Abstract
The diversity of nicotinic acetylcholine receptor (nAChR) subtypes was explored by measuring the effects of gene deletion and pharmacological diversity of epibatidine binding sites in mouse brain. All epibatidine binding sites require expression of either the α7, β2, or β4 subunit. In agreement with general belief, the α4β2*-nAChR and α7-nAChR subtypes are major components of the epibatidine binding sites. α4β2*-nAChR sites account for approximately 70% of total high- and low-affinity epibatidine binding sites, while α7-nAChR accounts for 16% of the total sites all of which have lower affinity for epibatidine. The other subtypes are structurally diverse. Although these minor subtypes account for only 14% of total binding in whole brain, they are expressed at relatively high concentrations in specific brain areas indicating unique functional roles.
Keywords: Neuronal nicotinic acetylcholine receptors, Epibatidine, Null mutant mice, α-Bungarotoxin, Cytisine, α-Conotoxin MII
Introduction
Ligand binding assays have been used for over 40 years to identify and characterize nicotinic cholinergic receptors (nAChRs) in brain. Indeed, the first studies that showed that [125I]-α-bungarotoxin (Patrick and Stallcup 1977) and [3H]-nicotine (Romano and Goldstein 1980) bind with high affinity to membranes derived from rat brain provided some of the first evidence that suggested that nAChRs might be expressed in brain. Subsequent studies that compared the anatomical distributions and biochemical properties of these binding sites in rat (Clarke et al. 1985) and mouse (Marks and Collins 1982) brain yielded the first evidence that more than one nAChR subtype is expressed in brain. When the nine nAChR subunit genes that are expressed in mammalian brain (α2–α7, β2–β4) were cloned and sequenced nearly 20 years ago, the number of potential subtypes expanded dramatically. Much of the recent research in the nAChR field is attempting to identify the subunit compositions of those receptor subtypes that are actually expressed (i.e., native receptors) in brain as well as other tissues (reviewed in Millar and Gotti 2008).
Pharmacological approaches have been used for many years in attempts to identify different nAChR subtypes. For example, the findings that α-bungarotoxin and decamethonium are potent inhibitors of the nAChR expressed at the neuromuscular junction whereas mecamylamine and hexamethonium are potent inhibitors of nAChR(s) expressed at autonomic ganglia (reviewed in Collins et al. 2009) were cornerstones of the growing data set that indicated that the nAChRs expressed at these two peripheral sites are different. These successes prompted several groups to develop and characterize additional nicotinic ligands as potential new tools for studying nAChRs. Early studies that used [3H]-methylcarbamylcholine (Abood and Grassi 1986), [3H]-acetylcholine (Martino-Barrows and Kellar 1987), and [3H]-cytisine (Pabreza et al. 1991) demonstrated that these compounds bind with high affinity to rodent brain membranes, but subsequent studies showed that these ligands bind to the same sites that had been successfully measured using [3H]-nicotine (Anderson and Arneric 1994). These ligands had several advantages when compared with [3H]-nicotine but were quickly replaced by radiolabeled epibatidine shortly after Badio and Daly (1994) reported that (+/−)epibatidine binds with extraordinarily high affinity (pM Kd) to rat brain membranes. The first binding studies done with epibatidine (Badio and Daly 1994; Houghtling et al. 1994) suggested that it binds to a single high-affinity site, the same sites that are measured with [3H]-nicotine. However, saturation analyses that used a broader range of ligand concentrations demonstrated that both high- and low-affinity epibatidine binding sites can be detected in rat, human (Houghtling et al. 1995), and mouse (Marks et al. 1998, 1999) brain. These findings, coupled with the report that [3H]-epibatidine binding is significantly greater than [3H]-cytisine binding in many brain regions (Perry and Kellar 1995), that it is a potent activator of α3β2, α3β4, α4β2, α7, and α8 nAChRs expressed in Xenopus laevis oocytes (Gerzanich et al. 1995), and that it binds to heteromeric receptors expressed in Xenopus (Parker et al. 1998), suggest that the ligand might be useful for measuring several, perhaps many, different nAChR subtypes.
The studies reported here review our use of pharmacological (competition binding) and genetic (null mutant or gene knockout) strategies to characterize epibatidine binding in mouse brain. The results of these experiments indicate that epibatidine can be used to measure a large number of different binding sites in brain.
Materials and Methods
Mice
All procedures involving mice were reviewed and approved by the Animal Care and Utilization Committee of the University of Colorado, Boulder. Mice were bred in the Specific Pathogen-Free Colony at the Institute for Behavioral Genetics, University of Colorado, weaned at 25 days of age and housed with like-sexed littermates. Animals were maintained on a 12-h light/12-h dark cycle (lights on 7 a.m.–7 p.m.) and allowed free access to food and water. Mice differing in β2 nAChR genotype were originally obtained from Marina Picciotto, Yale University (Picciotto et al. 1995), were derived by mating heterozygotes, and had been backcrossed to C57BL/6 J for at least ten generations. Tail clippings were obtained from mice about 40 days of age, and genotype was determined as described previously (Marks et al. 1999).
[3H]Epibatidine Binding to Tissue Homogenates
C57BL/6J mice were sacrificed by cervical dislocation and whole brains were quickly removed and placed in 4-ml ice-cold hypotonic buffer (NaCl, 14 mM; KCl, 0.15 mM; CaCl2, 0.2 mM; MgSO4, 0.1 mM; HEPES ½ Na, 2.5 mM; pH=7.5). Each brain was homogenized using a glass/Teflon tissue grinder and the homogenate was centrifuged at 20,000×g for 20 min. Following centrifugation, the supernatant was discarded, and the pellet was suspended in 4 ml of hypotonic buffer. The centrifugation/suspension cycle was repeated four times. Following the final centrifugation step, the supernatant was discarded, and the pellet was overlaid with 1 ml of hypotonic buffer and stored frozen until assay.
