Abstract
Purpose
Case series have shown a Fournier’s gangrene mortality rate of 20% to 40% with an incidence of as high as 88% in some studies. Because to our knowledge there are no population based data, we used a national database to investigate the epidemiology of Fournier’s gangrene.
Materials and Methods
We used the State Inpatient Databases, the largest hospital based database available in the United States, which includes 100% of hospital discharges from participating states. Inpatients diagnosed with Fournier’s gangrene (ICD-9 CM 608.83) who underwent genital/perineal débridement or died in the hospital were identified from 13 participating states in 2001 and from 21 in 2004. Population based incidence, regional trends and case fatality rates were estimated.
Results
We identified 1,641 males and 39 females with Fournier’s gangrene. Cases represented less than 0.02% of hospital admissions. The overall incidence was 1.6/100,000 males, which peaked in males who were 50 to 79 years old (3.3/100,000) with the highest rate in the South (1.9/100,000). The overall case fatality rate was 7.5%. Patients with Fournier’s gangrene were rarely treated at hospitals (mean ± SD 0.6 ± 1.2 per year, median 0, range 0 to 23). Overall 0 to 4 and 5 or greater cases were treated at 66%, 17%, 10%, 4%, 1% and 1% of hospitals, respectively.
Conclusions
Patients with Fournier’s gangrene are rarely treated at most hospitals. The population based mortality rate of 7.5% was substantially lower than that reported in case series from tertiary care centers.
Keywords: urology, male, female, gangrene, mortality
Fournier’s gangrene is a urological emergency characterized by progressive necrotizing infection of the external genitalia or perineum.1 Most studies indicate a mortality rate of 20% to 40% with some studies showing a fatality rate of as high as 88% (table 1).2 These data are from tertiary referral centers with the largest series including only 80 patients.3
Table 1.
Contemporary Fournier’s gangrene case series
| References | % Mortality | No. Cases |
|---|---|---|
| Present series | 7.5 | 1,641 |
| Corcoran et al20 | 10 | 68 |
| Basoglu et al14 | 8.8 | 45 |
| Carvalho et al3 | 16.3 | 80 |
| Djè K: Afr J Urol 2006; 12: 44 | 17.9 | 78 |
| Ayan et al4 | 21.9 | 41 |
| Edino ST: Afr J Urol 2005; 11: 1 | 12.5 | 24 |
| Yeniyol et al19 | 24 | 25 |
| Norton S: Am Surg 2002; 68: 709 | 9 | 33 |
| Daali M: Afr J Urol 2002; 8: 157 | 17 | 60 |
| Eke N: Int Surg 2000; 85: 77 | 9.5 | 21 |
| Eke16 | 16 | 1,726* |
| Yaghan RJ: Dis Colon Rectum 2000; 43: 1300 | 20 | 10 |
| Corman JM: BJU Int 1999; 84: 85 | 4 | 23 |
| Kouadio K: Med Trop 1998; 58: 245 | 27 | 30 |
| Brissiaud JC: Chirurgie 1998; 123: 387 | 34 | 44 |
| Pizzorno et al6 | 0 | 11 |
| Benchekroun A: J Urol (Paris) 1997; 103: 27 | 9 | 55 |
| Picramenos D: Prog Urol 1995; 5: 701 | 30 | 10 |
| Palmer LS: Br J Urol 1995; 76: 208 | 43 | 30 |
| Laor et al18 | 43 | 30 |
| Attah CA: Br J Urol 1992; 70: 78 | 0 | 13 |
| Baskin LE: J Urol 1990; 144: 984 | 21 | 29 |
| Clayton MD: Surg Gynecol Obstet 1990; 170: 49 | 18 | 57 |
| Wolach MD: Br J Urol 1989; 64: 310 | 20 | 10 |
| Fahal AH: Br J Urol 1988; 61: 451 | 25 | 9 |
| Enriquez J: Dis Colon Rectum 1987; 30: 33 | 25 | 28 |
| Spirnak JP: J Urol 1984; 131: 289 | 45 | 20 |
| Stone and Martin2 | 88 | 33 |
Review article.
