Stewart H. Gray A. Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieber MJ. Luckhart S. Transforming growth factor-betas and related gene products in mosquito vectors of human malaria parasites: signaling architecture for immunological crosstalk. Mol Immunol. 2004;41:965–977. doi: 10.1016/j.molimm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Lim J. Gowda DC. Krishnegowda G. Luckhart S. Induction of nitric oxide synthase in Anopheles stephensi by Plasmodium falciparum: mechanism of signaling and the role of parasite glycosylphosphatidylinositols. Infect Immun. 2005;73:2778–2789. doi: 10.1128/IAI.73.5.2778-2789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luckhart S. Riehle MA. The insulin signaling cascade from nematodes to mammals: insights into innate immunity of Anopheles mosquitoes to malaria parasite infection. Dev Comp Immunol. 2007;31:647–656. doi: 10.1016/j.dci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luckhart S. Vodovotz Y. Cui L. Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci USA. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahadev K. Wu X. Zilbering A. Zhu L. Lawrence JT. Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001;276:48662–48669. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- 28.Marshall JM. Taylor CE. Malaria control with transgenic mosquitoes. PLoS Med. 2009;6:e20. doi: 10.1371/journal.pmed.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molina-Cruz A. DeJong RJ. Charles B. Gupta L. Kumar S. Jaramillo-Gutierrez G. Barillas-Mury C. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem. 2008;283:3217–3223. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- 30.Oriente F. Andreozzi F. Romano C. Perruolo G. Perfetti A. Fiory F. Miele C. Beguinot F. Formisano P. Protein kinase C-alpha regulates insulin action and degradation by interacting with insulin receptor substrate-1 and 14–3-3 epsilon. J Biol Chem. 2005;280:40642–40649. doi: 10.1074/jbc.M508570200. [DOI] [PubMed] [Google Scholar]
- 31.Owusu-Ansah E. Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson TM. Gow AJ. Luckhart S. Nitric oxide metabolites induced in Anopheles stephensi control malaria parasite infection. Free Radic Biol Med. 2007;42:132–142. doi: 10.1016/j.freeradbiomed.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson TM. Luckhart S. A mosquito 2-Cys peroxiredoxin protects against nitrosative and oxidative stresses associated with malaria parasite infection. Free Radic Biol Med. 2006;40:1067–1082. doi: 10.1016/j.freeradbiomed.2005.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci. 2008;13:7210–7226. doi: 10.2741/3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riehle MA. Brown JM. Characterization of phosphatase and tensin homolog expression in the mosquito Aedes aegypti: six splice variants with developmental and tissue specificity. Insect Mol Biol. 2007;16:277–286. doi: 10.1111/j.1365-2583.2007.00724.x. [DOI] [PubMed] [Google Scholar]
- 36.Schonwasser DC. Marais RM. Marshall CJ. Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoelson SE. Lee J. Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sim C. Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc Natl Acad Sci USA. 2008;105:6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinden RE. Plasmodium differentiation in the mosquito. Parassitologia. 1999;41:139–148. [PubMed] [Google Scholar]
- 40.Snow RW. Guerra CA. Noor AM. Myint HY. Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone JR. Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 42.Surachetpong W. Singh N. Cheung KW. Luckhart S. MAPK ERK signaling regulates the TGF-beta1-dependent mosquito response to Plasmodium falciparum. PLoS Pathog. 2009;5:e1000366. doi: 10.1371/journal.ppat.1000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ushio-Fukai M. Alexander RW. Akers M. Yin Q. Fujio Y. Walsh K. Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 44.Vaughan JA. Noden BH. Beier JC. Sporogonic development of cultured Plasmodium falciparum in six species of laboratory-reared Anopheles mosquitoes. Am J Trop Med Hyg. 1994;51:233–243. doi: 10.4269/ajtmh.1994.51.233. [DOI] [PubMed] [Google Scholar]
- 45.Zhu S. Lai DH. Li SQ. Lun ZR. Stimulative effects of insulin on Toxoplasma gondii replication in 3T3-L1 cells. Cell Biol Int. 2006;30:149–153. doi: 10.1016/j.cellbi.2005.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.