On the day of assay, the samples were thawed; the pellet was suspended in the overlying buffer, and the homogenate was centrifuged at 20,000×g for 20 min. The supernatant was discarded, and the pellet was suspended in water for assay. Saturation curves for binding were determined by incubating samples with [3H]epibatidine (specific activity=48 Ci/mmol; Perkin-Elmer NEN, Shelton, CT, USA) for 2.5 h at 22°C in incubation buffer (NaCl, 140 mM; KCl, 1.5 mM; CaCl2, 2 mM; MgSO4, 1 mM; HEPES ½ Na, 25 mM; bovine serum albumin, 0.1%; pH=7.5). In the lower concentration range (0.005–1 nM), incubation volume was 500 µl, while in the higher concentration range (0.25–32 nM) incubation volume was 65 µl. Different incubation conditions were used to reduce problems with ligand depletion encountered with this high-affinity ligand. Concentrations were chosen such that the three highest concentrations in the low range and the three lowest concentrations in the high range were the same to assure that binding in the two incubation conditions was similar. Inhibition of high-affinity binding sites by cytisine (3×10−10 to 1×10−5 M) and d-tubocurarine (1×10−8 to 1×10−2 M) was measured using 0.4 nM [3H]epibatidine, and inhibition of low-affinity binding by cytisine (1×10−8 to 5×10−4 M) and by d-tubocurarine (1×10−8 to 1×10−2 M) was measured using 10 nM [3H]epibatidine. Blanks were established by including nicotine (10 µM or 1 mM used with the 0.4 nM and 10 nM [3H]epibatidine concentrations, respectively). Samples were collected by filtration at 4°C onto two glass fiber filters that had been soaked in 0.5% polyethylenimine (top filter type GB100, Microfiltration Systems, Dublin, CA, USA; bottom filter type A/E, Gelman Sciences, Ann Arbor, MI, USA) using a 96 place manifold (Inotech Biosystems, Rockville, MD, USA). Samples were washed five times with ice-cold incubation buffer without added albumin. Filters were placed in 5-ml scintillation vials and after addition of 1.5 ml of BudgetSolve (RPI, Arlington Heights, IL, USA) counted at 45% efficiency using a Packard 1600 liquid scintillation counter.
Saturation binding data were fit to a two site model: B=Bmax-H×Epi/(KD-H+Epi)+Bmax-L×Epi/(KD-L+Epi), where B is binding at each Epi concentration; Bmax-H and Bmax-L are maximal binding site density to the high- and low-affinity sites with apparent binding affinities of KD-H and KD-L, respectively.
Inhibition curves were also fit to a two-site model: B=BHA/(1+I/IC50-HA)+BLA/(1+I/IC50-LA), where B is binding at each inhibitor concentration; I, BHA, and BLA are binding site densities inhibited with IC50-HA and IC50-LA, respectively. Nonlinear curve fitting was accomplished using Sigma Plot.
Autoradiography Mice
(β2+/+, β2+/−, and β2−/−) were sacrificed by cervical dislocation; the brains were rapidly removed and quickly frozen by immersion in isopentane (−35°C). Brains were stored at −70°C until sectioning (14-µM thickness) using an IEC cryostat. Sections were thaw mounted on Fisher SupraFrost Plus microscope slides. Slides containing the sections were stored at −70°C until use.
On the day of the assay, samples were warmed to room temperature under desiccation, rehydrated before use, and treated with 1 mM phenylmethylsulfonyl fluoride during rehydration. High-affinity binding was measured using [125I]epibatidine (2,200 Ci/mmol, Perkin-Elmer NEN, Shelton, CT, USA). The ligand was mixed with unlabeled iodoepibatidine (a generous gift from Kenneth Kellar, Georgetown University) to reduce the specific activity to 220 Ci/mmol. The final total epibatidine concentration was 0.2 nM. Incubations under these conditions were done in the binding buffer to which 5 mM EDTA, 5 mM ethylene glycol tetraacetic acid, and 10 µg/ml each of aprotin, leupeptin, and pepstatin had been added. Samples were incubated for 2 h at 22°C with either no addition, addition of 100 nM cytisine, or addition of 100 nM cytisine plus 50 nM α-conotoxin MII. Binding to low- and high-affinity sites was measured using [3H]epibatidine (48 Ci/mmol, Perkin-Elmer NEN, Shelton, CT, USA). Final epibatidine concentration was 10 nM. Incubation under these conditions was done in binding buffer with no further additions. Samples were incubated at 22°C for 2 h with either no addition, addition of 1 µM α-bungarotoxin, or addition of 1 µM α-bungarotoxin plus 300 µM d-tubocurarine. Following the incubation, the slides were washed by immersion in ice-cold binding buffer (2×30 s), ice-cold 0.1× binding buffer (2×10 s), and ice-cold 5 mM HEPES, pH 7.5 (2×5 s each). Samples were then dried under a gentle stream of air and desiccated overnight before exposure to Kodak MR film (125I) or Amersham β-max film (3H). Samples adjacent to those used for high-affinity binding were used to measure the binding of [125I]α-conotoxin MII (0.5 nM). Incubation conditions were the same as those used for [125I]epibatidine. Slides were washed by immersion in binding buffer (22°C, 1×, 30 s), binding buffer (4°C, 1×30 s), 0.1× binding buffer (4°C, 2× 5 s), and 5 mM HEPES, pH 7.5 (4°C, 2×5 s). Samples were air-dried and desiccated overnight before exposure to Kodak MR film. Digital images were captured, and pseudocolor images were generated using NIH Image.