The generalizability of these data is limited. Previous reports reflect differences in referral patterns, surgical management, clinical volumes and many other institutional differences. For example, reports diverge widely in recommendations for urinary and fecal diversion, hyperbaric oxygen use and early skin grafting.3–7 There are sparse data from community hospitals and to our knowledge no population based data on incidence, regional trends or case fatality rates.
To better understand epidemiology and outcomes in patients with Fournier’s gangrene we examined a large, population based database to determine patient characteristics, and the incidence of and hospital experience with Fournier’s gangrene. We hypothesized that previous case series from tertiary referral centers do not reflect the clinical spectrum and outcomes in the general population.
MATERIALS AND METHODS
Population
We used SID, an all payer inpatient database containing 14 million hospital stays each year. SID was established by the Healthcare Cost and Utilization Project, a partnership of federal and state governments, hospital associations and private data organizations. SID, which contains data obtained from the hospital discharge abstract, including data collected on 100% of discharges from all adult and pediatric civilian hospitals in participating states, can be used to produce population based estimates.8 SID is a national information resource of patient level health care data that represents the largest collection of hospital care data in the United States.
We analyzed data purchased from 13 select states for 2001 and from 21 states for 2004. For 2001 data were purchased from Colorado, Florida, Iowa, Massachusetts, Maryland, Maine, North Carolina, New Jersey, New York, Oregon, Utah, Washington and West Virginia. For 2004 data were additionally purchased from Arizona, Kentucky, Michigan, Nebraska, Nevada, Rhode Island, South Carolina, Vermont and Wisconsin. Data from Maine were not available for 2004.
Case Definition and Data Abstraction Strategy
Cases were selected using the ICD-9 CM9 code for Fournier’s gangrene (608.83). Due to the requirement for surgical management included male patients had a Fournier’s gangrene diagnosis as well as a débridement procedure of the external genitalia or perineum unless the patient died in the hospital. Surgical débridement procedures were defined using ICD-9 procedure codes for incisions, excisions or débridement in the anatomical areas of interest (48.8–48.82, 48.9, 49.0, 49.01, 49.02, 49.04, 49.39, 49.93, 54.0, 54.3, 61.0–61.99, 62.0–62.19, 62.2–62.42, 63.3, 63.4, 64.0, 64.2, 64.3, 64.92, 64.98, 71.0, 71.09, 71.22, 71.24, 71.29, 71.3, 71.5, 71.6–71.62, 71.8, 71.9, 83.0–83.09, 83.19, 83.21, 83.3–83.39, 83.4, 83.42, 83.44–83.49, 86.0, 86.04, 86.09, 86.22, 86.28, 86.3, 86.4, 86.9 and 86.99).
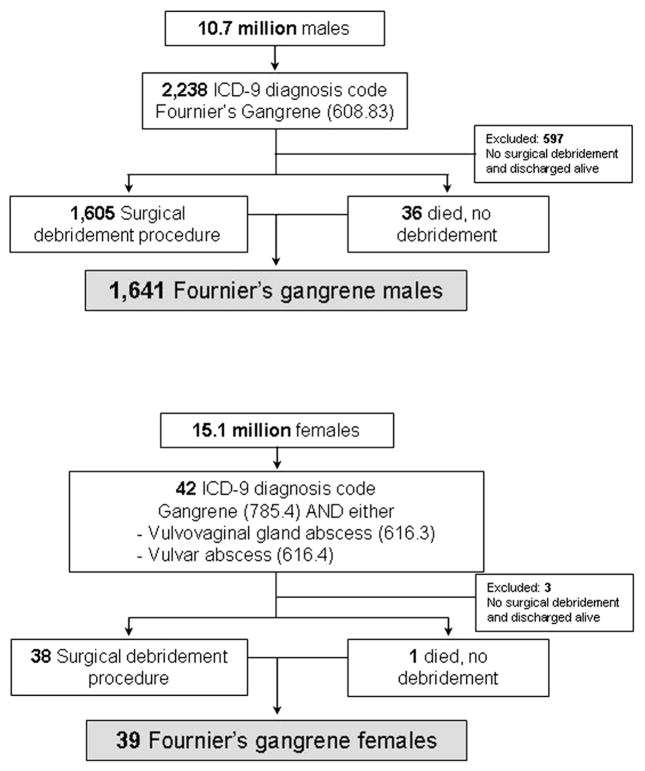
The ICD-9 code for Fournier’s gangrene (608.83) is found under the diseases of the male genital organs subheading. There is no Fournier’s gangrene diagnostic code for females. To identify female patients we searched for patients with diagnosis codes for gangrene (785.4) and vulvovaginal gland abscess (616.3) or vulvar abscess (616.4). Female patients were also required to have undergone a débridement procedure unless they died in the hospital. Due to the differential identification and the low yield of female patients with Fournier’s gangrene analysis of female patients was limited to demographic and descriptive factors (fig. 1).
