Abstract
Introduction
Male circumcision is being promoted for HIV prevention in high-risk heterosexual populations. However, there is a concern that circumcision may impair sexual function.
Aim
To assess adult male circumcision’s effect on men’s sexual function and pleasure.
Methods
Participants in a controlled trial of circumcision to reduce HIV incidence in Kisumu, Kenya were uncircumcised, HIV negative, sexually active men, aged 18–24 years, with a hemoglobin ≥9.0 mmol/L. Exclusion criteria included foreskin covering less than half the glans, a condition that might unduly increase surgical risks, or a medical indication for circumcision. Participants were randomized 1:1 to either immediate circumcision or delayed circumcision after 2 years (control group). Detailed evaluations occurred at 1, 3, 6, 12, 18, and 24 months.
Main Outcome Measures
(i) Sexual function between circumcised and uncircumcised men; and (ii) sexual satisfaction and pleasure over time following circumcision.
Results
Between February 2002 and September 2005, 2,784 participants were randomized, including the 100 excluded from this analysis because they crossed over, were not circumcised within 30 days of randomization, did not complete baseline interviews, or were outside the age range. For the circumcision and control groups, respectively, rates of any reported sexual dysfunction decreased from 23.6% and 25.9% at baseline to 6.2% and 5.8% at month 24. Changes over time were not associated with circumcision status. Compared to before they were circumcised, 64.0% of circumcised men reported their penis was “much more sensitive,” and 54.5% rated their ease of reaching orgasm as “much more” at month 24.
Conclusions
Adult male circumcision was not associated with sexual dysfunction. Circumcised men reported increased penile sensitivity and enhanced ease of reaching orgasm. These data indicate that integration of male circumcision into programs to reduce HIV risk is unlikely to adversely effect male sexual function.
Keywords: Male Circumcision, HIV Infection, Sexual Dysfunction, Sexual Satisfaction, Erectile Dysfunction, Ejaculatory Dysfunction, Balanitis
Introduction
Male circumcision is now being promoted in many areas, particularly in eastern and southern Africa, as a public health measure to reduce HIV risk. Three randomized clinical trials [1–3] support epidemiological data [4] showing that adult male circumcision reduces the risk for HIV acquisition in men by 51–76%. Possible mechanisms by which male circumcision may protect against HIV infection include that circumcised men have more penile cornification, lower rates of penile injury during intercourse, fewer HIV receptors, and lower rates of inflammation and of some sexually transmitted infections (STIs) [1–3]. The clinical trials documented acceptable surgery-related adverse event rates [1–3], and the World Health Organization now recommends male circumcision as one element of HIV prevention programs [5]. Neonatal circumcision reduces urinary tract infection rates substantially [6–8], and other data suggest that male circumcision is associated with lower rates of STIs [9–15]. Circumcised males do not develop phimosis or para-phimosis, and they are at lower risk for balanitis, human papilloma virus infection [16], and penile cancer [17–19]. In addition, female sexual partners of circumcised men have been shown to have reduced risk of cervical cancer [13] and chlamydial infection [20].
Despite these benefits, there is a concern that male circumcision may decrease male sexual function and satisfaction. Ritualistic male circumcision has been practiced in West Africa and the Middle East for over 4,000 years [21]. In the West, circumcision started to be promoted in the late 19th century for a wide variety of public health reasons, from the reduction of syphilis risk to the prevention of masturbation [22,23]. Male circumcision has been advocated in some societies to reduce pubescent males’ excitability and sexual arousal, while in others, it is seen as enhancing sexual prowess [24]. Proposed mechanisms by which circumcision may decrease sexual function include reorganization/atrophy of neural circuitry [25], keratinization of the glans penis resulting in lower sensitivity [26–29], decreased erectile function [27], increased ejaculatory latency time [30], removal of the smegma with lower pheromone levels, and less, “normal gliding action” [24].
Previous studies have described variable and inconsistent effects of circumcision on male sexual function. Some case control studies have reported reduced sexual sensation, masturbatory pleasure, and sexual enjoyment among circumcised men compared with uncircumcised men [26,28], but higher fine-touch pressure thresholds in the glans among circumcised men compared with uncircumcised men [29]. Some before and after studies of men circumcised as adults have reported decreased penile sensitivity [27,31], while others have reported no change in penile sensitivity and satisfaction [32]. One before and after study found an increased ejaculatory latency time after circumcision, which was deemed an advantage [30]. A probability sample of 10,173 men in Australia aged 16–59 years found circumcised men were less likely to report trouble keeping an erection or physical pain during intercourse [33]. Payne and associates found no difference during genital sensory testing as a function of sexual arousal between 20 circumcised and 20 uncircumcised men [34]. In the U.S. National Health and Social Life Survey (NHSLS), circumcised men were found to have a more elaborate set of sexual practices [35]. A recent randomized clinical trial found that circumcised adult men experienced no clinically significant adverse effects on sexual satisfaction or sexual function [36].
To better understand the risks and benefits of circumcision, we prospectively evaluated sexual function and sexual satisfaction among adult men participating in a randomized, controlled clinical trial of adult male circumcision to prevent HIV infection in Kisumu, Kenya.
Methods
Study Design and Participants
The trial design, circumcision technique, adverse events, and primary outcome (HIV infection) have been described [2,37,38]. Briefly, participants were recruited from sexually transmitted disease clinics, workplaces, social events, and youth organizations. Interested men were given an appointment for randomization and possible circumcision within 1 week of screening. For inclusion, men had to be uncircumcised, HIV negative, sexually active in the last 12 months, aged 18–24 years, have a hemoglobin ≥9.0 mmol/L, and be residents of Kisumu District. Exclusion criteria included foreskin covering less than half of the glans, a bleeding disorder, keloid formation, other conditions that might unduly increase the risks of elective surgery, or a medical indication for circumcision. Institutional review boards of the University of Illinois at Chicago, the Kenyatta National Hospital, RTI International, the University of Manitoba, and the University of Washington approved the study.
Clinical Procedures and Follow-Up
Following written informed consent, the participants were randomized 1:1 to either immediate circumcision or delayed circumcision after a 2-year follow-up period (the control group). The men in both groups were counseled extensively on STIs and HIV risk reduction, and were provided unlimited supplies of free condoms.
The circumcision group had a standard “forceps guided procedure” as described previously [38]. Under local anesthesia, the prepuce was grasped at the 3 and 9 o’clock positions using two mosquito clamps, then was pulled over the glans. After outlining the incision with a marking pen, a Kocher clamp was applied below the planned incision, taking care to avoid injury to the glans. The prepuce was excised by cutting above the Kocher clamp, which was then removed. Bleeders were ligated using 3/0 plain. Skin and mucosal incisions were approximated using interrupted 3–0 and 4–0 chromic sutures. Follow-up visits were scheduled on postoperative days 3, 8, and 30. Surgical results, adverse events, resumption of activities of daily living, and participants’ high degree of satisfaction with their surgical procedures have been described in detail in our previous reports [2,37,38].
Detailed evaluations were conducted at 1, 3, 6, 12, 18, and 24 months from randomization for both the circumcision and the control groups. At each visit, the participants underwent a standardized medical history and physical examination, plus a personal interview to obtain sociodemo-graphic and health information, and to assess behavioral risk factors. Trained counselors interviewed the participants in their language of choice (English, Dholuo, or Kiswahili). Extensive data were collected on sexual function and satisfaction, including the items outlined in Tables 1 and 2.
