Abstract
Objective
To develop the techniques needed for the specific gene/protein targeting transfection experiments in isolated lymphatic vessels, we completed two major tasks: 1) optimize the experimental conditions to maintain the viability of isolated rat lymphatic vessels in culture for sufficiently long periods of time to permit knockdown or overexpression of selected proteins/genes; 2) develop effective transfection protocols for lymphatic muscle and endothelial cells in intact lymphatic vessels without nonspecific impairment of lymphatic contractile function due to the transfection protocol itself.
Methods
Experimental protocols were developed for the maintenance of isolated lymphatic vessels under non-pressurized and pressurized conditions for 3-12 days in culture and for adenoviral gene transfection of the lymphatic muscle and endothelial cells.
Results
The data demonstrate the effectiveness of the newly developed experimental protocols for the maintenance of isolated rat mesenteric lymphatic vessels and thoracic duct in culture up to 3-12 days without significant impairment of the parameters of their pumping and effective adenoviral/GFP transfection of lymphatic endothelial and muscle cells in isolated rat mesenteric lymphatic vessels.
Conclusions
These experimental techniques will extend the set of the modern experimental tools available to researchers investigating the physiology of lymphatic function.
Keywords: lymphatic vessel, tissue culture, transportation, adenoviral transfection
INTRODUCTION
It is an accepted paradigm of modern lymphology that all major functions of the lymphatic system, namely the maintenance of fluid and macromolecular homeostasis, lipid uptake from gut, and immune cell trafficking, require an effective transport of lymph (containing fluid, macromolecules and cells). These processes cannot be maintained without the normal contractility of the muscular collecting lymphatic vessels (2,15,20,33,39,40). Lymphatic muscle cells regulate lymph flow by influencing both the resistance to lymph flow and the generation of lymph propulsion. Lymphatic vessel resistance is determined largely by the level of lymphatic muscle tone, whereas the force of lymph propulsion critically depends on the strength and frequency of the rapid phasic contractions of lymphatic muscle cells (see recent reviews in (15,20,31)). Both of these tonic and phasic components of lymphatic muscle contractility are regulated by multiple mechanisms, thereby constantly adjusting lymphatic pumping ability to the moment-by-moment combinations of extrinsic and intrinsic influences (15,20,31). To date, only a few publications (reviewed in (31)) provide sparse and incomplete information on molecular regulatory mechanisms of tonic and phasic lymphatic contractions.
To further investigate these mechanisms over the past several years we have invested significant effort in developing methodologies to study the regulatory mechanisms of tonic and phasic lymphatic contractions. Because lymphatic muscle cells possess a mixture of smooth and striated contractile protein isoforms different from any other muscle-containing tissues (30), it is not easy to perform the same types of studies which have previously been conducted on other tissue types. In addition, the potential of genetically-modified mouse models, which are widely used in modern biological studies, is critically diminished for studies on regulatory mechanisms of the lymphatic muscle contractility because: 1) the literature (28,29,32,37) and our own unpublished data suggest that murine vessels are not highly or consistently contractile, 2) spontaneous contractions have only been demonstrated in the DDY strain (28,29,32,37), which is not typically used for transgenic crosses, 3) genetically-modified mouse models suffer from the problem that genes other than the targeted one may contribute to compensatory phenotypic alterations (7,8,11,21,27,42,43). Recently, a new method was described to image intrinsic lymph pumping in mice (26) to: “(1) assess lymphatic function in transgenic mouse models to better understand the role that specific gene expression has on lymphatic function; and (2) investigate pharmacologic agents that stimulate the formation of functional lymphatics as well as stimulate the contractile apparatus of existing lymphatics” (41). Unfortunately, careful review of those studies raises several serious questions and concerns about their conclusions. These data and the accompanying online video clips (26) were time compressed 3-5 fold above real time for “demonstration purposes” and would be more accurately interpreted as the slow tonic contractions of mouse lymphatic vessels. Additionally, the possibility of over-distension of mouse lymphatic vessels by abnormal volume expansion could not be excluded in that study. Moreover, it is well known that near infra-red fluorophores including Indocyanine Green (ICG) ICG can distribute freely in the body (13) and have particularly high rates of cellular uptake and laser-induced photooxidation (1). This seems likely to be important given the high sensitivity of lymphatic muscle cells to biologically active substances, in particular to free radicals/oxidative agents (45,46) that can be produced during photooxidation. While the laser irradiation in studies by Abels et al. (1) was more intense than that in the work by Kwon and Sevick-Muraca (26), no pharmacological tests were done to evaluate or exclude this potential influence of ICG on their ability to recapitulate normal functioning lymphatic vessels. We conclude that this work (26) did not provide strong support for the principle of using the mouse models to study phasic lymphatic contractility. Because of all currently available data described above, the potential power and specificity of using lymphatic preparations from genetically modified mice for studies of the regulation of lymphatic function has been unattainable despite the expenditure of significant time and effort.