Figure 1.
Enrollment approach identifying patients in SID with diagnosis and surgical débridement, leading to 1,641 males and 39 females with Fournier’s gangrene.
Comorbidities were abstracted from SID and the Charlson comorbidity index was calculated based on 17 weighted indicators of coexisting conditions.10 Individual comorbidities in patients with Fournier’s gangrene were compared to those in the more than 10 million male patients hospitalized in those states during the study period for any other reason using age adjusted logistic regression analysis.
Data Management and Analysis
Total hospital charges from SID include the sum of all supplied detailed charges. Charges do not include professional fees and noncovered charges because they are removed from the charges during data processing.
Population based incidence rates were calculated using United States Census Bureau data for the mid year male population in the 13 states included in 2001 and the 21 states included in 2004.11 Fournier’s gangrene incidence and mortality rates were determined for the 4 United States Census Bureau defined regions of the Northeast, South, Midwest and West.12 Trends in incidence were compared to 2005 Behavioral Risk Factor Surveillance System prevalence rates for the diagnoses of diabetes and obesity using linear regression analysis.13 To evaluate possible differences in patient characteristics or outcomes in transferred patients subgroup analysis was performed of the 110 (7%) admitted to the hospital via transfer.
When appropriate, chi-square analysis was performed for binary variables and Student’s t test allowing for unequal variance was performed for continuous variables. All p values are 2-tailed with significance considered at <0.05. Analysis was done using SAS®, version 9 and Stata®, version 10.
RESULTS
Patient and Hospital Demographics
Of the more than 10 million male hospital admissions in the 2001 and 2004 SID from select states we identified 1,641 male patients with Fournier’s gangrene (fig. 1). The overall case fatality rate in males was 7.5% (124 deaths per 1,641 cases). Males had a mean ± SD age of 50.9 ± 18.6 years, 51% were white and many had comorbidities (table 2). Compared to the other male patients in SID those with Fournier’s gangrene were more likely to have diabetes (OR 3.3, 95% CI 2.9–3.7) and obesity (OR 3.7, 95% CI 3.1–4.3) but they had similar rates of hypertension (OR 0.9, 95% CI 0.8–1.0) and tobacco and alcohol use (OR 1.0, 95% CI 0.8–1.2), and were less likely to use illicit drugs (OR 0.5, 95% CI 0.3–0.8) after adjustment for age.
Table 2.