Table 1.
Sexual function by circumcision status and study visit
| Sexual function measures: | Baseline, n (%) | Follow-up, n/N (%) |
|||
|---|---|---|---|---|---|
| 6 months, n (%) | 12 months, n (%) | 18 months, n (%) | 24 months, n (%) | ||
| From behavioral questionnaire* | N = 2,304 | N = 2,032 | N = 2,014 | N = 1,604 | N = 1,195 |
| Inability to ejaculate | |||||
| Control group | 47/1,178 (4.0) | 28/1,031 (2.7) | 11/1,015 (1.1) | 7/808 (0.9) | 7/582 (1.2) |
| Circumcision group | 55/1,124 (4.9) | 30/1,001 (3.0) | 12/999 (1.2) | 9/796 (1.1) | 8/613 (1.3) |
| Premature ejaculation | |||||
| Control group | 226/1,177 (19.2) | 95/1,030 (9.2) | 41/1,012 (4.1) | 32/807 (4.0) | 27/582 (4.6) |
| Circumcision group | 185/1,122 (16.5) | 72/1,000 (7.2) | 37/999 (3.7) | 29/796 (3.6) | 24/613 (3.9) |
| Pain during intercourse | |||||
| Control group | 86/1,179 (7.3) | 33/1,031 (3.2) | 18/1,015 (1.8) | 6/808 (0.7) | 7/582 (1.2) |
| Circumcision group | 87/1,125 (7.7) | 33/1,000 (3.3) | 12/999 (1.2) | 7/796 (0.9) | 4/613 (0.7) |
| Sex not pleasurable | |||||
| Control group | 96/1,179 (8.1) | 40/1,029 (3.9) | 19/1,015 (1.9) | 12/808 (1.5) | 6/582 (1.0) |
| Circumcision group | 87/1,122 (7.8) | 27/998 (2.7) | 9/1,000 (0.9) | 15/796 (1.9) | 11/613 (1.8) |
| Difficulty achieving/maintaining erection | |||||
| Control group | 87/1,179 (7.4) | 34/1,030 (3.3) | 22/1,014 (2.2) | 13/808 (1.6) | 8/582 (1.4) |
| Circumcision group | 74/1,125 (6.6) | 29/1,001 (2.9) | 21/1,000 (2.1) | 16/796 (2.0) | 14/612 (2.3) |
| Any of the above reported sexual difficulties | |||||
| Control group | 304/1,176 (25.9) | 150/1,027 (14.6) | 82/1,011 (8.1) | 54/807 (6.7) | 34/582 (5.8) |
| Circumcision group | 263/1,116 (23.6) | 127/996 (12.8) | 71/999 (7.1) | 49/796 (6.2) | 38/612 (6.2) |
| From medical history* | N =2,684 | N = 2,438 | N = 2,384 | N = 1,916 | N = 1,431 |
| Erections feel normal | |||||
| Control group | 1,356/1,371 (98.9) | 1,244/1,248 (99.7) | 1,204/1,208 (99.7) | 969/974 (99.5) | 712/714 (99.7) |
| Circumcision group | 1,303/1,313 (99.2) | 1,180/1,190 (99.2) | 1,168/1,176 (99.3) | 939/942 (99.7) | 716/717 (99.9) |
| Deviation during erection | |||||
| Control group | 15/1,371 (1.1) | 1/1,248 (0.1) | 0/1,208 (0.0) | 0/974 (0.0) | 0/714 (0.0) |
| Circumcision group | 17/1,313 (1.3) | 3/1,190 (0.3) | 0/1,176 (0.0) | 0/942 (0.0) | 0/717 (0.0) |
| Difficulty achieving erection because skin is too tight | |||||
| Control group | 1/1,371 (0.1) | 0/1,248 (0.0) | 0/1,208 (0.0) | 0/974 (0.0) | 0/714 (0.0) |
| Circumcision group | 1/1,313 (0.1) | 0/1,190 (0.0) | 0/1,176 (0.0) | 0/942 (0.0) | 0/717 (0.0) |
Sample sizes vary by characteristic because of missing responses.
Table 2.
Sexual satisfaction and pleasure among circumcised men by follow-up visit
| Characteristic* | Follow-up, n/N (%) |
|||
|---|---|---|---|---|
| 6 months | 12 months | 18 months | 24 months | |
| N = 1,186 | N = 1,170 | N = 938 | N = 706 | |
| Ever avoided sex because of being circumcised | ||||
| No | 1101 (93.3) | 1129 (96.7) | 919 (98.0) | 691 (97.9) |
| Yes | 79 (6.7) | 38 (3.3) | 19 (2.0) | 15 (2.1) |
| Penile sensitivity | ||||
| Much more | 594 (50.1) | 652 (55.7) | 597 (63.7) | 451 (64.0) |
| Somewhat more | 155 (13.1) | 142 (12.1) | 68 (7.3) | 55 (7.8) |
| About the same | 256 (21.6) | 239 (20.4) | 182 (19.4) | 136 (19.3) |
| Somewhat less | 66 (5.6) | 58 (5.0) | 45 (4.8) | 37 (5.3) |
| Much less | 18 (1.5) | 21 (1.8) | 15 (1.6) | 11 (1.6) |
| Don’t know | 97 (8.2) | 58 (5.0) | 30 (3.2) | 15 (2.1) |
| Easiness of reaching orgasm | ||||
| Much more | 439 (37.1) | 535 (45.7) | 476 (50.8) | 385 (54.5) |
| Somewhat more | 192 (16.2) | 134 (11.5) | 93 (9.9) | 61 (8.6) |
| About the same | 244 (20.6) | 258 (22.1) | 203 (21.7) | 158 (22.4) |
| Somewhat less | 125 (10.6) | 115 (9.8) | 85 (9.1) | 62 (8.8) |
| Much less | 40 (3.4) | 50 (4.3) | 32 (3.4) | 15 (2.1) |
| Don’t know | 145 (12.2) | 78 (6.7) | 48 (5.1) | 25 (3.5) |
| Frequency of sex | ||||
| Much more | 144 (12.2) | 227 (19.4) | 245 (26.1) | 205 (29.0) |
| Somewhat more | 154 (13.0) | 139 (11.9) | 100 (10.7) | 69 (9.8) |
| About the same | 474 (40.1) | 437 (37.4) | 352 (37.5) | 245 (34.7) |
| Somewhat less | 154 (13.0) | 149 (12.8) | 130 (13.9) | 89 (12.6) |
| Much less | 210 (17.8) | 193 (16.5) | 87 (9.3) | 85 (12.0) |
| Don’t know | 46 (3.9) | 24 (2.1) | 24 (2.6) | 13 (1.8) |
| How protected do you feel against sexual diseases? | ||||
| Much more | 642 (54.1) | 704 (60.2) | 583 (62.2) | 479 (67.9) |
| Somewhat more | 242 (20.4) | 188 (16.1) | 147 (15.7) | 110 (15.6) |
| About the same | 158 (13.3) | 158 (13.5) | 117 (12.5) | 73 (10.3) |
| Somewhat less | 28 (2.4) | 22 (1.9) | 14 (1.5) | 8 (1.1) |
| Much less | 21 (1.8) | 20 (1.7) | 13 (1.4) | 8 (1.1) |
| Don’t know | 95 (8.0) | 78 (6.7) | 64 (6.8) | 28 (4.0) |
| Sexual partners’ reaction to circumcision | ||||
| Not aware been circumcised | 231 (19.6) | 133 (11.5) | 94 (10.1) | 53 (7.6) |
| Very Pleased | 478 (40.7) | 559 (48.4) | 503 (54.0) | 415 (59.6) |
| Somewhat pleased | 73 (6.2) | 46 (4.0) | 42 (4.5) | 21 (3.0) |
| Neutral or expressed no opinion | 368 (31.3) | 387 (33.5) | 276 (29.7) | 199 (28.6) |
| Somewhat displeased | 6 (0.5) | 8 (0.7) | 2 (0.2) | 4 (0.6) |
| Very displeased | 2 (0.2) | 0 (0.0) | 1 (0.1) | 0 (0.0) |
| Don’t know | 18 (1.5) | 23 (2.0) | 13 (1.4) | 4 (0.6) |
| Easiness of condom use* | ||||
| Have not used a condom since circumcision | 290 (32.8) | 264 (27.4) | 233 (25.1) | 165 (23.7) |
| Easier to use | 412 (46.7) | 500 (51.9) | 524 (56.4) | 413 (59.3) |
| Not as easy to use | 29 (3.3) | 33 (3.4) | 32 (3.4) | 13 (1.9) |
| Same as before | 152 (17.2) | 166 (17.2) | 141 (15.2) | 106 (15.2) |
Sample sizes vary slightly by characteristic because of a few missing responses.