Therefore, in an effort to attain alternatives to genetically-modified mouse lymphatic models, we began to develop techniques in which protein/gene function is altered over the course of up to 2 weeks in lymphatic muscle and/or endothelium using isolated rat lymphatic vessels. Rat vessels are particularly suited for gene transfection since the muscle layer is only 1-2 cell layers thick. To develop these techniques, we focused on two major tasks: 1) Optimize the experimental conditions to maintain the viability of isolated “normal” rat lymphatic vessels in culture for sufficiently long periods of time to permit effective knockdown or overexpression of selected proteins/genes. 2) Develop effective transfection protocols for lymphatic muscle cells in intact lymphatic vessels without nonspecific impairment of lymphatic contractile function due to the transfection protocol per se. In the present manuscript we describe the details of experimental techniques that meet these goals and which now are available for use in studies on molecular mechanisms of regulation of lymph transport.
METHODS
Animals and Surgery
Mesenteric lymphatic vessels and thoracic ducts were isolated from male Sprague-Dawley rats (weighing between 300 and 400 g). The animal facilities used for these studies were accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care and adhered to the regulations, policies and principles detailed in the Public Health Service Policy for the Humane Care and Use of Laboratory Animals (PHS Policy, 1996) and the United States Department of Agriculture’s Animal Welfare Regulations (Animal Welfare Act, AWA, 9CFR, 1985, 1992). All animal procedures performed for this study were reviewed and approved by our Institutional Animal Care and Use Committee.
To isolate the thoracic duct, rats were euthanized with pentobarbital (120 mg/kg body weight IP). The animal was positioned on its back, the ventral chest wall was opened by lateral incision and the sternum and approximately half of the ribs were excised. The inferior vena cava was ligated and cut close to the diaphragm. The lungs and heart were pulled to the left side of the animal so as to expose the thoracic duct between the aorta and vertebral column. The thoracic duct was then carefully cleared of all surrounding tissue using a dissecting microscope. Extreme caution was used to prevent direct contact with the thoracic duct at all times, thereby reducing the likelihood of damage. The segment of interest was kept moist for the period of dissection using standard Dulbecco’s phosphate-buffered saline (DPBS) (Invitrogen Corp., Cat. # 14040-133). Sections of thoracic duct 1-2 cm long were dissected and used for these experiments.
To isolate mesenteric lymphatic vessels, the rat was anesthetized with a solution containing a combination of Fentanyl/Droperidol (0.3ml/kg IM) and Diazepam (2.5mg/kg IM). A 4-cm long midline abdominal incision was made through the skin, underlying fascia and muscle layers. A small loop of intestine, 6-7 cm in length was exteriorized through the incision. A section of the mesentery containing lymphatic vessels was positioned in a dissection chamber within the field of view of a dissecting microscope and continuously suffused with DPBS. Suitable mesenteric lymphatic vessels were identified and cleared of all surrounding tissue. Sections of mesenteric lymphatic vessels 1-1.5 cm in length were carefully dissected and used for experiments. After isolating mesenteric lymphatic vessels, the rat was euthanized with pentobarbital (120 mg/kg body weight IP). Throughout the experiments we measured the diameters of the thoracic duct and mesenteric lymphatic vessel segments used for these studies. At a transmural pressure of 3 cm H2O, the outer diastolic diameters of thoracic duct segments were ~500-600 μm; the outer diastolic diameters for mesenteric lymphatic vessel segments at a transmural pressure of 5 cm H2O were ~130-160 μm.