Results
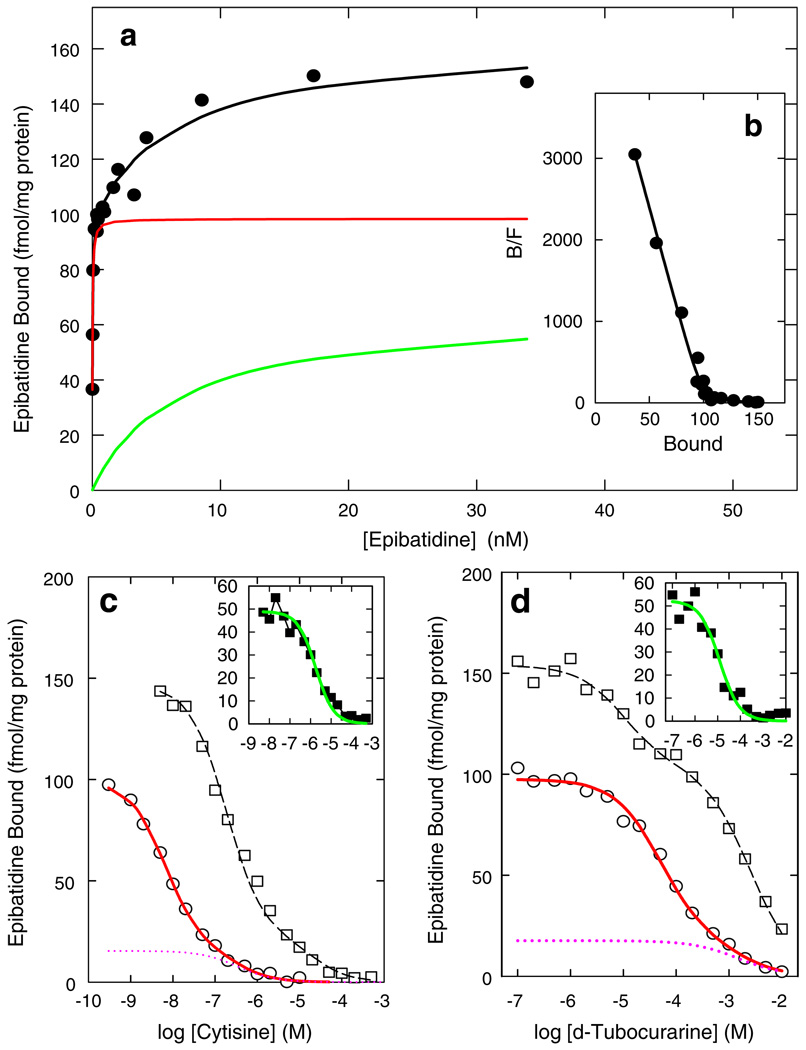
The biphasic nature of the binding of [3H]epibatidine to whole mouse brain membranes was reported by Marks et al. (1999). Figure 1 illustrates the biphasic nature of [3H] epibatidine binding to membranes derived from whole mouse brain and the inhibition of [3H]epibatidine binding by cytisine and d-tubocurarine. The saturation curve for [3H]epibatidine binding can be resolved into two components with apparent KD values of 0.02 and 6.4 nM, which are illustrated by the red and green lines in Fig. 1a. The biphasic nature of the binding is clearly demonstrated by the nonlinear Scatchard plot shown in Fig. 1b. The inhibition of high-affinity [3H]epibatidine binding by cytisine is also biphasic as illustrated in Fig. 1c. In whole brain, the component with high affinity for cytisine accounts for 85% of total binding. The estimated KI for cytisine at the site with higher affinity is 0.3 nM. The component with lower affinity for cytisine accounts for the remaining 15%. The estimated KI at this site is 13 nM. The proportion of cytisine-sensitive and cytisine-resistant [3H]epibatidine binding sites varies markedly among brain regions (Marks et al. 1998). The cytisine inhibition of low-affinity [3H]epibatidine binding sites was calculated and is illustrated in the inset to Fig. 1c. The KI for cytisine at the low-affinity [3H]epibatidine sites is estimated to be 0.6 µM. The inhibition of high-affinity [3H]epibatidine binding by d-tubocurarine is also biphasic with proportions of high-and low-sensitivity sites very similar to those measured with cytisine. However, the affinity of d-tubocurarine for these sites is much lower than that for cytisine (KI values of 2.2 and 62 µM, respectively). The d-tubocurarine inhibition of low-affinity [3H]epibatidine binding sites was calculated and is illustrated in the inset to Fig. 1d. In contrast to the large difference in affinity for the high- and low-affinity [3H]epibatidine binding sites exhibited by cytisine, the KI value for d-tubocurarine estimated for the lower-affinity [3H]epibatidine binding sites (4 µM) is very similar to that calculated for high-affinity [3H]epibatidine binding. This property makes d-tubocurarine an effective reagent to measure low-affinity [3H]epibatidine binding by differential inhibition as illustrated by the black curve in Fig. 1d. Therefore, both [3H]epibatidine saturation binding and differential inhibition of the high- and low-affinity binding sites reveal that [3H]epibatidine binds to several diverse sites.
Figure 1.
