Introduction
The abuse of crystal methamphetamine (CM) has reached epidemic proportions in the US, with widespread health consequences for a wide segment of the population. Of US residents older than 12 years, almost 5% (12 million) have reported using CM at least once. Between 1993 and 2003, the rate of admissions for treatment for CM abuse in the US increased from 13 to 56 admissions per 100,000 individuals.1 CM, a stimulant street drug, is closely associated with party use in an attempt to increase the sociability of party participants. Its use is an independent risk factor for both acquisition of and propagation of HIV infection.2–4
To deal effectively with the effects of CM on patients, clinicians need to understand its use and its role in HIV risk, its neurobiologic effects, and some of the risk-intervention methods currently used with patients who abuse it.
Crystal Methamphet-amine and Crack Cocaine
CM, known by a number of street names, including meth, speed, ice, Tina, crystal, tweak, crank, and glass, is a methamphetamine powder that can be white, yellow, orange, pink, or brown. Color variations are a result both of different contaminants or additives included by the preparer and of the preparer's expertise. Ice and glass are methamphetamine of a higher purity (concentration). It is generally translucent to white, sometimes with a green, blue, or pink tinge. Methamphetamines are derived from the parent compound ephedrine or pseudoephedrine hydrochloride and pseudoephedrine sulfate. Methamphetamines of abuse potential are purchased on the street in the form of powder and then used by inhalation and smoking, by snorting into the nostril, or by solubilizing and injecting intravenously and even rectally.1
Neurobiologic Effects
The brains of people addicted to methamphetamine are different from those of nonaddicts. The pleasure center of the brain is the nucleus accumbens, where the active neurotransmitter is dopamine. Both crack cocaine and methamphetamine prevent the reuptake of dopamine, which allows it to collect and thus prolongs and increases its effects. Although crack cocaine works only at the synapse level, methamphetamine can also penetrate the neuron, and thus cause permanent cell damage.5
A wide variety of stimuli affect dopamine levels. Natural rewards such as food and sex elevate dopamine output by 150% to 300% above basal output.4 Stimulant drugs, however, are more efficient than natural rewards at increasing the release of dopamine. Methamphetamine increases dopamine release to >1000% above basal levels within the first hour of taking the drug, with levels returning to basal after three hours. Similar increases are seen with cocaine, nicotine, and ethanol, of >300%, >200%, and approximately 200%, respectively.6,7 Brain-imaging studies in both animals and humans show profound, long-lasting alterations of brain chemistry after relatively brief exposures to CM.8
Physical and Psychological Effects
The impact of altered brain chemistry is illustrated by a variety of cognitive impairments. One study focused on word and picture recognition and recall at baseline when study subjects stopped taking CM and thereafter.9 At three months and six months after stopping use of the drug, word recall and recognition continued to worsen, whereas picture recall and recognition began to improve slightly. This finding may have implications for communication strategies when interacting with current or recent methamphetamine users: A more pictorial approach to communication may be more effective.
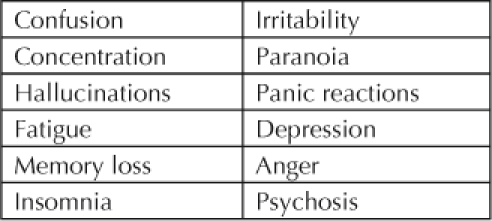
The acute physical effects of CM mimic those of other stimulant drugs (Table 1). Heart rate increases, as do blood pressure, pupil size, respiratory activity, sensory acuity, and energy levels. Reaction time, the need for sleep, and appetite decrease. Acute psychological effects are increased confidence and alertness, elevations of mood and sex drive, and increased energy level and talkativeness and a decreased sense of boredom, loneliness, and timidity, all effects that make the drug desirable. Chronically, the drug causes tremor, weakness, and dry mouth (Table 2). The anorexia and diarrhea it causes often lead to weight loss. Those who snort and inhale the drug are at increased risk for respiratory infections. Chronic psychologic effects include confusion, decreased concentration, irritability, and panic reactions (Table 3).
Table 1.