Hospital and male patient characteristics
| Pt Demographics | Males with Fournier’s Gangrene* | Males in SID for Any Other Reason* | p Value |
|---|---|---|---|
| No. pts | 1,641 | 11.2 Million | |
| Age: | <0.0001 | ||
| No. younger than 40 (%) | 379 (23) | 3,606,816 (32) | |
| No. 40–49 (%) | 341 (21) | 1,286,656 (12) | |
| No. 50–59 (%) | 407 (25) | 1,481,863 (13) | |
| No. 60–69 (%) | 250 (15) | 1,584,606 (14) | |
| No. 70+ (%) | 264 (16) | 3,224,827 (29) | |
| Mean ± SD | 50.9 ± 18.6 | 48.5 ± 28.3 | |
| No. race/ethnicity (%) | <0.0001 | ||
| White | 836 (51) | 6,042,041 (54) | |
| Black | 307 (19) | 1,316,227 (12) | |
| Hispanic | 109 (7) | 801,713 (7) | |
| Other | 49 (3) | 477,827 (4) | |
| Missing | 340 (20) | 2,547,388 (23) | |
| Mean ± SD Charlson comorbidity index | 1.2 ± 1.4 | ||
| % Comorbidities: | |||
| Diabetes | 37 | 14 | <0.0001 |
| Obesity | 11 | 4 | <0.0001 |
| Hypertension | 31 | 31 | 0.64 |
| Alcohol abuse | 5 | 3 | <0.0001 |
| Tobacco smoker | 15 | 15 | 0.69 |
| Illicit drug abuse | 1.5 | 2.3 | 0.0007 |
| No. admission source (%): | <0.0001 | ||
| Primary presentation | 1,502 (91) | 10,547,415 (94) | |
| Transferred from elsewhere | 110 (7) | 451,481 (4) | |
| No. deaths (%) | 124 (7.5) | 298,313 (2.7) | <0.0001 |
Values may not total 100% due to missing data.
We identified only 39 women who met our case definition for Fournier’s gangrene. Females with Fournier’s gangrene had mean age, race, comorbidity prevalence, number of surgical débridement procedures and discharge needs that were similar to those in males. The females who were identified likely represented a more acutely ill group because they had double the requirement for mechanical ventilation and dialysis, longer mean and median length of stay, greater total hospital charges and a higher case fatality rate of 12.8% (5 of 39 cases) than males, although none of these factors attained statistical significance.
Admissions for Fournier’s gangrene were rare, representing less than 0.02% of admissions. At the 1,764 hospitals analyzed an average of 0.6 ± 1.2 Fournier’s gangrene cases per year (median 0, range 0 to 23) were treated. No Fournier’s gangrene cases were treated at 1,171 of the 1,764 evaluated hospitals (66%) during the 2 study years. Figure 2 shows the distribution of hospitals where patients with Fournier’s gangrene received care. Results were similar when 2001 and 2004 data were analyzed individually.
Figure 2.
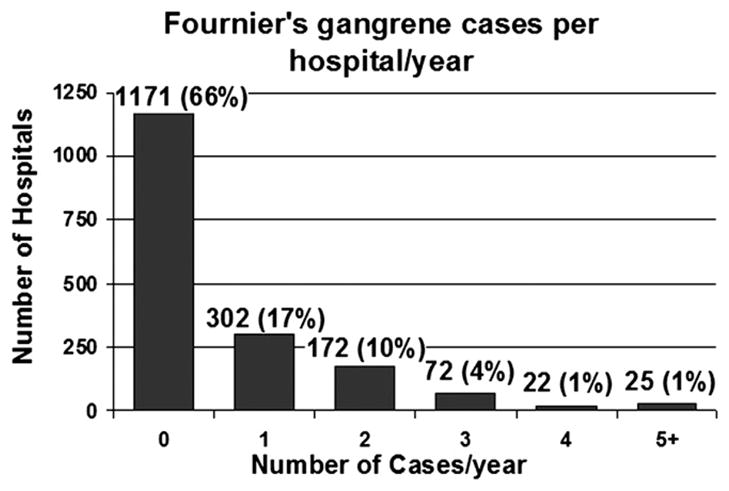
Number of Fournier’s gangrene cases treated at each hospital per year. At 66% of institutions no cases of Fournier’s gangrene were treated per year, while 5 or more per year were treated at only 1% of hospitals.
Population Based Epidemiology and Regional Trends
The overall incidence rate was 1.6 Fournier’s gangrene cases per 100,000 males per year (table 3). Fournier’s gangrene was rare in pediatric patients but the incidence increased with increasing age. The incidence peaked and remained steady after age 50 years at 3.3 cases per 100,000 males.
Table 3.