Isolated Lymphatic Vessel Procedures for Functional Tests
Once the lymphatic segment was exteriorized, it was used for one of three protocols: 1) acute functional tests, 2) controlled-pressure, isolated vessel culture experiments, or 3) transfection experiments followed by subsequent functional tests. For all functional tests, the lymphatic segment was transferred to an isolated vessel chamber (a modified Living Systems Instrumentation single vessel chamber, model CH/1, with homemade multi-channel temperature controller system), filled with room temperature Dulbecco’s modified Eagle medium: nutrient mixture F12 (D-MEM/F12) (Invitrogen, cat.# 11039). The isolated lymphatic vessel was cannulated and tied onto glass pipettes. The inflow and outflow pipettes were connected to independently adjustable pressure reservoirs filled with D-MEM/F12. Great care was taken to ensure that there were no air bubbles in the tubing or the pipettes. Once the vessel was cannulated, a slight positive transmural pressure (2-3 cm H2O) was applied to detect leaks and to ensure that the vessel was undamaged and untwisted. The vessel was set to its approximate in situ length and positioned just above the glass coverslip comprising the chamber bottom. The chamber was transferred to the stage of a microscope. The vessel was set to an equilibration transmural pressure of 3 cm H2O and warmed to 38° C over 15-20 minutes. Once tone or spontaneous contractions developed, the vessel was allowed to equilibrate at 3 cm H2O for another 30 minutes. A video camera, hi-resolution monitor and DVD/HDD recorder were used to observe and record activity of the lymphatic segments continuously in all experiments.
For every experiment requiring functional tests, we evaluated the pressure-induced contractile responses of the lymphatic segments. Segments of mesenteric lymphatic vessels were exposed to a range of transmural pressures: 1, 3, 5 and 7 cm H2O for 5 minutes at each pressure; thoracic duct segments were exposed to pressures of 1, 2, 3 and 5 cm H2O. We chose these sets of transmural pressures because isolated rat mesenteric lymphatic vessels and thoracic duct display maximal active pumping at 5 cm H2O and 3 cm H2O, respectively (14,16,18,20). At the end of each experiment, the passive (relaxed) diameter was measured at each level of transmural pressure after the vessel was exposed to a nominally calcium-free, EDTA (3.0 mM)-supplemented physiological salt solution (PSS) (in mM: 145.0 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 5.0 dextrose, 2.0 sodium pyruvate, and 3.0 MOPS) for 15 minutes.
Data Analysis and Statistics for Isolated Vessel Experiments
Lymphatic diameters were tracked from the DVD records of experiments using “Vessel Track” software developed previously (9,10). We used cardiac pump analogies to define systole and diastole in reference to the lymphatic contractile cycle (3,16,23,46) and the end-diastolic and end-systolic points in the diameter tracings were recorded for each 5-minute interval for each set of pressures. To normalize changes in diameter from vessel to vessel, diastolic and systolic diameters were normalized to the passive lymphatic diameter in Ca-free PSS at the corresponding transmural pressure. The anatomy and basal contraction frequencies of lymphatic vessels in many species (rats, dogs, guinea pigs, humans etc.) are quite variable from vessel to vessel, even when similar vessels from age, weight and sex matched animals are studied (4,22,44). The normalization of lymphatic diameters minimizes animal-to-animal variability in basal lymphatic parameters and allows for a more sensitive investigation of the controlled parameters by minimizing the effect of random variation in vessel size between animals (17,20). From the lymphatic end-diastolic and end-systolic diameters, the following lymph pump parameters were calculated: lymphatic tone index (the difference between the passive lymphatic diameter in Ca-free PSS and end-diastolic diameter expressed as a percentage of the passive lymphatic diameter in Ca-free PSS), contraction amplitude (the difference between the normalized diastolic and systolic diameters), contraction frequency (number of contractions per minute), ejection fraction (the fraction of end-diastolic volume ejected during a single phasic lymphatic contraction) and fractional pump flow (an index of minute lymph pump flow, calculated as the ejection fraction times the contraction frequency).
Statistical differences were determined by two-way ANOVA, regression analysis and Student’s t-test (JMP software version 5.0.1.2. for Windows), as appropriate, and considered significant at P ≤ 0.05. In the Results section, the numbers of lymphatic vessels used in the reported data are shown separately for each group of experiments, where n depicts the number of lymphatic segments used for each experimental protocol.