“Don’t know” and “refused” (no more than two or three responses by follow-up interval) are excluded from the frequencies for “easiness of condom use.”
Statistical Analyses
Data for this analysis were collected as part of a randomized, controlled trial designed to assess the effect of male circumcision on reducing HIV seroconversion. The trial’s target sample size was 2,776 enrolled men to be able to detect a 50% difference in a 2-year HIV seroincidence, assuming 15% noninformative loss to follow-up, 5% nonadherence to treatment assignment, and a 2.5 per 100 person-years annual seroincidence in the control group (overall two-sided type I error rate = 0.05, power = 80%) [2]. As a result of an interim analysis conducted in October 2006 (with 87% follow-up completed), the data and safety monitoring board stopped the trial in December 2006. The data presented here include the follow-up through October 2006.
Data collection and management procedures have been described in detail previously [2,37,38]. We conducted two primary analyses here. The first compared sexual function over time between the circumcised and uncircumcised groups. There were five measures of sexual dysfunction (Table 1). “Any sexual dysfunction” was defined as a positive response to any of these five measures. The second analysis assessed sexual satisfaction and pleasure over time among circumcised men only. As secondary analyses, we compared the standardized clinical assessments by circumcision status, and penile complaints after circumcision among circumcised men.
The magnitude of the association between different dichotomous variables and circumcision status with time was estimated with odds ratios (ORs) and their corresponding 95% confidence intervals (CIs). Predicted odds were used to quantify relative change in sexual satisfaction and pleasure from baseline among the men who were circumcised. Generally, time (baseline, 6-, 12-, 18-, and 24-month follow-ups for sexual dysfunction, and 6-, 12-, 18-, and 24-month follow-ups for sexual satisfaction and pleasure) was included as categories in the models. However, additional models were run using orthogonal polynomials to assess trends with time and accounting for the same parameter space as in the models with time treated categorically (fourth-order polynomials for sexual dysfunction, and third-order polynomials for sexual satisfaction and pleasure). The generalized estimating equations (GEE) extension of generalized linear models was used to incorporate the within-subject correlation among the repeated measures, assuming binomial distributions with log link. Standard errors were obtained using an exchangeable correlation structure with robust (empirical) estimate of standard error. This GEE method takes into account the incomplete follow-up experience because of the early stoppage of the trial under the assumption that the data are missing at random. No consideration was given to multiple test issues. Data were analyzed using STATA/SE 9.2 for Windows (Stata Corp., College Station, TX, USA).
Results
Study Sample
Between February 2002 and September 2005, 2,784 participants were randomized, including 1,391 in the circumcision group and 1,393 in the control group. Screening results and reasons for exclusion and nonparticipation in the main trial have been reported [2,39]. There were no differences in the timing of the follow-up visits by group [2]. Of the 1,738 participants randomized at least 24 months plus 2 weeks prior to the October 2006 analysis, 1,501 (86%) had completed the 24-month follow-up [2]. For earlier study visits, the number of follow-ups and percentages among participants reaching the time lapse since randomization were 2,569 (92%) for month 1, 2,440 (88%) for month 3, 2,520 (91%) for month 6, 2,474 (89%) for month 12, and 2,003 (87%) for month 18 [2]. Among the 2,784 men enrolled, 100 were excluded from this analysis: 5 who did not complete the baseline interview, 3 who were outside the age range, 16 control participants who were circumcised, 57 men who were randomized to circumcision but were not circumcised, and 19 men randomized to circumcision but were not circumcised within 30 days of randomization.
Baseline Sociodemographic Characteristics and Behavioral Risk Factors
Interviews were conducted in English (60%), Dholuo (38%), and Kiswahili (2%), with the baseline assessments completed prior to randomization. Participants’ median age was 20 years. Over 86% of men were sexually active in the past 6 months, and their median number of lifetime sex partners was four. The median age at first sex was 16 years, and the median number of years being sexually active was five. The two study arms were well balanced in terms of sociodemographic characteristics and sexual behaviors [2,39]. Selected sociodemographic and behavioral characteristics are shown in Table 3.
Table 3.
Selected baseline sociodemographic characteristics and behaviors by circumcision status
| Characteristic* | Circumcision group | Control group |
|---|---|---|
| N = 1,313 | N = 1,371 | |
| n (%) | n (%) | |
| Reported age in years | ||
| 18–20 | 670 (51.0) | 698 (50.9) |
| 21–24 | 643 (49.0) | 673 (49.1) |
| Marital status | ||
| Not married or living with a female sex partner | 1,239 (94.6) | 1,292 (94.4) |
| Married or living with a female sex partner | 71 (5.4) | 76 (5.6) |
| Highest education completed | ||
| None, primary | 443 (33.7) | 473 (34.5) |
| Secondary 1–3 | 243 (18.5) | 230 (16.8) |
| Secondary 4, postsecondary | 627 (47.8) | 668 (48.7) |
| Employment | ||
| Salaried | 123 (9.4) | 132 (9.6) |
| Self-employed | 354 (27.0) | 352 (25.7) |
| Unemployed | 836 (63.7) | 887 (64.7) |
| Number of sex partners in the past 6 months | ||
| 0 | 183 (14.0) | 190 (13.9) |
| 1 | 575 (43.9) | 611 (44.6) |
| 2+ | 553 (42.2) | 570 (41.6) |
Sample sizes vary slightly by characteristic because of a few missing responses.