Age stratified incidence rate in men with Fournier’s gangrene
| Age | No. Pts | Population | Incidence Rate/100,000 Males |
|---|---|---|---|
| 0–9 | 36 | 14,050,384 | 0.3 |
| 10–19 | 83 | 14,864,893 | 0.6 |
| 20–29 | 85 | 14,187,958 | 0.6 |
| 30–39 | 175 | 15,238,114 | 1.1 |
| 40–49 | 341 | 15,905,127 | 2.1 |
| 50–59 | 407 | 12,363,771 | 3.3 |
| 60–69 | 250 | 7,685,933 | 3.3 |
| 70–79 | 176 | 5,362,421 | 3.3 |
| 80+ | 88 |
2,731,291 |
3.2 |
| Totals | 1,641 | 102,389,892 | 1.6 |
The incidence of Fournier’s gangrene was highest in the South, and lowest in the West and Midwest United States (table 4). When these regional trends were compared to 2005 Behavioral Risk Factor Surveillance System prevalence rates using linear regression analysis, an increased prevalence of diabetes was associated with an increased incidence of Fournier’s gangrene (an increase of 0.2/100,000 males for each 1% increase in diabetes prevalence, p = 0.02). There was no association between the Fournier’s gangrene incidence and the prevalence of obesity (p = 0.95) or between the regional mortality rate of Fournier’s gangrene and the regional prevalence of diabetes (p = 1.00) or obesity (p = 0.35). Similar findings were observed when individual states were analyzed, rather than regions of the United States.
Table 4.
Incidence rate of Fournier’s gangrene by region vs 2005 diabetes and obesity diagnoses prevalence rates
| United States Region |
Totals | ||||
|---|---|---|---|---|---|
| Northeast | Midwest | South | West | ||
| No. male pts | 651 | 150 | 567 | 273 | 1,641 |
| Male population | 39,850,544 | 11,451,728 | 30,532,528 | 20,555,092 | 102,389,892 |
| Male incidence/100,000 | 1.6 | 1.3 | 1.9 | 1.3 | |
| % Mortality | 8.8 | 6.7 | 6.2 | 8.1 | |
| Estimated % prevalence:* | |||||
| Diabetes | 7.1 | 7.4 | 9.1 | 6.3 | |
| Obesity | 22.3 | 26.5 | 27.1 | 22.9 | |
2005 Behavioral Risk Factor Surveillance System prevalence rates.
Transferred patients were similar in age and ethnicity distribution, the frequency of surgical procedures, length of stay, total hospital charges and discharge needs to patients who were not transferred. However, there was a trend toward a higher fatality rate in transferred cases than cases that were not transferred (14 deaths per 110 cases or 12.7% vs 110/1,531 or 7.1%, p = 0.09).
To evaluate our case definition we analyzed the 633 patients with a diagnosis of Fournier’s gangrene who did not undergo surgical débridement. These patients had a lower mortality rate (5%) and an average length of stay that was almost half as long (7 vs 13 days) with many patients being observed overnight and discharged home the following day. These patients were less likely to require intensive care unit level care, mechanical ventilation or dialysis during hospitalization. These observations led us to conclude that the survivors in this group of patients were unlikely to truly have had Fournier’s gangrene. Thus, they were excluded from the study population.
DISCUSSION
In 1883 Fournier described the gangrene as idiopathic, of sudden presentation and rapidly developing in previously healthy young males.1 This definition has changed substantially. Today an underlying etiology can almost always be identified and the disease is not limited to young people or to males.14,15
This population based epidemiological study provides a different perspective than that provided by previous studies. We confirmed that Fournier’s gangrene is rare by providing data that Fournier’s gangrene represents less than 0.02% of hospital admissions with an overall incidence of 1.6 cases per 100,000 males. Overall no patients with Fournier’s gangrene were treated at 66% of hospitals during a given year and 5 or more per year were treated at only 1% of hospitals. Thus, even at the highest volume centers a patient with Fournier’s gangrene was treated only every few months.