[3H]Epibatidine binding to whole mouse brain membranes. The saturation curve for [3H]epibatidine binding is shown in a. The black circles represent the data points. Total binding, represented by the black curve, was resolved into high-affinity (red) and low-affinity (green) components. b displays the Scatchard plot for the data in a. Inhibition of [3H] epibatidine binding (0.4 nM, open circles; 12.5 nM open squares) by cytisine is shown in c. The solid red curve displays the biphasic fit for inhibition using 0.4 nM [3H]epibatidine and the dotted red line illustrates the estimated cytisine-resistant component of this binding. The inset to c shows the inhibition of the lower-affinity [3H]epibatidine binding sites. Inhibition of [3H]epibatidine binding (0.4 nM, open circles; 12.5 nM open squares) by d-tubocurarine is shown in d. The solid red curve displays the biphasic fit for inhibition using 0.4 nM [3H] epibatidine and the dotted red line illustrates the estimated d-tubocurarine-resistant component of this binding. The inset to d shows the inhibition of the lower-affinity [3H]epibatidine binding sites. This figure was modified from Marks et al. (1999)
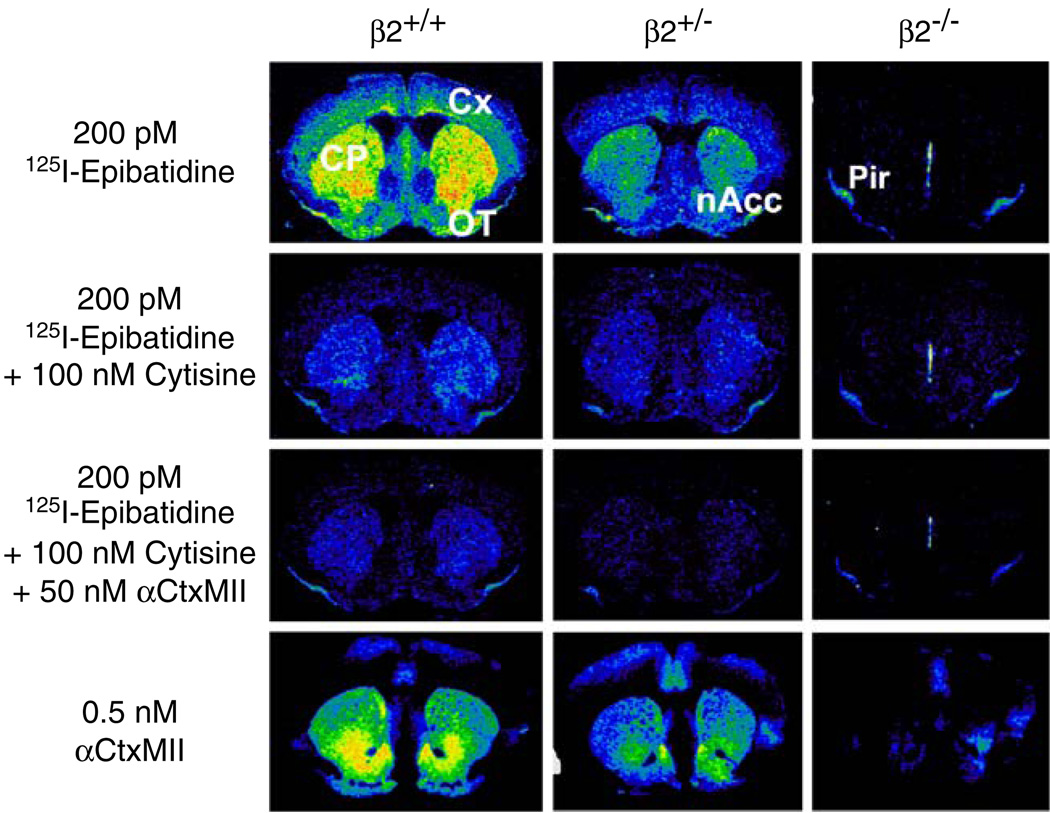
In order to analyze further the diversity of epibatidine binding sites, the effect of deletion of specific nAChR subunits on these sites has been evaluated using autoradiography. The autoradiograms shown in Fig. 2 illustrate the effect of differential inhibition by cytisine and α-conotoxin MII as well as of the deletion of the β2 nAChR subunit on high-affinity [125I]epibatidine binding at the level of the nucleus accumbens and caudate putamen. High-affinity binding in these brain regions is heterogeneous as illustrated by the effects of cytisine and α-conotoxin MII in wild-type β2+/+ mice. The binding of [125I]epibatidine is substantially inhibited when 100 nM cytisine is included in the incubation, although significant signal persists in the caudate putamen, nucleus accumbens, and olfactory tubercles. Binding is further reduced, but not completely eliminated, by addition of 50 nM α-conotoxin MII. Deletion of the β2 subunit eliminates most [125I]epibatidine binding in these sections. However, piriform cortex and medial septum of β2−/− samples retain low levels of [125I] epibatidine binding, indicating the presence of non-β2 sites. [125I]Epibatidine binding in β2+/− mice was intermediate between that of wild-type and null mutants. The pattern of inhibition was similar to that of wild type, consistent with a gene dose-dependent expression of these sites. Deletion of the β2 subunit eliminated specific binding of [125I]α-conotoxin MII, consistent with the results seen with inhibition of [125I]epibatidine binding by this toxin.
Figure 2.