Acute physical effects of methamphetamine
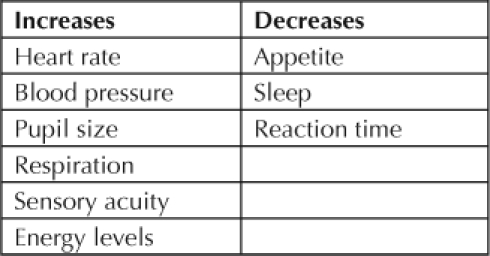
Table 2.
Chronic physical effects of methamphetamine
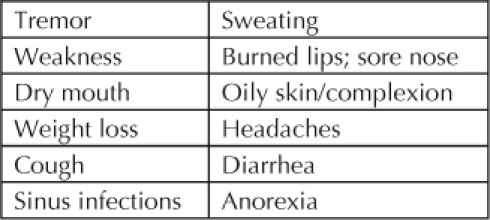
Table 3.
Chronic psychological effects of methamphetamine
Patients taking the drug often have the sensation that they have bugs under their skin, leading to picking. This tendency contributes to ongoing and pronounced skin and soft tissue infections (Figure 1). Users engage in bruxism because of the hyperactivity produced by the drug. This and the decreased saliva production and toxic effects of the drug itself cause pronounced degradation of the teeth and “crystal meth mouth syndrome.”
Figure 1.

(upper) Example of skin and soft tissue changes after only three months of CM use. (lower) Example of appearance changes after several (2.5) years of CM use. www.facesofmeth.us [Web page on the Internet]. Portland (OR): Multnomah County Sheriff's Office; 2005 [cited 2007 Sep 24]. Available from: www.facesofmeth.us. Reprinted with permission from the Multnomah County Sheriff's Office.
Prevention Strategies and Drug Treatment
It is evident that there is a severe need for preventing CM use. Use of the drug is a risk factor for HIV acquisition, especially for men who have sex with men.1–3 In this population, the general probability of HIV positivity is 8%. The prevalence of HIV among recreational users of the drug is 26%, whereas it is 41% among untreated chronic users. The likelihood of HIV infection is 62% in individuals seeking detoxification in outpatient psychosocial clinics. Among those requiring residential treatment for their addiction, HIV prevalence is 90%. The longer someone is using CM, as seen by these indicators, the greater the likelihood of acquiring HIV.
Prevention of HIV in this population has been approached in a number of different ways. One strategy is postexposure prophylaxis with antiretroviral agents, which has been shown to be effective if done within 72 hours of exposure and is considered to be the standard of care, with numerous evidence-based guidelines available.10
Another approach centers on treatment of individuals known to be HIV positive. Those who are being successfully treated for HIV and have a low viral load are much less likely to transmit the virus to others. Also, when patients are in treatment for HIV, they are much more likely to receive appropriate risk-reduction counseling regarding both drug use and sex in addition to counseling about their medications.
Early work in developing prevention strategies for both patients without HIV and those already infected with it revolves around risk counseling and patients' and clinicians' perceptions of risk. Patients expect that if they are at risk because of a certain behavior, their clinicians will ask about that behavior. Conversely, the significant barriers on the part of clinicians are their general comfort level with discussing particular risks and the expectation that patients will initiate discussion if they are at risk. It is not difficult to see that this dynamic is ineffective. A key point to remember is that patients will disclose risk to their clinicians if given the opportunity.
As part of the “Prevention for Positives” program, one novel intervention tool has been developed: the “video doctor.”11 The video doctor was part of the prevention strategy, called Positive Choices, in which patients spend 10 to 15 minutes of discussion time with a computer-generated physician and receive risk-reduction counseling (unpublished data from Barbara Gerbert, PhD, et al).a Each discussion is oriented to patients' risk profiles as disclosed to the video doctor. The program also assesses patients' readiness to change and their sex. The system then generates a “cueing sheet” that is given to clinicians prior to patients' visits.
The Gerbert trial randomized 476 HIV-positive patients (376 men [79%] and 100 women [21%]) to either receive standard counseling by clinicians or spend time with the video doctor before seeing their clinician. Study retention was good, with 371 (78%) completing three months of follow-up care and 395 (83%) completing six months. Of the 476, 243 (51%) had the HIV risk factor of being men who had sex with men, 100 (21%) had other sexual risk factors, and 76 (16%) had the risk factor of intravenous drug use.