Culture of isolated lymphatic vessels
Culture of lymphatic vessels under non-pressurized conditions
Following dissection, isolated segments of rat thoracic duct and mesenteric lymphatic vessels, sufficiently clean to perform subsequent functional tests after vessel culture, were gently rinsed ablumenally in sterile DPBS. This procedure was performed in standard 35-mm plastic Petri dishes filled with DPBS under a dissecting microscope to diminish possible vessel contamination. After this procedure, all subsequent manipulations were performed using sterile instruments, dishes, and solutions under a laminar flow hood. After two rinses in new Petri dishes, each lymphatic segment assigned for organ culture was transferred to another sterile 35-mm Petri dish filled with pre-warmed (38°C) D-MEM/F12 (Invitrogen Corp., cat.# 11039) supplemented with antibiotic mixture (Invitrogen, cat.# 15140) to achieve a concentration of 100 units of Penicillin/100  g of Streptomycin per ml of D-MEM/F12 (hereafter referred to as D-MEM/F12/a/b). Each lymphatic segment was placed (unpressurized) in a separate Petri dish and gently pressed to the bottom of the dish by lightly touching the edges with forceps to keep the segment in a nearly straight position on the dish bottom. Afterwards the Petri dishes were transferred into a cell culture incubator with 10% CO2 at 37°C and maintained for 3 to 16 days. During culture, all segments were checked daily and dishes with visible signs of contamination were discarded. The culture media was not changed. After the vessel culture period was complete, the lymphatic vessels were used for functional tests.
g of Streptomycin per ml of D-MEM/F12 (hereafter referred to as D-MEM/F12/a/b). Each lymphatic segment was placed (unpressurized) in a separate Petri dish and gently pressed to the bottom of the dish by lightly touching the edges with forceps to keep the segment in a nearly straight position on the dish bottom. Afterwards the Petri dishes were transferred into a cell culture incubator with 10% CO2 at 37°C and maintained for 3 to 16 days. During culture, all segments were checked daily and dishes with visible signs of contamination were discarded. The culture media was not changed. After the vessel culture period was complete, the lymphatic vessels were used for functional tests.
Culture of lymphatic vessels under pressurized conditions and the development of a model simulating chronically high lymph pressure
In separate protocols, isolated lymphatic segments were maintained in organ culture (up to 12 days) under conditions of controlled intralymphatic pressure. Rat mesenteric lymphatic vessel segments were isolated, cannulated and maintained in an isolated vessel chamber similar to that which used routinely for functional tests (19,20). The chamber, which was sterilized by overnight exposure to 100% alcohol, was filled with pre-warmed sterile (38°C) D-MEM/F12/a/b. The isolated lymphatic segment was cannulated and tied onto two alcohol-sterilized glass pipettes in the same manner as described above for acute functional tests. The chamber was transferred to the stage of a microscope. In one set of experiments, pressurized vessel segments were maintained for 3-4 days at an intraluminal pressure of 3 cm H2O (with a single case of daily observation for 12 days). In a second set of experiments, vessel segments were maintained overnight at an intraluminal pressure of 3 cm H2O to confirm the preservation of normal contractility, and then maintained for 3-4 days at an intraluminal pressure of 10 cm H2O. In those experiments, elevated intralymphatic pressure was used to simulate conditions that occur in different diseases of the lymphatic system involving partial or complete insufficiency of regional lymphatic drainage (5,12,34-36,38). Because pressurized, cultured lymphatic vessels were maintained outside of a laminar flow hood in a standard isolated vessel chamber attached to a microscope specifically designed for functional tests, we were able to perform functional tests (described above) at any time point during the culture conditions. We performed such tests at least two times daily throughout the protocol.
The following preventive measures reduced the likelihood of contamination in pressurized lymphatic vessel culture: 1. We used an internal culture room without windows, with diminished air flow in the room and with the room door closed whenever possible. 2. Everything in contact with the vessel was alcohol-sterilized (instruments, sutures, vessel chamber, glass pipettes and glass reservoirs). 3. Plastic tubing, stopcocks and connectors were replaced after use in every experiment. 4. The upper cover of the vessel chamber was closed during culture. 5. The input and output reservoirs were covered with parafilm to prevent contamination by airborne particles. 6. To eliminate the need to recannulate the vessel when air bubbles formed inside the pipettes or tubing, we continuously heated the D-MEM/F12/a/b solution in the input pressure line to 38°C. 7. A slow-speed (0.15 ml/min) peristaltic pump was used to constantly replenish D-MEM/F12/a/b at 38°C through the isolated vessel chamber and each opened bottle of freshly prepared D-MEM/F12/a/b was used within a 24-hour period with any remaining solution discarded.