Effect of deletion of the β2 subunit on subsets of high-affinity [125I]epibatidine binding sites and [125I]-α-conotoxin MII binding. [125I] Epibatidine (0.2 nM) was incubated with sections derived from β2+/+, β2+/−, and β2−/− mice in the absence of added ligands, in the presence of 100 nM cytisine, and in the presence of 100 nM cytisine plus 50 nM α-conotoxin MII as indicated. Near adjacent sections were incubated with 0.5 nM [125I] α-conotoxin MII. Labels: CP, caudate putamen; Cx, cerebral cortex; nAcc, nucleus accumbens; OT, olfactory tubercles; Pir, piriform cortex
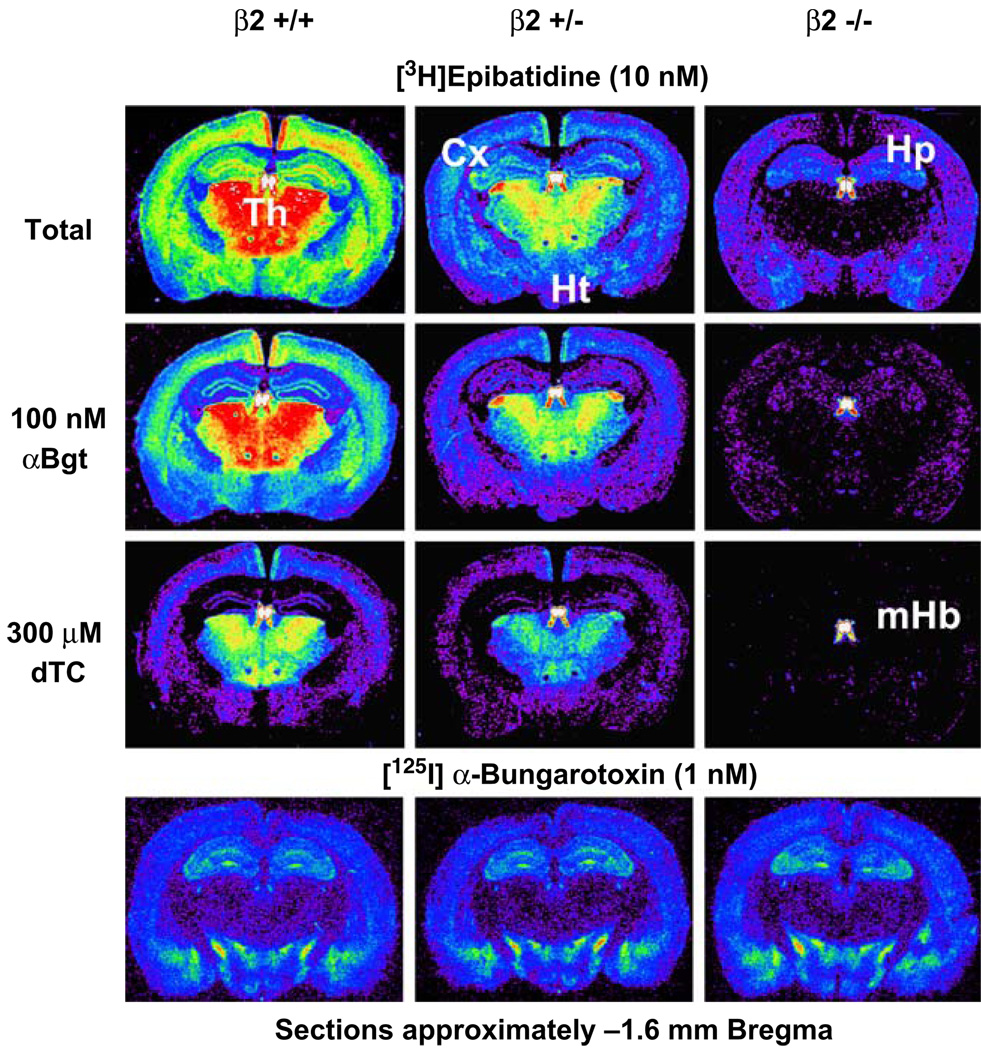
The autoradiograms shown in Fig. 3 illustrate the effects of differential inhibition of binding by α-bungarotoxin and d-tubocurarine and the effects of β2 gene deletion on binding of 10 nM [3H]epibatidine at the level of the thalamus, medial habenula, and dorsal hippocampus. Total [3H]epibatidine binding is heterogeneous as illustrated by the differential inhibition by several competitive ligands and by gene deletion. In wild-type (β2+/+) mice, incubation in the presence of α-bungarotoxin significantly reduces labeling in hippocampus, demonstrating that [3H]epibatidine will bind to α7-nAChR at this high concentration. Addition of 300 µM d-tubocurarine generally reduces binding and the resulting pattern resembles that seen for samples incubated with a lower concentration of [3H] epibatidine (0.4 nM). Deletion of the β2 subunit reduces [3H]epibatidine binding throughout the brain, but significant α-bungarotoxin-sensitive [3H]epibatidine binding is noted in hippocampus, cerebral cortex, and hypothalamus. The only detectable [3H]epibatidine binding persisting in the presence of 300 µM d-tubocurarine in these sections of β2−/− mice is found in the medial habenula and fasciculus retroflexus and almost certainly due to β4*nAChR.
Figure 3.
Effect of deletion of the β2 subunit on subsets on [3H]epibatidine binding sites and [125I]α-bungarotoxin binding. [125I]Epibatidine (0.2 nM) was incubated with sections derived from β2+/+, β2+/−, and β2−/− mice in the absence of added ligands, in the presence of 1,000 nM α-bungarotoxin, and in the presence of 300 µM d-tubocurarine as indicated. Near adjacent sections were incubated with 1 nM [125I]α-bungarotoxin. Labels: Cx, cerebral cortex; Hp, hippocampus; Ht, hypothalamus; mHb, medial habenula; Th, thalamus
Epibatidine binding in the β2+/− mice is intermediate between that of wild-type and knockout mice. This figure also illustrates that deletion of β2 has no effect on the binding of [125I]α-bungarotoxin binding as expected. Note the similarity of the patterns of [3H]epibatidine and [125I]α-bungarotoxin binding in the β2−/− mice.
Discussion
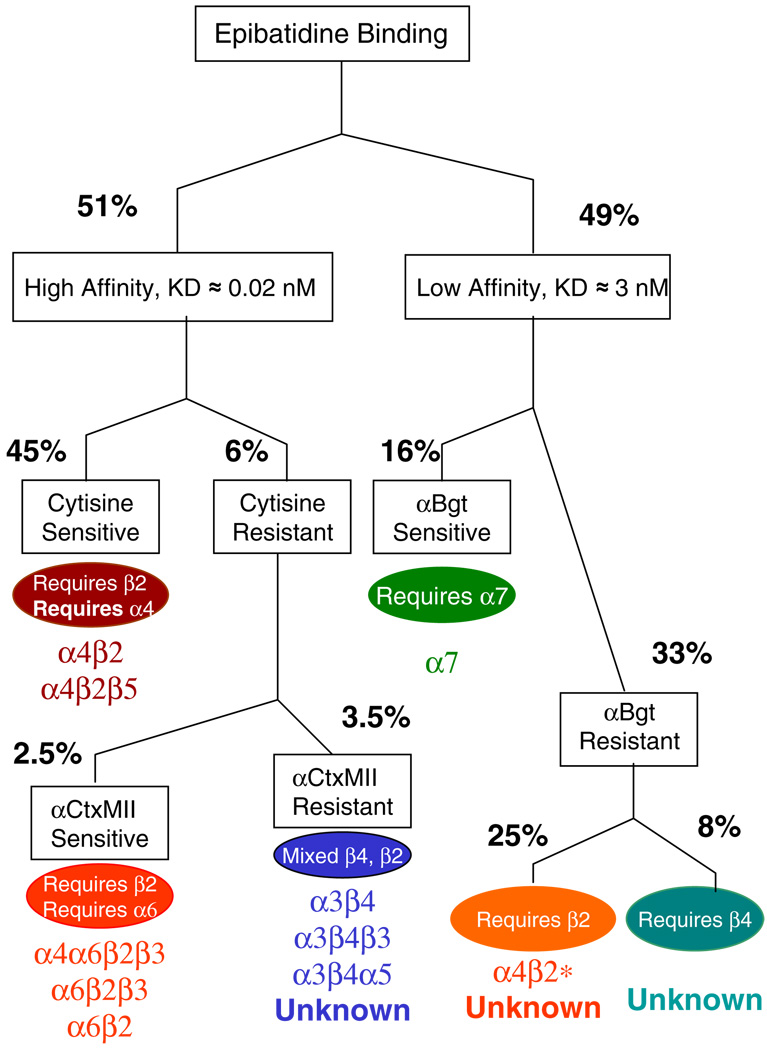
The results presented here summarize how pharmacological and genetic approaches have been used to investigate the diversity of epibatidine binding sites in rodent brain and to begin to establish the subunit composition of the nAChR subtypes measured with epibatidine. The results presented in this article provide a general description of nAChR subtypes identified by epibatidine binding. Greater detail is provided in previous publications cited where appropriate. Figure 4 presents these results diagrammatically.