Sexual Dysfunction at Baseline
Among the 2,684 participants, 2,292 answered all five questions about sexual dysfunction at baseline, and 567 (24.7%; 95% CI: 23.0–26.5%) reported any sexual dysfunction. These included 411 men (17.9%) reporting ejaculating too quickly, 183 men (8.0%) reporting no pleasure during sex, 173 men (7.6%) reporting pain during sex, 161 men (7.0%) reporting difficulty achieving or maintaining erection, and 102 men (4.4%) reporting inability to ejaculate (Table 1). A marginally smaller percentage of men in the intervention group reported premature ejaculation at baseline (P = 0.09, Pearson chi-square test). Baseline reports of other sexual dysfunction measures did not differ between the treatment arms.
Sexual Functions by Circumcision Status and Follow-Up Visit
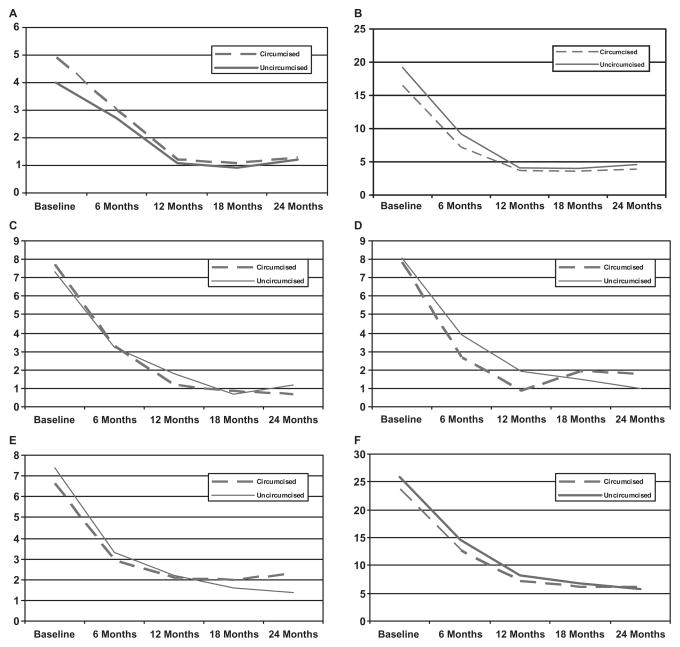
During the 2-year period from randomization, the circumcision group and the control group both experienced dramatic decreases in reported sexual dysfunction (Table 1, Figure 1A–F). For the circumcision and control groups, respectively, the percent reporting any of the five sexual dysfunction items decreased from 23.6% and 25.9% at baseline, to 6.2% and 5.8% at the 24-month follow-up visit.
Figure 1.
Percent reporting sexual dysfunctions by circumcision status and study visit. (A) Inability to ejaculate; (B) premature ejaculation; (C) pain during intercourse; (D) sex is not pleasurable; (E) difficulty achieving or maintaining erection; and (F) reporting any sexual dysfunction.
GEE was used to model sexual dysfunction by treatment and visit, taking into account the correlation of repeated measures on individual participants (Table 4). Changes from baseline in reports of any sexual dysfunction, or with any of the five individual sexual dysfunction items, did not differ by circumcision status (test for interaction was not significant on any measure). At 6 months, there were fewer reports of premature ejaculation among the men who were circumcised, but this difference was related to an imbalance at baseline, and the change from baseline was not significantly different by circumcision status. The decrease in sexual dysfunction over time in both study arms was significant with a tapering of the downward trend after 12 months (Table 4; P < 0.001 for the linear component on each item, and P < 0.02 for the quadratic component on each item).
Table 4.
Adjusted odds ratios of sexual dysfunctions for circumcision (vs. control) and follow-up (vs. baseline)*
| Characteristic | Odds ratio (95% CI) |
|---|---|
| Inability to ejaculate | |
| Circumcision group (vs. control) | 1.19 (0.89–1.61) |
| Visit | |
| Baseline | Reference |
| 6-month follow-up | 0.63 (0.46–0.86) |
| 12-month follow-up | 0.25 (0.16–0.38) |
| 18-month follow-up | 0.21 (0.12–0.36) |
| 24-month follow-up | 0.25 (0.14–0.44) |
| Premature ejaculation | |
| Circumcision group (vs. control) | 0.83 (0.69–0.99) |
| Visit | |
| Baseline | Reference |
| 6-month follow-up | 0.41 (0.34–0.49) |
| 12-month follow-up | 0.18 (0.14–0.23) |
| 18-month follow-up | 0.17 (0.12–0.22) |
| 24-month follow-up | 0.16 (0.11–0.23) |
| Pain during intercourse | |
| Circumcision group (vs. control) | 0.99 (0.77–1.28) |
| Visit | |
| Baseline | Reference |
| 6-month follow-up | 0.41 (0.31–0.54) |
| 12-month follow-up | 0.19 (0.13–0.27) |
| 18-month follow-up | 0.08 (0.05–0.17) |
| 24-month follow-up | 0.10 (0.05–0.20) |
| Sex not pleasurable | |
| Circumcision group (vs. control) | 0.90 (0.70–1.15) |
| Visit | |
| Baseline | Reference |
| 6-month follow-up | 0.39 (0.30–0.52) |
| 12-month follow-up | 0.16 (0.11–0.40) |
| 18-month follow-up | 0.19 (0.12–0.28) |
| 24-month follow-up | 0.14 (0.08–0.25) |
| Difficulty achieving/maintaining erection | |
| Circumcision group (vs. control) | 0.96 (0.75–1.23) |
| Visit | |
| Baseline | Reference |
| 6-month follow-up | 0.43 (0.32–0.57) |
| 12-month follow-up | 0.29 (0.21–0.40) |
| 18-month follow-up | 0.24 (0.16–0.36) |
| 24-month follow-up | 0.23 (0.14–0.37) |
| Any of the above reported sexual difficulties | |
| Circumcision group (vs. control) | 0.88 (0.76–1.03) |
| Visit | |
| Baseline | Reference |
| 6-month follow-up | 0.48 (0.42–0.55) |
| 12-month follow-up | 0.25 (0.21–0.30) |
| 18-month follow-up | 0.19 (0.15–0.24) |
| 24-month follow-up | 0.15 (0.11–0.21) |
Results are based on descriptive data given in Table 2. For each characteristic, the log odds of exhibiting the characteristic are modeled as a linear function of circumcision group and visit, using the generalized estimating equations extension of generalized linear models. Predicted odds ratios and their 95% confidence intervals (CIs) are reported from each model. There are no significant interactions to report. Trends with time were assessed through orthogonal polynomials: P < 0.001 for the linear component on each item, P < 0.02 for the quadratic component on each item, no higher-order trend was found.
During their follow-up evaluations, almost all circumcised men reported that their erections felt normal, that their penis did not deviate with erection, that they had little or no difficulty inserting their penis during intercourse, and that they had little or no difficulty achieving erection because their skin was too tight (Table 1). On physical examination, no circumcised man had painful lumps along the suture line, significant scarring, twisting of the penis, or penile pain. Almost all men were satisfied with their circumcisions (as reported by 98.9% of 1,186 men at month 6, increasing to 99.9% of 706 men reporting at month 24). Of the 1,332 uncircumcised men, 9 (0.7%) had symptoms or signs of balanitis during follow-up, including 1 man who had both balanitis and phimosis. None of these findings were detected by physical examination among the circumcised men. Based on follow-up time, the rate of symptomatic balanitis among uncircumcised men was 0.40 cases per 100 person-years (95% CI: 0.14–0.67).