Transfections of isolated rat mesenteric lymphatic vessels: development of protocol using a GFP reporter gene
To develop protocols that would effectively transfect lymphatic muscle cells in intact vessels without impairment of lymphatic contractile function, we used recombinant adenoviral vectors to express a fluorescent reporter signal, green fluorescent protein (GFP). Transfected vessels were imaged confocally to determine the intensity of the signal in cells of the lymphatic wall. The segments were observed at 40-50× magnification using a Leica AOBS SP2 Confocal-Multiphoton Microscope System and confocal images were acquired at 0.3 um intervals via 489 nm of peak excitation wavelength and 508 nm peak emission wavelength in order to completely image the entire lymphatic vessel. The image stacks were reconstructed using different 3D perspectives to produce the projections using the Leica confocal software. After preliminary tests to optimize the adenoviral GFP (AdGFP) transfections, we were able to transfect more than 90% of lymphatic muscle cells. A subset of the AdGFP-transfected vessels was checked functionally as described above in Isolated Lymphatic Vessel Procedures for Functional Tests: Experimental Techniques and Protocols.
Experimental protocol for adenoviral transfection of isolated mesenteric lymphatic vessels by AdGFP constructs
The GFP gene was cloned into the pShuttleCMV plasmid and used to generate a recombinant adenovirus (AdGFP) under the control of the cytomegalovirus immediate-early promoter, using the AdEasy system (25), as previously described (6). Purified virus was used to transfect lymphatic muscle cells in whole isolated lymphatic vessels as described below. In total we performed more than 50 transfections and are providing here a summary of the optimal experimental protocol. Our criteria for successful transfection included finding 85-90% GFP-positive muscle cells in lymphatic wall; 12 out of the last 14 transfected lymphatic vessels met these criteria.
We performed adenoviral GFP (AdGFP) transfections in the smallest possible volumes as practical. We slightly modified for these purposes the standard 0.65 ml plastic tubes (VWR Cat. # 53550-100). Their caps were separated; three holes were penetrated through them: one hole allowed insertion of a small diameter plastic tube ~10 cm length (VWR #63010-007), two other holes helped to relieve pressure inside tube after closure, therefore preventing the prolapse of the lymphatic wall inside the micropipette. Plastic tubing from the inner cap side was connected with ~1 cm length glass micropipette with a tip diameter of ~ 100-120  m (made from capillary glass tubes; Drummon Scientific #9-000-3000); the other end of the tubing was connected to a 1 ml syringe. After the plastic tubing and pipette were filled by D-MEM/F12/a/b and positioned in a large plastic Petri dish, the lymphatic segments were cannulated at the upstream end onto the micropipette and the cannulated end of the vessel was secured by 6-0 suture with the output end remaining open. This method of handling the lymphatic segment allowed us to replace solutions inside the vessel.
m (made from capillary glass tubes; Drummon Scientific #9-000-3000); the other end of the tubing was connected to a 1 ml syringe. After the plastic tubing and pipette were filled by D-MEM/F12/a/b and positioned in a large plastic Petri dish, the lymphatic segments were cannulated at the upstream end onto the micropipette and the cannulated end of the vessel was secured by 6-0 suture with the output end remaining open. This method of handling the lymphatic segment allowed us to replace solutions inside the vessel.
The solution containing the AdGFP construct was prepared by the following method. Immediately before loading the isolated vessels for transfection, the adenoviral constructs were mixed with antennapedia leader peptide CT (ALP-CT) (Anaspec, Inc., catalog # 61376) by mixing equal volumes of ALP-CT stock solution to non-diluted adenoviral construct stock solution (1*1013 viral particles/ml) for 30 minutes at room temperature. Antennapedia leader peptide is known to increase viral transfection efficiency in intact vessels (24). In our experience, supplementation of viral particles with this peptide increased AdGFP efficiency of lymphatic muscle cells from ~10-15% to ~85-90%. After pretreatment of AdGFP constructs by ALP-CT, we diluted this mixture by D-MEM/F12/a/b to produce a final titer of viral particles ranging between 1.1 and 1.5*1010 viral particles/ml.