Figure 4.
Contribution of nAChR subunits to epibatidine binding sites. Subunit composition of epibatidine binding sites determined by pharmacological, genetic, and immunochemical approaches. This figure has been expanded from Marks et al. (2006)
Epibatidine was first isolated by John Daly from the skin of the Ecuadorean tree frog, Epipedobates tricolor (Daly et al. 1978) and was identified as a very potent nAChR agonist. The extraordinary affinity of epibatidine has made it a particularly useful reagent with which to identify multiple nAChR binding sites (Badio and Daly 1994; Houghtling et al. 1994, 1995; Davila-Garcia et al. 1997; Marks et al. 1998, 1999; Zoli et al. 1998; Whiteaker et al. 2000; Perry and Kellar 1995, 2002). Because of epibatidine’s potency, it has been possible to label not only high-affinity sites that are amenable to measurement with other ligands such as nicotine and cytisine but also to label sites with lower affinity that evade measurement with other ligands as illustrated in Fig. 1.
Gene knockout (null mutant) mouse strains have been developed for all nine nAChR subunit genes that are expressed in mammalian brain. These knockout strains have been very useful for determining subunits that are required to form specific binding sites. Examination of the effect of β2, β4, or α7 subunit deletion established that both high- and low-affinity epibatidine binding sites require expression of either α7, which assembles into homomeric pentamers, or β2 or β4, which are required for the assembly of heteromeric receptors (Marks et al. 2006).
The higher-affinity sites (KD≈0.02 nM) account for approximately one half of total epibatidine binding and are relatively well characterized. At least three families of nAChR subtypes are included in the higher affinity [3H] epibatidine binding sites. Each of these families is heterogeneous. The major subtype, accounting for approximately 90% of high-affinity [3H]epibatidine binding (45% of total), is sensitive to inhibition by cytisine and is eliminated by deletion of β2 as illustrated in Fig. 2. The cytisine-sensitive site is also eliminated by deletion of the α4 (Marks et al. 2007), establishing this site as an α4β2*-nAChR. This site can also be measured by direct binding of [3H]cytisine or [3H]nicotine (Anderson and Arneric 1994). Consistent with the effects of gene deletion on cytisine-sensitive epibatidine binding, it has previously been demonstrated that [3H] nicotine binding is eliminated in α4 (Marubio et al. 1999) and β2 (Picciotto et al. 1995) null mutant mice. Furthermore, gene deletion and immunoprecipitation establish that some of these sites also contain the α5 subunit (Brown et al. 2007).
In contrast to the complete dependence of cytosine-sensitive [3H]epibatidine binding sites on expression of the β2 subunit, only some of the cytisine-resistant sites require expression of β2, while the remainder require expression of β4. Most of the cytisine-resistant β2*-nAChR sites bind α-conotoxin MII with high affinity and correspond to α6β2*-nAChR (see Fig. 2). These receptors are heterogeneous and include α6α4β2β3-, α6β2β3-, and α6β2-nAChR subtypes as demonstrated by assays of ligand binding (Salminen et al. 2005) and nAChR-mediated dopamine release (Salminen et al. 2007). However, a minor subset of α-conotoxin MII binding, particularly in the medial habenula and interpeduncular nucleus, is likely α3β2*-nAChR (Whiteaker et al. 2002; Grady et al. 2009). Relatively few α6β2*-nAChR are measurable in whole-brain preparations, but this subtype is expressed in significant levels in dopaminergic and visual pathways (Champtiaux et al. 2003; Salminen et al. 2005; Gotti et al. 2005a, b; Marritt et al. 2005; Cox et al. 2008).
The β4*-nAChR also have restricted distribution. However, β4*-nAChR are highly expressed in several brain regions including medial habenula, interpeduncular nucleus, inferior colliculus, and accessory olfactory bulb (Whiteaker et al. 2000). Most of these receptors are α3β4*-nAChR (Marks et al. 2002; Grady et al. 2009), and ganglionic versions of this subtype often include the α5 subunit (Conroy and Berg 1995; Mao et al. 2006). It has recently been shown that β3 can assume the role of the accessory subunit in this subtype in the interpeduncular nucleus (Grady et al. 2009).
The lower-affinity [3H]epibatidine binding sites (KD≈5 nM) have been less well characterized. These sites are nicotinic as demonstrated by their elimination by deletion of the β2, β4, or α7 subunit (Marks et al. 2006). These lower-affinity sites represent approximately 50% of total [3H]epibatidine binding measured at high (30–40 nM) ligand concentrations.
It has previously been shown that [125I]α-bungarotoxin binding is eliminated by deletion of α7 (Orr-Urtreger et al. 1997), which also selectively eliminates the α-bungarotoxin-sensitive component of lower-affinity [3H] epibatidine binding. Thus, α7-nAChR sites can be measured with [3H]epibatidine and represent approximately one third of low-affinity sites (16% of the total). Deletion of either β2 or β4 (or α4) has no measurable effect on these sites.