Clinical variables assessed in the study included antiretroviral use for treatment of HIV, which was at the level of 67% and 16%, respectively, for current and former users. Undetectable viral loads were found in 45%. Twenty-one percent had viral loads <10,000 copies/mL, 11% had between 10,001 and 50,000 copies/mL, and 6% had >50,000 copies/mL. Sixty-one percent reported having unprotected sex (45% with their main partners and 35% with casual partners). Forty-three percent reported some drug use, with crack cocaine and methamphetamine being most common at 24% and 15%, respectively. Thirty-nine percent reported heavy drinking of alcohol.
The Positive Choices intervention was found to significantly reduce high-risk behaviors. At three and six months, respectively, overall drug use decreased from 85% to 66% and from 88% to 59% of study subjects, methamphetamine use decreased from 83% to 58% and from 73% to 48%, heavy drinking decreased from 78% to 53% from 51% to 43%, and unprotected sex with casual partners decreased from 87% to 69% and from 94% to 81%.
Conclusions
The recreational use of methamphetamine is highly prevalent among populations at risk for acquiring HIV infection, especially men who have sex with men. Although the health consequences, in addition to HIV infection and its subsequent morbidity, are serious and affect a great many people, there are significant barriers on the part of both clinicians and patients to assessing risk and providing appropriate risk-reduction counseling. Appropriate and novel screening tools for assessing risk help overcome these barriers and contribute to substantial reductions in high-risk behavior.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Original presentation by Michael Allerton, MS, at the 2007 Fifth National Kaiser Permanente HIV/AIDS, STD, and Hepatitis Conference in Napa Valley, CA, April 2007.


Acknowledgments
The author would like to thank Barbara Gerbert, PhD, and her staff, University of California, San Francisco Division of Behavioral Sciences for their generous assistance in providing prepublication outcome data from the Positive Choices intervention study.
Katharine O'Moore-Klopf of KOK Edit provided editorial assistance.
Footnotes
a The Positive Choices trial, Barbara Gerbert, PhD, University of California, San Francisco Division of Behavioral Sciences, principal investigator.
References
- The NSDUH report: methamphetamine use, abuse, and dependence: 2002, 2003, and 2004 [monograph on the Internet] Rockville (MD): Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 2005 Sep 16. [cited 2007 Oct 2]. Available from: http://oas.samhsa.gov/2k5/meth/meth.pdf. [Google Scholar]
- Buchacz K, McFarland W, Kellogg TA, et al. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS. 2005 Sep 2;19(13):1423–4. doi: 10.1097/01.aids.0000180794.27896.fb. [DOI] [PubMed] [Google Scholar]
- Molitor F, Truax SR, Ruiz JD, Sun RK. Association of methamphetamine use during sex with risky sexual behaviors and HIV infection among non-injection drug users. West J Med. 1998 Feb;168(2):93–7. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control & Prevention. Methamphetamine use and HIV risk behaviors among heterosexual men—preliminary results from five northern California counties, December 2002–November 2003. MMWR Morb Mortal Wkly Rep. 2006 Mar 17;55(10):273–7. [PubMed] [Google Scholar]
- Jaffe JA, Ling W, Rawson RA. Amphetamines. In: Sadock BJ, Sadock VA, editors. Kaplan & Sadock's comprehensive textbook of psychiatry. Baltimore, MD: Lippincott Williams & Wilkins; 2002. pp. 458–84. p. [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after d-amphetamine-induced behavioral sensitization. J Neurosci. 1999 Jan 1;19(1):456–63. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara GD, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations on the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, et al. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004 Feb;161(2):242–8. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000 Summer;9(3):222–31. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR. 2005;54(No. RR-2):1–20. [PubMed] [Google Scholar]
- Gerbert B, Johnston K, Bleecker T, Allerton M. Interactive multimedia sexual risk assessment: using a video doctor to screen patients. MD Comput. 1996 Sep–Oct;13(5):416–22. [PubMed] [Google Scholar]