After the vessel segment was cannulated and secured at its upstream end with suture to the glass micropipette (110-130  m diameter of the tip), the vessel lumen was flushed with ~0.2 ml of D-MEM/F12/a/b using a 1 ml syringe. For denudation of the endothelium, an air bolus was inserted into the vessel lumen to replace all the fluid inside of the vessel with air (the syringe was disconnected from the opened input tubing and ~0.05 ml of air was sucked into it). The air bolus was maintained inside the vessel for 10-12 sec, and then the vessel was immediately flushed with ~0.2 ml D-MEM/F12/a/b from the syringe to allow complete replacement of air with fluid. Whether or not endothelial denudation was performed in different vessels, the D-MEM/F12/a/b solution inside of the lymphatic vessel was replaced by ALP-CT / AdGFP -solution from another 1 ml syringe and the vessel was tied at its output end after it was filled with the virus suspension. The syringe was disconnected from the plastic tubing, leaving the input end of the vessel open. The 0.65
m diameter of the tip), the vessel lumen was flushed with ~0.2 ml of D-MEM/F12/a/b using a 1 ml syringe. For denudation of the endothelium, an air bolus was inserted into the vessel lumen to replace all the fluid inside of the vessel with air (the syringe was disconnected from the opened input tubing and ~0.05 ml of air was sucked into it). The air bolus was maintained inside the vessel for 10-12 sec, and then the vessel was immediately flushed with ~0.2 ml D-MEM/F12/a/b from the syringe to allow complete replacement of air with fluid. Whether or not endothelial denudation was performed in different vessels, the D-MEM/F12/a/b solution inside of the lymphatic vessel was replaced by ALP-CT / AdGFP -solution from another 1 ml syringe and the vessel was tied at its output end after it was filled with the virus suspension. The syringe was disconnected from the plastic tubing, leaving the input end of the vessel open. The 0.65  l plastic tube was filled by D-MEM/F12/a/b (with or without virus at 1.1*1010 viral particles/ml – see Results), and then the vessel, pipette and plastic cap were rapidly transferred from the Petri dish and inserted into the plastic tube. Care was taken to avoid puncturing the vessel or bending it at the pipette tip. Next, the plastic tube, containing the cannulated vessel, was positioned into a tube rack so that the open end of the plastic tubing, filled with fluid, was placed ~5 cm above the pipette tip to allow the vessel to be pressurized at all times during the subsequent culture. The tube rack was placed into a cell culture incubator (10% CO2, 37°C) for the required number of hours for transfection with subsequent confocal imaging and functional tests as described above. The vessels were checked daily. Any vessels with visible signs of contamination were discarded.
l plastic tube was filled by D-MEM/F12/a/b (with or without virus at 1.1*1010 viral particles/ml – see Results), and then the vessel, pipette and plastic cap were rapidly transferred from the Petri dish and inserted into the plastic tube. Care was taken to avoid puncturing the vessel or bending it at the pipette tip. Next, the plastic tube, containing the cannulated vessel, was positioned into a tube rack so that the open end of the plastic tubing, filled with fluid, was placed ~5 cm above the pipette tip to allow the vessel to be pressurized at all times during the subsequent culture. The tube rack was placed into a cell culture incubator (10% CO2, 37°C) for the required number of hours for transfection with subsequent confocal imaging and functional tests as described above. The vessels were checked daily. Any vessels with visible signs of contamination were discarded.