The remaining lower-affinity binding sites represent a significant fraction of total binding (33% of total, two thirds of the low-affinity sites).Most of these sites require expression of β2 (Marks et al. 2006) and α4 (Marks et al. 2007). These low-affinity [3H]epibatidine binding sites are expressed throughout the brain, and their distribution resembles that of high affinity α4β2*-nAChR [3H]epibatidine binding sites (see Fig. 3). These non-α7 sites have not been well characterized. It is not yet established whether they are assembled functional receptors, partially assembled receptors, or dead-end assembly/folding intermediates. It was speculated that these binding sites correspond to receptors with lower sensitivity to activation by agonists and corresponding to α4β2-nAChR with a stoichiometry of (α4)3(β2)2. However, studies of binding to heterologously expressed receptors with this stoichiometry establish that cytisine binds to them with high affinity (Moroni et al. 2006).
In summary, pharmacological, genetic, and immunochemical methods have successfully been used to identify many diverse epibatidine binding sites reflecting a diverse population of nAChR. These approaches confirm the often-made assertion that α4β2*-nAChR and α7*-nAChR are the most highly expressed subtypes but also indicate that there are a large number of less common subtypes with diverse subunit composition. The tendency for these unusual subtypes to be expressed in discrete brain nuclei suggests that they may play roles in specific nicotinic responses in these areas as indicated by the important role of α6β2*-nAChR in dopaminergic pathways and α3β4*-nAChR in the habenulo-interpeduncular nucleus pathway. Indeed, α6α4β2(β3)-nAChR seem to be particularly important in regulating nicotine intake (Pons et al. 2008), and α3β4*-nAChR are involved in nicotine-induced convulsions (Adams et al. 2004; Salas et al. 2004). Identification and functional characterization of native nAChR subtypes will be a continuing effort.
Acknowledgments
Supported by grants DA003194, DA012242, and DA015663 to ACC, MJM, and PW and GM048677 and MH053631 to JMM
Footnotes
Proceedings of the XIII International Symposium on Cholinergic Mechanisms
Contributor Information
Michael J. Marks, Email: Michael.Marks@Colorado.EDU, Institute for Behavioral Genetics, University of Colorado, Boulder, CO, USA.
Duncan S. Laverty, Institute for Behavioral Genetics, University of Colorado, Boulder, CO, USA
Paul Whiteaker, Division of Neurobiology, Barrow Neurological Institute, Phoenix, AZ, USA.
Outi Salminen, Faculty of Pharmacy, University of Helsinki, Helsinki, Finland.
Sharon R. Grady, Institute for Behavioral Genetics, University of Colorado, Boulder, CO, USA
J. Michael McIntosh, Departments of Biology and Psychiatry, University of Utah, Salt Lake City, UT, USA.
Allan C. Collins, Institute for Behavioral Genetics, University of Colorado, Boulder, CO, USA
References
- Abood LG, Grassi S. [3H]Methylcarbamylcholine, a new radioligand for studying brain nicotinic receptors. Biochemical Pharmacology. 1986;35:4199–4202. doi: 10.1016/0006-2952(86)90695-7. [DOI] [PubMed] [Google Scholar]
- Adams MR, Nikkel AL, Donnelly-Roberts DL, Watt AT, Johnston JF, Cowsert LM, et al. In vitro and in vivo effects of an alpha3 neuronal nicotinic acetylcholine receptor antisense oligonucleotide. Brain Research. Molecular Brain Research. 2004;129:67–79. doi: 10.1016/j.molbrainres.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Arneric SP. Nicotinic receptor binding of [3H]cytisine, [3H]nicotine and [3H]methylcarbamylcholine in rat brain. European Journal of Pharmacology. 1994;253:261–267. doi: 10.1016/0014-2999(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Badio B, Daly JW. Epibatidine, a potent analgetic and nicotinic agonist. Molecular Pharmacology. 1994;45:563–569. [PubMed] [Google Scholar]
- Brown RW, Collins AC, Lindstrom JM, Whiteaker P. Nicotinic alpha5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. Journal of Neurochemistry. 2007;103:204–215. doi: 10.1111/j.1471-4159.2007.04700.x. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. Journal of Neuroscience. 2003;23:7820–78299. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PBS, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: Autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. Journal of Neuroscience. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Salminen O, Marks MJ, Whiteaker P, Grady SR. The road to discovery of nicotinic receptor subtypes. Handbook of Experimental Pharmacology. 2009;192:85–112. doi: 10.1007/978-3-540-69248-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. Journal of Biological Chemistry. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- Cox BC, Marritt AM, Perry DC, Kellar KJ. Transport of multiple nicotinic acetylcholine receptors in the rat optic nerve: High densities of receptors containing alpha6 and beta3 subunits. Journal of Neurochemistry. 2008;105:1924–1938. doi: 10.1111/j.1471-4159.2008.05282.x. [DOI] [PubMed] [Google Scholar]
- Daly JW, Brown GB, Mennah-Dwumah H, Myers CW. Classification of skin alkaloids from neotropical poison-dart frogs (Dendrobatidae) Toxicon. 1978;16:163–188. doi: 10.1016/0041-0101(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Dávila-García MI, Musachio JL, Perry DC, Xiao Y, Horti A, London ED, et al. [125I]IPH, an epibatidine analog, binds with high affinity to neuronal nicotinic cholinergic receptors. Journal of Pharmacology and Experimental Therapeutics. 1997;282:445–451. [PubMed] [Google Scholar]
- Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, et al. Comparative pharmacology of epibatidine: A potent agonist for neuronal nicotinic acetylcholine receptors. Molecular Pharmacology. 1995;48:774–782. [PubMed] [Google Scholar]
- Gotti C, Moretti M, Zanardi A, Gaimarri A, Champtiaux N, Changeux JP, et al. Heterogeneity and selective targeting of neuronal nicotinic acetylcholine receptor (nAChR) subtypes expressed on retinal afferents of the superior colliculus and lateral geniculate nucleus: Identification of a new native nAChR subtype alpha3beta2(alpha5 or beta3) enriched in retinocollicular afferents. Molecular Pharmacology. 2005a;68:1162–1171. doi: 10.1124/mol.105.015925. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, et al. Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Molecular Pharmacology. 2005b;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, et al. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. Journal of Neuroscience. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghtling RA, Davila-Garcia MI, Hurt SD, Kellar KJ. [3H]Epibatidine binding to nicotinic cholinergic receptors in brain. Medicinal Chemistry Research. 1994;4:538–546. [Google Scholar]