Sexual Function and Satisfaction Following Circumcision
At their 6-, 12-, 18-, and 24-month visits, the circumcised men were asked six questions to assess sexual function and pleasure compared to before being circumcised (Table 2). At their 6-month follow-up, 50.1% of 1,186 men reported that their penis was “much more” sensitive, increasing to 64.0% of 706 men reporting at 24 months. In contrast, 6–7% reported that their penis was “somewhat less” or “much less” sensitive consistently across follow-up visits. Ease of reaching orgasm was rated as “much more” by 37.1% of men at their 6-month visit, increasing to 54.5% among the subset reporting at the 24-month visit. The same measure was rated “somewhat less” or “much less” by approximately 13.9% of men at 6 months, and decreased to approximately 10.9% at 24 months. At 6 months, 12.2% of the circumcised men reported having sex “much more” often than prior to circumcision, a rate that increased to 29% at 24 months. In contrast, the frequency of sex was rated as “much less” by 17.8% at 6 months, and 12.0% at 24 months. Condoms were reported as “easier to use” by 46.7% of men at month 6, increasing to 59.3% of men at month 24. Few men reported ever avoiding sex because of being circumcised. The increases over time in ease of reaching orgasm, penile sensitivity, and more frequent sex were statistically significant (P < 0.001 for the linear component on each item, not significant for the quadratic component on any item) in GEE analyses (Table 5).
Table 5.
Predicted odds of sexual satisfaction and pleasure for 6-, 12-, 18-, and 24-month follow-up among circumcised men*
| Characteristic | Odds (95% CI) |
|---|---|
| Ever avoided sex because of being circumcised | |
| 6-month follow-up | 0.07 (0.06–0.09) |
| 12-month follow-up | 0.03 (0.02–0.05) |
| 18-month follow-up | 0.02 (0.01–0.03) |
| 24-month follow-up | 0.02 (0.01–0.03) |
| Compared to before you were circumcised . . . | |
| Penile sensitivity: “more vs. same,” “less,” “don’t know” | |
| 6-month follow-up | 1.70 (1.52–1.92) |
| 12-month follow-up | 2.10 (1.86–2.37) |
| 18-month follow-up | 2.51 (2.19–2.88) |
| 24-month follow-up | 2.83 (2.40–3.33) |
| Easiness of reaching orgasm: “more vs. same,” “less,” “don’t know” | |
| 6-month follow-up | 1.14 (1.01–1.27) |
| 12-month follow-up | 1.33 (1.19–1.89) |
| 18-month follow-up | 1.62 (1.43–1.84) |
| 24-month follow-up | 1.94 (1.67–2.26) |
| Frequency of sex: “more vs. same,” “less,” “don’t know” | |
| 6-month follow-up | 0.33 (0.29–0.38) |
| 12-month follow-up | 0.45 (0.40–0.51) |
| 18-month follow-up | 0.59 (0.52–0.68) |
| 24-month follow-up | 0.68 (0.59–0.79) |
| How protected do you feel against sexual diseases: “more vs. same,” “less,” “don’t know” | |
| 6-month follow-up | 2.93 (2.57–3.34) |
| 12-month follow-up | 3.21 (2.80–3.67) |
| 18-month follow-up | 3.50 (3.01–4.07) |
| 24-month follow-up | 5.03 (4.14–6.12) |
| Sexual partners’ reaction to circumcision (among those whose sex partners were aware of their circumcision): “pleased vs. neutral,” “displeased,” or “don’t know” | |
| 6-month follow-up | 1.36 (1.20–1.55) |
| 12-month follow-up | 1.43 (1.26–1.62) |
| 18-month follow-up | 1.85 (1.62–2.13) |
| 24-month follow-up | 1.94 (1.67–2.26) |
| Ease of using a condom (among those who had used a condom since circumcision): “easier to use a condom” vs. “not as easy” or “same as before” | |
| 6-month follow-up | 2.34 (1.96–2.79) |
| 12-month follow-up | 2.53 (2.14–2.97) |
| 18-month follow-up | 2.99 (2.52–3.54) |
| 24-month follow-up | 3.31 (2.71–4.03) |
Results are based on descriptive data given in Table 4. “More” includes responses “much more” and “somewhat more.” “Less” includes responses “much less” and “somewhat less.” “Pleased” includes responses “very pleased” and “somewhat pleased.” “Displeased” includes responses “somewhat displeased” and “very displeased.” For each characteristic, the log odds of exhibiting the characteristic are modeled as a linear function of visit using the generalized estimating equations extension of generalized linear models. Predicted odds of exhibiting the characteristic at the follow-up visits and the 95% confidence intervals (CIs) are reported from each model. Trends with time were assessed through orthogonal polynomials: P < 0.001 for the linear component on each item, no higher-order trend was found.
We examined the possibility that increased penile sensitivity might be related to premature ejaculation, by conducting an analysis relating penile sensitivity to premature ejaculation status and time (GEE, binomial distribution, and log link). Premature ejaculation was not associated with penile sensitivity over time (OR = 0.83, 95% CI 0.59–1.17; P = 0.293). Additionally, we examined text comments provided by the few men reporting dissatisfaction with circumcision. One dissatisfied man reported reduced sensation, and one reported difficulty maintaining erection. Other reasons for dissatisfaction were related to the circumcision procedure itself (e.g., pain, itching, and “marks” at site). The one remaining man did not report a reason for dissatisfaction.
Discussion
Adult male circumcision was not associated with sexual dysfunction in this study. We found no significant difference between circumcised and uncircumcised men with respect to the frequency of erectile dysfunction, inability to ejaculate, pain during intercourse, lack of pleasure with inter-course, or these dysfunctions combined. On careful clinical evaluation over 2 years of follow-up, the circumcised men did not have evidence of penile deformities or long-term surgical complications. More than 99% were “satisfied” with their circumcisions. Only six men reported they were “dissatisfied” at the 6-month follow-up; each of these men reported being satisfied at either the 12-month or the 18-month visit, with no further dissatisfaction. Our findings support and substantially extend findings from another randomized trial of adult male circumcision that also found no significant difference in sexual function between circumcised men and uncircumcised controls [36]. These critical findings are reassuring in view of current efforts to promote male circumcision to prevent HIV infections in some countries, particularly in eastern and southern Africa [40]. We hope that these data can be used to inform public health recommendations for male circumcisions in other settings. In contrast to many other HIV prevention measures evaluated in clinical trials, male circumcision has proven to be effective, with approximately 60% reduction in HIV incidence among circumcised men.