In 1972 Stone and Martin reported an 88% mortality rate in 33 patients with Fournier’s gangrene.2 Although contemporary published series indicate a fatality rate in the 20% to 40% range, this population based study showed a substantiality lower mortality rate of 7.5% (table 1). Even in transferred patients, possibly representing the most acutely ill patients, the mortality rate was only 12.7%. In the largest study of Fournier’s gangrene Eke reviewed 1,726 cases from the English literature and reported an overall mortality rate of 16%,16 more than double the 7.5% rate in our study. The case fatality rate in patients with Fournier’s gangrene in our study is less than the 24% rate in previous reports of group A streptococcal necrotizing soft tissue infections based on the Centers for Disease Control and Prevention population based surveillance system, supporting the often less lethal course of Fournier’s gangrene compared to that of other necrotizing soft tissue infections.17 Using a population based approach allowed us to identify how a large number of Fournier’s gangrene cases were managed at multiple centers, including tertiary care referral hospitals and nonreferral hospitals, limiting case selection and publication bias.
Contrary to the original description of Fournier, only 26% of patients in our study had no coded comorbidity and only 23% were younger than 40 years old. Fournier’s gangrene does not occur exclusively in males but our efforts to identify a comparable cohort of females revealed only 39 patients. Because of the differences in case ascertainment, our analysis focused on male cases.
This series has important limitations. The study was retrospective, using administrative data, and subject to the inherent biases of these study designs. There were no available clinical or microbiological variables in the data set18–20 to allow us to confirm the diagnosis, determine the degree/severity of infection or the percent of surface area of skin involvement, or calculate a Fournier’s gangrene severity index. We were unable to explore urethral stricture disease as a precipitating comorbidity since this was not captured as comorbidity in the data set. Differential coding of comorbidities was possible with comorbidities more likely to be captured in more severely ill patients and in those who died. We may have excluded patients with Fournier’s gangrene who were treated with antibiotics only because our case definition required surgical débridement. However, our subgroup analysis revealed that these patients likely did not merit a diagnosis of Fournier’s gangrene. It is possible that the case fatality rate was inflated because we included all patients who died with a Fournier’s gangrene diagnosis code but required all survivors to have a Fournier’s gangrene diagnosis code as well as genital/perineal débridement. We have limited information on women due to ICD-9 coding limitations.
To our knowledge this is the largest study of Fournier’s gangrene and the first population based study allowing an accurate estimation of incidence and case fatality. Our study provides needed data on managing this complex condition in the United States. Unfortunately to our knowledge there are no comparable data with regard to incidence rates in other parts of the world. We provide new insights into the rarity and hospital experience with Fournier’s gangrene, regional trends and the association of Fournier’s gangrene with other comorbidities. Our findings agree with prior literature on age at onset and comorbid risk factors, although we found substantially lower mortality than reported in case series from tertiary referral centers. Further study is necessary to explore potential differences in treatments and outcomes in patients with Fournier’s gangrene at different types and sizes of hospitals, and explore the impact of hospital experience with Fournier’s gangrene.
CONCLUSIONS
Fournier’s gangrene is a rare but serious necrotizing soft tissue infection. At 66% of hospitals no patients with Fournier’s gangrene were treated during the 2 study years and 5 or more patients per year were treated at only 1% of hospitals. The incidence of Fournier’s gangrene was 1.6 cases per 100,000. It varied by region and was associated with the regional prevalence of diabetes. The contemporary population based case fatality rate of 7.5% is lower than that previously reported in tertiary referral series in the literature.
Acknowledgments
Dr. Jonathan Wright provided statistical expertise. Study was done at Harborview Injury Prevention and Research Center.
Supported by Centers for Disease Control Grant R49/CE000197.
Abbreviations and Acronyms
- SID
State Inpatient Databases
Footnotes
Study received University of Washington institutional review board approval.