- Houghtling RA, Davila-Garcia MI, Hurt SD, Kellar KJ. Characterization of [3H]epibatidine binding to nicotinic cholinergic receptors in rat and human brain. Molecular Pharmacology. 1995;48:280–287. [PubMed] [Google Scholar]
- Mao D, Yasuda RP, Fan H, Wolfe BB, Kellar KJ. Heterogeneity of nicotinic cholinergic receptors in rat superior cervical and nodose ganglia. Molecular Pharmacology. 2006;70:1693–1699. doi: 10.1124/mol.106.027458. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Collins AC. Characterization of nicotine binding in mouse brain and comparison with the binding of α-bungarotoxin and quinuclidinyl benzilate. Molecular Pharmacology. 1982;22:554–564. [PubMed] [Google Scholar]
- Marks MJ, Smith KW, Collins AC. Differential agonist inhibition identifies multiple epibatidine binding sites in mouse brain. Journal of Pharmacology and Experimental Therapeutics. 1998;285:377–386. [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, et al. Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the β2 subunit. Journal of Pharmacology and Experimental Therapeutics. 1999;289:1090–1103. [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Grady SR, Picciotto MR, McIntosh JM, Collins AC. Characterization of [125I] epibatidine binding and nicotinic agonist-mediated 86Rb+ efflux in interpeduncular nucleus and inferior colliculus of β2 null mutant mice. Journal of Neurochemistry. 2002;81:1102–1115. doi: 10.1046/j.1471-4159.2002.00910.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Collins AC. Deletion of the α7, β2 or β4 nicotinic receptor subunit genes identifies highly expressed subtypes with relatively low affinity for [3H]epibatidine. Molecular Pharmacology. 2006;70:947–959. doi: 10.1124/mol.106.025338. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Meinerz NM, Drago J, Collins AC. Gene targeting demonstrates that α4 nicotinic acetylcholine receptor subunits contribute to the expression of diverse [3H] epibatidine binding sites and components of biphasic 86Rb+ efflux with high and low sensitivity to stimulation by acetylcholine. Neuropharmacology. 2007;53:390–405. doi: 10.1016/j.neuropharm.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marritt AM, Cox BC, Yasuda RP, McIntosh JM, Xiao Y, Wolfe BB, et al. Nicotinic cholinergic receptors in the rat retina: Simple and mixed heteromeric subtypes. Molecular Pharmacology. 2005;68:1656–1668. doi: 10.1124/mol.105.012369. [DOI] [PubMed] [Google Scholar]
- Martino-Barrows AM, Kellar KJ. [3H]Acetylcholine and [3H](−)nicotine label the same recognition site in brain. Molecular Pharmacology. 1987;31:169–174. [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d’Exaerde A, et al. Nature (London) 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2008;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. α4β2 nicotinic receptors with high and low acetylcholine sensitivity: Pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Molecular Pharmacology. 2006;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo T, Goldberg T, DeBiasi M, et al. Mice deficient in the α7 neuronal nicotinic receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic currents. Journal of Neuroscience. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabreza LA, Dhawan S, Kellar KJ. [3H]Cytisine binding to nicotinic cholinergic receptors in brain. Molecular Pharmacology. 1991;39:9–12. [PubMed] [Google Scholar]
- Parker MJ, Beck A, Leutje CW. Neuronal nicotinic receptor β2 and β4 subunits confer large differences in agonist binding affinity. Molecular Pharmacology. 1998;54:1132–1139. [PubMed] [Google Scholar]
- Patrick J, Stallcup WB. α-Bungarotoxin binding and cholinergic receptor function on a rat sympathetic nerve cell line. Journal of Biological Chemistry. 1977;252:8629–8633. [PubMed] [Google Scholar]
- Perry DC, Kellar KJ. [3H]Epibatidine labels nicotinic receptors in rat brain: An autoradiographic study. Journal of Pharmacology and Experimental Therapeutics. 1995;275:1030–1034. [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Dávila-García MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. Journal of Neurochemistry. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, LeNovere N, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. Journal of Neuroscience. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Goldstein A. Stereospecific nicotine receptors on rat brain membranes. Science. 1980;210:647–650. doi: 10.1126/science.7433991. [DOI] [PubMed] [Google Scholar]
- Salas R, Cook KD, Bassetto L, De Biasi M. The alpha3 and beta4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004;47:401–407. doi: 10.1016/j.neuropharm.2004.05.002. Erratum in: (2004) Neuropharmacology, 47, 1113. [DOI] [PubMed] [Google Scholar]
- Salminen O, Whiteaker P, Grady SR, Collins AC, McIntosh JM, Marks MJ. The subunit composition and pharmacology of alpha-conotoxin MII-binding nicotinic acetylcholine receptors studied by a novel membrane-binding assay. Neuropharmacology. 2005;48:696–705. doi: 10.1016/j.neuropharm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of α-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Molecular Pharmacology. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Jimenez M, McIntosh JM, Collins AC, Marks MJ. Identification of a novel nicotinic binding site in mouse brain using [125I]-epibatidine. British Journal of Pharmacology. 2000;131:729–739. doi: 10.1038/sj.bjp.0703616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker P, Peterson CG, Xu W, McIntosh JM, Paylor R, Beaudet AL, et al. Involvement of the alpha3 subunit in central nicotinic binding populations. Journal of Neuroscience. 2002;22:2522–2529. doi: 10.1523/JNEUROSCI.22-07-02522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux J-P. Identification of four classes of nicotinic receptors using beta2 mutant mice. Journal of Neuroscience. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]