Overall, 24.7% of the healthy 18- to 24-year-old men in our study reported at least one sexual dysfunction at baseline. Few studies have examined sexual dysfunction in young men. The items we used to assess sexual dysfunction are similar to the United States (NHSLS) [41], British National Survey of Sexual Attitudes and Lifestyles (NATSAL) [42], and Global Study of Sexual Attitudes and Behaviors [43,44]. Among 18- to 29-year-olds in the NHSLS [41], inability to reach orgasm was 7% (vs. 4.4% at baseline in our study), and erectile dysfunction was 7% (vs. 7% at baseline in our study). The NHSLS found a 30% prevalence of premature ejaculation in this age group [41], almost double our 18% prevalence at baseline. The multinational Premature Ejaculation Prevalence and Attitudes survey also found an 18% prevalence of premature ejaculation among 18- to 24-year-olds [45]. Although not age-stratified, excluding the question on lack of interest in sex, the NATSAL prevalence of any sexual dysfunction was 24% [43], comparable with our 25% rate. In contrast to these reports, a randomized trial of male circumcision among men aged 15–49 in Uganda found that self-reported sexual dysfunctions were infrequent at enrollment, as assessed using four items: “difficulty to achieve and maintain an erection” reported by 1.3%, “difficulty with vaginal penetration” reported by 1.8%, “difficulty with ejaculation” reported by 0.6%, and “pain during or after intercourse” reported by 1.2% [36]. Thus, the rate of sexual dysfunction in our study is generally comparable with the rates in young men surveyed in the United States [41], Britain [42], and most other countries [43,44].
Besides documenting that circumcision had no significant adverse effect on male sexual function, our data suggest potential changes in sexual pleasure for some circumcised men. The circumcised men reported increased penile sensitivity and enhanced ease of reaching orgasm, subjective findings that may be considered to be either a potential benefit or an adverse effect by individual men. The circumcised men had progressively higher rates of sexual satisfaction over time, as well as a lower rate of balanitis. Reduced rates of reported sexual dysfunction in both the circumcised and control men over the course of the study may have a number of different interpretations, including regression to the mean, increased familiarity with the study questions, or another effect of repeated assessment. Alternatively, it may be that as these young men aged and became sexually more experienced, their sexual difficulties lessened, or they became better able to deal with them. Furthermore, they received regular counseling at frequent intervals from the study counselors. Counseling was mainly directed at HIV risk reduction, but general psychological counseling and support was provided, which may have had some impact in terms of handling sexual dysfunction, although no specific treatment for sexual dysfunction was provided. Most importantly, the reduction in sexual dysfunction was observed in both study arms. Having an uncircumcised control group allowed for the observation of such unanticipated factors, in contrast to other studies that were limited to evaluating adult men before and after circumcision [27,30,31].
Over time, a large and increasing proportion of circumcised men reported having sex more frequently compared to before they were circumcised. This could be due to a perceived reduction in the risk of HIV acquisition (i.e., engaging in risk compensation). However, increased frequency of sexual activity may not necessarily reflect increased risk of HIV acquisition, if it is associated with having more sex with a regular partner or more sex with a condom. In any case, no difference was observed between the circumcised and uncircumcised men with regard to risky sexual practices (including unprotected sexual intercourse, recent sex with a casual sex partner, and inconsistent condom use), and there was a significant decrease in these behaviors in the circumcision group from before circumcision to after [2]. Additionally, the circumcised men reported that condom use was easier after circumcision, and the proportion reporting this increased over time. Continued HIV/STI evaluation and counseling in HIV/STI risk reduction remain critical as male circumcision is introduced as an HIV prevention intervention.
This study has several limitations. We did not have direct observation of sexual function, partner reports, or physiologic or laboratory indicators of sexual dysfunction. While we assessed sexual dysfunction using questions similar to those used in other large population-based surveys [41], we did not use validated instruments, such as the International Index of Erectile Function [46–48] or a recently validated sexual quality of life questionnaire for use in men with premature ejaculation or erectile dysfunction [49]. To translate and validate these instruments would have entailed substantial linguistic and cultural complexities. Although self-reported symptoms of sexual dysfunction differ from clinical diagnosis, self-report of erectile dysfunction correlates strongly (0.80) with urologic examination results [50]. The prevalence of sexual dysfunction is subject to the definition and period of recall used [42,48]. Lack of validated instruments may prove especially difficult in assessing items such as premature ejaculation, increased sensitivity, and the enhanced ease of reaching orgasm reported in our study; the latter might be another way of describing undesired premature ejaculation. However, premature ejaculation was not associated with increased penile sensitivity (P = 0.293) or with ease of reaching orgasm (P = 0.588). We did not have measures of diabetes, vascular disease, stress, and mental or emotional health. However, the young age of our population and the active lifestyle of participants in this trial, as well as medical screening for conditions contraindicating surgery, make it likely that few cases of sexual dysfunction resulted from chronic illnesses or medication use. Stability of the male–female relationship may influence the incidence of sexual dysfunction. Although 94.6% of participants in the circumcision group and 94.4% of participants in the control group were not married or living with a female partner, we did not have any other measure of relationship stability. We did not evaluate homosexual activities in our population, which might be influenced by circumcision status [51]. Finally, men who were excluded from randomization because of medical indications for circumcision or genital abnormalities might have had higher rates of sexual dysfunction than the men enrolled in our trial.
Advantages of this study include the large number of men randomly assigned to circumcision evaluated against a control group prospectively; use of thorough medical histories and physical exams; use of global questions to assess multiple aspects of sexual function; and extensive data on sexual behavioral risks and STI diagnoses.
In summary, the circumcised men did not experience an increased risk of sexual dysfunction when compared with the uncircumcised control men. Among the circumcised men, penile sensitivity and ability to reach orgasm increased. The similar rates of sexual dysfunction between the circumcised and uncircumcised men suggest that integration of male circumcision into programs to reduce HIV transmission will not have adverse effects on male sexual function.
Acknowledgments
Foremost, we thank the young men of Kisumu who volunteered to participate in this study. We also thank Richard Muga and Allan Ronald for their helpful advice and constant support; George Magoha and James Otieno for their surgical expertise and support; Ian Maclean for laboratory organization and advice; Carolyn Williams and Melanie Bacon for advice and medical oversight; and the staff of the Universities of Nairobi, Illinois, and Manitoba (UNIM) Project. This research was supported by grant number AI50440 from the Division of AIDS, National Institute of Allergies and Infectious Disease of the United States National Institutes of Health, and by grant number HCT 44180 from the Canadian Institutes of Health Research (CIHR). S. Moses was supported by a CIHR Investigator Award. The sponsors reviewed and commented on the study design, and monitored the study management and analyses. The sponsors had no role in the final analyses, interpretation of the data, or in the preparation, review, or approval of the manuscript. C. Parker and S. Mehta “had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.”
The project described was supported by Award Number U01AI050440 from the National Institute Of Allergy And Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Allergy And Infectious Diseases or the National Institutes of Health.
Footnotes
Presented in part at the XVII International AIDS Conference, August 3–8, 2008, Mexico City, Mexico.
Conflict of Interest: None declared.