References
- 1.Fournier JA. Gangrene foudroyante de la verge (overwhelming gangrene) Sem Med. 1883;3:345. doi: 10.1007/BF02554904. [DOI] [PubMed] [Google Scholar]
- 2.Stone HH, Martin JD., Jr Synergistic necrotizing cellulitis. Ann Surg. 1972;175:702. doi: 10.1097/00000658-197205000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho JP, Hazan A, Cavalcanti AG, Favorito LA. Relation between the area affected by Fournier’s gangrene and the type of reconstructive surgery used. A study with 80 patients. Int Braz J Urol. 2007;33:510. doi: 10.1590/s1677-55382007000400008. [DOI] [PubMed] [Google Scholar]
- 4.Ayan F, Sunamak O, Paksoy SM, Polat SS, As A, Sakoglu N, et al. Fournier’s gangrene: a retrospective clinical study on forty-one patients. ANZ J Surg. 2005;75:1055. doi: 10.1111/j.1445-2197.2005.03609.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown DR, Davis NL, Lepawsky M, Cunningham J, Kortbeek J. A multicenter review of the treatment of major truncal necrotizing infections with and without hyperbaric oxygen therapy. Am J Surg. 1994;167:485. doi: 10.1016/0002-9610(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 6.Pizzorno R, Bonini F, Donelli A, Stubinski R, Medica M, Carmignani G. Hyperbaric oxygen therapy in the treatment of Fournier’s disease in 11 male patients. J Urol. 1997;158:837. doi: 10.1097/00005392-199709000-00039. [DOI] [PubMed] [Google Scholar]
- 7.Saffle JR, Morris SE, Edelman L. Fournier’s gangrene: management at a regional burn center. J Burn Care Res. 2008;29:196. doi: 10.1097/BCR.0b013e318160daba. [DOI] [PubMed] [Google Scholar]
- 8.HCUP. Overview, Healthcare Cost and Utilization Project. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 9.The International Classification of Diseases, 9th Revision, Clinical Modification. 7. Washington, D. C: United States Department of Health and Human Services, Public Health Service, United States Health Care Financing Administration; 2007. p. 2.p. v. [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.United States Census: Annual Estimates of the Population for the United States, Regions, States and Puerto Rico: April 1, 2000 to July 1, 20007. Washington, D. C: United States Census Bureau, Population Division; 2007. [Google Scholar]
- 12.Census Regions and Divisions of the United States. Washington, D. C: United States Census Bureau; [Google Scholar]
- 13.Chowdhury PP, Balluz L, Murphy W, Wen XJ, Zhong Y, Okoro C, et al. Surveillance of certain health behaviors among states and selected local areas—United States, 2005. MMWR Surveill Summ. 2007;56:1. [PubMed] [Google Scholar]
- 14.Basoglu M, Ozbey I, Atamanalp SS, Yildirgan MI, Aydinli B, Polat O, et al. Management of Fournier’s gangrene: review of 45 cases. Surg Today. 2007;37:558. doi: 10.1007/s00595-006-3391-6. [DOI] [PubMed] [Google Scholar]
- 15.Vick R, Carson CC., 3rd Fournier’s disease. Urol Clin North Am. 1999;26:841. doi: 10.1016/s0094-0143(05)70224-x. [DOI] [PubMed] [Google Scholar]
- 16.Eke N. Fournier’s gangrene: a review of 1726 cases. Br J Surg. 2000;87:718. doi: 10.1046/j.1365-2168.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 17.O’Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007;45:853. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- 18.Laor E, Palmer LS, Tolia BM, Reid RE, Winter HI. Outcome prediction in patients with Fournier’s gangrene. J Urol. 1995;154:89. [PubMed] [Google Scholar]
- 19.Yeniyol CO, Suelozgen T, Arslan M, Ayder AR. Fournier’s gangrene: experience with 25 patients and use of Fournier’s gangrene severity index score. Urology. 2004;64:218. doi: 10.1016/j.urology.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 20.Corcoran AT, Smaldone MC, Gibbons EP, Walsh TJ, Davies BJ. Validation of the Fournier’s gangrene severity index in a large contemporary series. J Urol. 2008;180:944. doi: 10.1016/j.juro.2008.05.021. [DOI] [PubMed] [Google Scholar]