Statement of Authorship
Category 1
-
Conception and DesignJohn N. Krieger; Jeckoniah O. Ndinya-Achola; Stephen Moses; Robert C. Bailey
-
Acquisition of DataJohn N. Krieger; Robert C. Bailey; Kawango Agot; Corette Parker; Stephen Moses
-
Analysis and Interpretation of DataJohn N. Krieger; Supriya D. Mehta; Corette Parker
Category 2
-
Drafting the ArticleJohn N. Krieger; Supriya D. Mehta
-
Revising It for Intellectual ContentJohn N. Krieger; Supriya D. Mehta; Robert C. Bailey; Kawango Agot; Jeckoniah O. Ndinya-Achola; Corette Parker; Stephen Moses
Category 3
-
Final Approval of the Completed ArticleJohn N. Krieger; Supriya D. Mehta; Robert C. Bailey; Kawango Agot; Jeckoniah O. Ndinya-Achola; Corette Parker; Stephen Moses
References
- 1.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: The ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, Williams CF, Campbell RT, Ndinya-Achola JO. Male circumcision for HIV prevention in young men in Kisumu, Kenya: A randomised controlled trial. Lancet. 2007;369:643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, Kiwanuka N, Moulton LH, Chaudhary MA, Chen MZ, Sewankambo NK, Wabwire-Mangen F, Bacon MC, Williams CF, Opendi P, Reynolds SJ, Laeyendecker O, Quinn TC, Wawer MJ. Male circumcision for HIV prevention in men in Rakai, Uganda: A randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.Bailey RC, Plummer FA, Moses S. Male circumcision and HIV prevention: Current knowledge and future research directions. Lancet Infect Dis. 2001;1:223–31. doi: 10.1016/S1473-3099(01)00117-7. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization and the Joint United Nations Programme on HIV/AIDS [press release] WHO and UNAIDS announce recommendations from expert consultation on male circumcision for HIV prevention. Mar 28, 2007. [Google Scholar]
- 6.Zorc JJ, Levine DA, Platt SL, Dayan PS, Macias CG, Krief W, Schor J, Bank D, Shaw KN, Kupper-mann N. Clinical and demographic factors associated with urinary tract infection in young febrile infants. Pediatrics. 2005;116:644–8. doi: 10.1542/peds.2004-1825. [DOI] [PubMed] [Google Scholar]
- 7.Singh-Grewal D, Macdessi J, Craig J. Circumcision for the prevention of urinary tract infection in boys: A systematic review of randomised trials and observational studies. Arch Dis Child. 2005;90:853–8. doi: 10.1136/adc.2004.049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig JC, Knight JF, Sureshkumar P, Mantz E, Roy LP. Effect of circumcision on incidence of urinary tract infection in preschool boys. J Pediatr. 1996;128:23–7. doi: 10.1016/s0022-3476(96)70423-7. [DOI] [PubMed] [Google Scholar]
- 9.Nasio JM, Nagelkerke NJ, Mwatha A, Moses S, Ndinya-Achola JO, Plummer FA. Genital ulcer disease among STD clinic attenders in Nairobi: Association with HIV-1 and circumcision status. Int J STD AIDS. 1996;7:410–4. doi: 10.1258/0956462961918374. [DOI] [PubMed] [Google Scholar]
- 10.Lavreys L, Rakwar JP, Thompson ML, Jackson DJ, Mandaliya K, Chohan BH, Bwayo JJ, Ndinya-Achola JO, Kreiss JK. Effect of circumcision on incidence of human immunodeficiency virus type 1 and other sexually transmitted diseases: A prospective cohort study of trucking company employees in Kenya. J Infect Dis. 1999;180:330–6. doi: 10.1086/314884. [DOI] [PubMed] [Google Scholar]
- 11.Diseker RA, 3rd, Peterman TA, Kamb ML, Kent C, Zenilman JM, Douglas JM, Jr, Rhodes F, Iatesta M. Circumcision and STD in the United States: Cross sectional and cohort analyses. Sex Transm Infect. 2000;76:474–9. doi: 10.1136/sti.76.6.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherpes TL, Meyn LA, Krohn MA, Hillier SL. Risk factors for infection with herpes simplex virus type 2: Role of smoking, douching, uncircumcised males, and vaginal flora. Sex Transm Dis. 2003;30:405–10. doi: 10.1097/00007435-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Castellsague X, Bosch FX, Munoz N, Meijer CJ, Shah KV, de Sanjose S, Eluf-Neto J, Ngelangel CA, Chichareon S, Smith JS, Herrero R, Moreno V, Franceschi S International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–12. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 14.Moses S, Bailey RC, Ronald AR. Male circumcision: Assessment of health benefits and risks. Sex Transm Infect. 1998;74:368–73. doi: 10.1136/sti.74.5.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss HA, Thomas SL, Munabi SK, Hayes RJ. Male circumcision and risk of syphilis, chancroid, and genital herpes: A systematic review and meta-analysis. Sex Transm Infect. 2006;82:101–9. doi: 10.1136/sti.2005.017442. discussion 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez BY, Wilkens LR, Zhu X, McDuffie K, Thompson P, Shvetsov YB, Ning L, Goodman MT. Circumcision and human papillomavirus infection in men: A site-specific comparison. J Infect Dis. 2008;197:787–94. doi: 10.1086/528379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoen EJ, Colby CJ, To TT. Cost analysis of neonatal circumcision in a large health maintenance organization. J Urol. 2006;175:1111–5. doi: 10.1016/S0022-5347(05)00399-X. [DOI] [PubMed] [Google Scholar]
- 18.Mallon E, Hawkins D, Dinneen M, Francics N, Fearfield L, Newson R, Bunker C. Circumcision and genital dermatoses. Arch Dermatol. 2000;136:350–4. doi: 10.1001/archderm.136.3.350. [DOI] [PubMed] [Google Scholar]
- 19.Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, Carter JJ, Porter PL, Galloway DA, McDougall JK, Krieger JN. Penile cancer: Importance of circumcision, human papillo-mavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606–16. doi: 10.1002/ijc.21009. [DOI] [PubMed] [Google Scholar]
- 20.Castellsague X, Peeling RW, Franceschi S, de Sanjose S, Smith JS, Albero G, Diaz M, Herrero R, Munoz N, Bosch FX. Chlamydia trachomatis infection in female partners of circumcised and uncircumcised adult men. Am J Epidemiol. 2005;162:907–16. doi: 10.1093/aje/kwi284. [DOI] [PubMed] [Google Scholar]
- 21.Warner E, Strashin E. Benefits and risks of circumcision. Can Med Assoc. 1981;125:967–76. [PMC free article] [PubMed] [Google Scholar]
- 22.Remondino P. History of circumcision: From the earliest times to the present: Moral and physical reasons for its performance, with a history of eunuchism, hermaphrodism, etc and of the different operations practiced upon the prepuce. Philadelphia, PA: FA Davis Co; 1891. (reprinted 1990) [Google Scholar]
- 23.Hutchinson J. On the influence of circumcision in preventing syphilis. Med Times Gaz. 1855;II:542–3. [Google Scholar]
- 24.Immerman RS, Mackey WC. A biocultural analysis of circumcision. Soc Biol. 1997;44:265–75. doi: 10.1080/19485565.1997.9988953. [DOI] [PubMed] [Google Scholar]
- 25.Immerman RS, Mackey WC. A proposed relationship between circumcision and neural reorganization. J Genet Psychol. 1998;159:367–78. doi: 10.1080/00221329809596158. [DOI] [PubMed] [Google Scholar]
- 26.Boyle GJ, Bensley GA. Adverse sexual and psychological effects of male infant circumcision. Psychol Rep. 2001;88:1105–6. doi: 10.2466/pr0.2001.88.3c.1105. [DOI] [PubMed] [Google Scholar]
- 27.Fink KS, Carson CC, DeVellis RF. Adult circumcision outcomes study: Effect on erectile function, penile sensitivity, sexual activity and satisfaction. J Urol. 2002;167:2113–6. [PubMed] [Google Scholar]
- 28.Kim D, Pang MG. The effect of male circumcision on sexuality. BJU Int. 2007;99:619–22. doi: 10.1111/j.1464-410X.2006.06646.x. [DOI] [PubMed] [Google Scholar]
- 29.Sorrells ML, Snyder JL, Reiss MD, Eden C, Milos MF, Wilcox N, Van Howe RS. Fine-touch pressure thresholds in the adult penis. BJU Int. 2007;99:864–9. doi: 10.1111/j.1464-410X.2006.06685.x. [DOI] [PubMed] [Google Scholar]
- 30.Senkul T, Iser IC, Sen B, KarademIr K, Saracoglu F, Erden D. Circumcision in adults: Effect on sexual function. Urology. 2004;63:155–8. doi: 10.1016/j.urology.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Masood S, Patel HR, Himpson RC, Palmer JH, Mufti GR, Sheriff MK. Penile sensitivity and sexual satisfaction after circumcision: Are we informing men correctly? Urol Int. 2005;75:62–6. doi: 10.1159/000085930. [DOI] [PubMed] [Google Scholar]
- 32.Collins S, Upshaw J, Rutchik S, Ohannessian C, Ortenberg J, Albertsen P. Effects of circumcision on male sexual function: Debunking a myth? J Urol. 2002;167:2111–2. [PubMed] [Google Scholar]
- 33.Richters J, Smith AM, de Visser RO, Grulich AE, Rissel CE. Circumcision in Australia: Prevalence and effects on sexual health. Int J STD AIDS. 2006;17:547–54. doi: 10.1258/095646206778145730. [DOI] [PubMed] [Google Scholar]
- 34.Payne K, Thaler L, Kukkonen T, Carrier S, Binik Y. Sensation and sexual arousal in circumcised and uncircumcised men. J Sex Med. 2007;4:667–74. doi: 10.1111/j.1743-6109.2007.00471.x. [DOI] [PubMed] [Google Scholar]
- 35.Laumann EO, Masi CM, Zuckerman EW. Circumcision in the United States. Prevalence, prophylactic effects, and sexual practice. JAMA. 1997;277:1052–7. [PubMed] [Google Scholar]
- 36.Kigozi G, Watya S, Polis CB, Buwembo D, Kiggundu V, Wawer MJ, Serwadda D, Nalugoda F, Kiwanuka N, Bacon MC, Ssempijja V, Makumbi F, Gray RH. The effect of male circumcision on sexual satisfaction and function, results from a randomized trial of male circumcision for human immunodeficiency virus prevention, Rakai, Uganda. BJU Int. 2008;101:65–70. doi: 10.1111/j.1464-410X.2007.07369.x. [DOI] [PubMed] [Google Scholar]
- 37.Krieger JN, Bailey RC, Opeya JC, Ayieko BO, Opiyo FA, Omondi D, Agot K, Parker C, Ndinya-Achola JO, Moses S. Adult male circumcision outcomes: Experience in a developing country setting. Urol Int. 2007;78:235–40. doi: 10.1159/000099344. [DOI] [PubMed] [Google Scholar]
- 38.Krieger JN, Bailey RC, Opeya J, Ayieko B, Opiyo F, Agot K, Parker C, Ndinya-Achola JO, Magoha GA, Moses S. Adult male circumcision: Results of a standardized procedure in Kisumu District, Kenya. BJU Int. 2005;96:1109–13. doi: 10.1111/j.1464-410X.2005.05810.x. [DOI] [PubMed] [Google Scholar]
- 39.Mehta SD, Moses S, Agot K, Agingu W, Parker C, Ndinya-Achola JO, Bailey RC. Herpes simplex virus type 2 infection among young uncircumcised men in Kisumu, Kenya. Sex Transm Infect. 2008;84:42–8. doi: 10.1136/sti.2007.025718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vardi Y, Sadeghi-Nejad H, Pollack S, Aisuodionoe-Shadrach OI, Sharlip ID. Male circumcision and HIV prevention. J Sex Med. 2007;4:838–43. doi: 10.1111/j.1743-6109.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 41.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA. 1999;281:537–44. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 42.Mercer CH, Fenton KA, Johnson AM, Copas AJ, Macdowall W, Erens B, Wellings K. Who reports sexual function problems? Empirical evidence from Britain’s 2000 National Survey of Sexual Attitudes and Lifestyles Sex. Transm Infect. 2005;81:394–9. doi: 10.1136/sti.2005.015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicolosi A, Laumann EO, Glasser DB, Moreira ED, Jr, Paik A, Gingell C. Sexual behavior and sexual dysfunctions after age 40: The Global Study of Sexual Attitudes and Behaviors. Urology. 2004;64:991–7. doi: 10.1016/j.urology.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 44.Laumann EO, Nicolosi A, Glasser DB, Paik A, Gingell C, Moreira E, Wang T. Sexual problems among women and men aged 40–80 y: Prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res. 2005;17:39–57. doi: 10.1038/sj.ijir.3901250. [DOI] [PubMed] [Google Scholar]
- 45.Porst H, Montorsi F, Rosen RC, Gaynor L, Grupe S, Alexander J. The Premature Ejaculation Prevalence and Attitudes (PEPA) survey: Prevalence, comorbidities, and professional help-seeking. Eur Urol. 2007;51:816–23. doi: 10.1016/j.eururo.2006.07.004. discussion 824. [DOI] [PubMed] [Google Scholar]
- 46.Yang CC, Porter MP, Penson DF. Comparison of the International Index of Erectile Function erectile domain scores and nocturnal penile tumescence and rigidity measurements: Does one predict the other? BJU Int. 2006;98:105–9. doi: 10.1111/j.1464-410X.2006.06246.x. discussion 109. [DOI] [PubMed] [Google Scholar]
- 47.Okulate G, Olayinka O, Dogunro AS. Erectile dysfunction: Prevalence and relationship to depression, alcohol abuse and panic disorder. Gen Hosp Psychiatry. 2003;25:209–13. doi: 10.1016/s0163-8343(03)00015-x. [DOI] [PubMed] [Google Scholar]
- 48.de Boer BJ, Bots ML, Lycklama a Nijeholt AA, Moors JP, Pieters HM, Verheij TJ. Impact of various questionnaires on the prevalence of erectile dysfunction. The ENIGMA-study. Int J Impot Res. 2004;16:214–9. doi: 10.1038/sj.ijir.3901053. [DOI] [PubMed] [Google Scholar]
- 49.Abraham L, Symonds T, Morris MF. Psychometric validation of a sexual quality of life questionnaire for use in men with premature ejaculation or erectile dysfunction. J Sex Med. 2008;5:595–601. doi: 10.1111/j.1743-6109.2007.00749.x. [DOI] [PubMed] [Google Scholar]
- 50.O’Donnell AB, Araujo AB, Goldstein I, McKinlay JB. The validity of a single-question self-report of erectile dysfunction. Results from the Massachusetts Male Aging Study. J Gen Intern Med. 2005;20:515–9. doi: 10.1111/j.1525-1497.2005.0076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao L, Templeton DJ, Crawford J, Imrie J, Prestage GP, Grulich AE, Donovan B, Kaldor JM, Kippax SC. Does circumcision make a difference to the sexual experience of gay men? Findings from the Health in Men (HIM) Cohort. J Sex Med. 2008 doi: 10.1111/j.1743-6109.2008.00845.x. [DOI] [PubMed] [Google Scholar]