RESULTS
Cultured non-pressurized lymphatic vessels
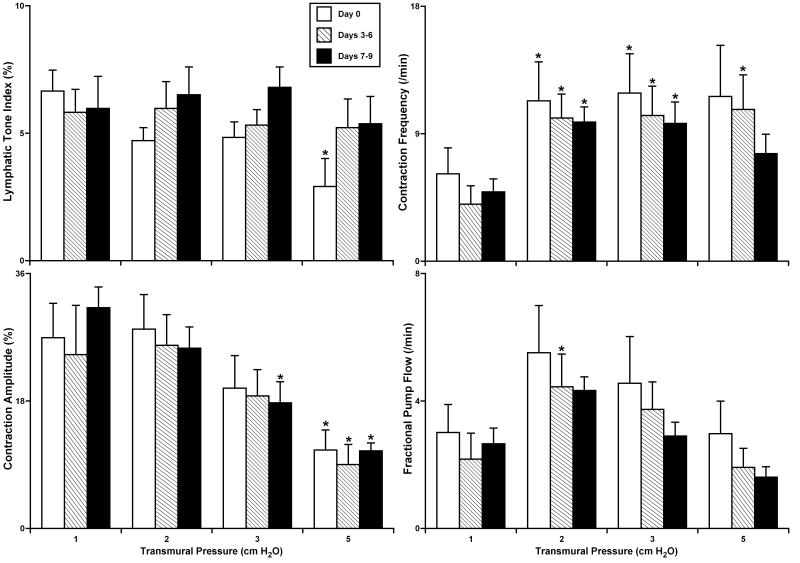
Fig. 1 summarizes the results of experiments with culture of non-pressurized isolated segments of rat thoracic duct. At day 0 (the control group), these vessels demonstrated typical contractile responses that have been well-characterized in the recent literature (19,20) with optimal lymphatic pumping obtained at 2-3 cm H2O intraluminal pressure. Vessel culture through day 9 did not significantly change any of the measured/calculated parameters of lymphatic contractility: lymphatic tone index, contraction amplitude (amplitude of phasic contractions), contraction frequency and fractional pump flow. At culture days 7-9 we observed only a small but insignificant trend of reductions in contraction frequency and pump flow, together with a slight increase in lymphatic tone. In the group of vessels cultured for 14-16 days (data not shown), we observed a significant decline of lymphatic phasic contraction amplitude in the thoracic duct segments yet without a complete cessation of spontaneous phasic contractions.
Figure 1.
The parameters of lymphatic contractile activity in isolated segments of the rat thoracic duct after culture under non-pressurized conditions.
Day 0 – parameters of lymphatic contractile activity in control group of non-cultured lymphatic segments (n=6); Days 3-6 - parameters of lymphatic contractile activity in group of lymphatic segments cultured for 3-6 days (n=7); Days 7-9 – similar parameters for segments cultured for 7-9 days (n=8). *Indicates significant differences (P ≤ 0.05) within each group between parameters at an initial level of transmural pressure of 1 cm H2O versus all other levels of transmural pressure during the acute functional tests.
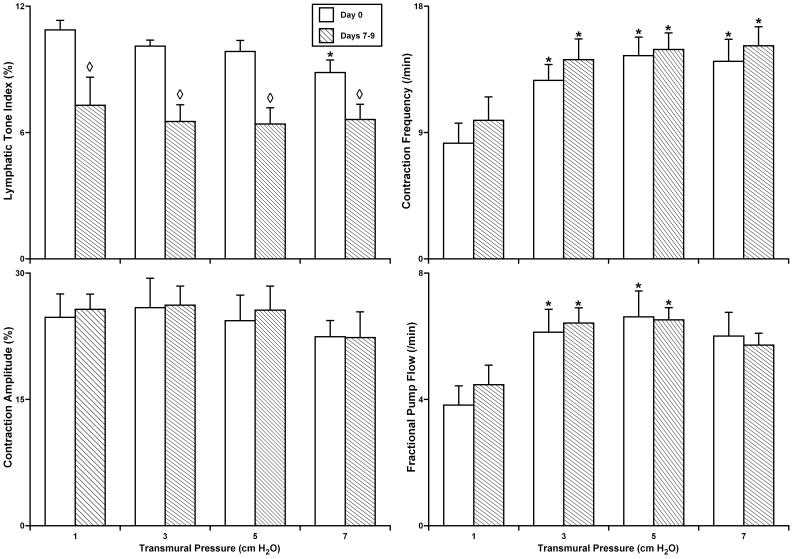
Fig. 2 summarizes the results of similar experiments with culture of non-pressurized rat isolated segments of the mesenteric lymphatic vessels. At day 0 (the control group) these vessels demonstrated the features of their contractile responses similar to those described recently (16), with optimal lymphatic pumping near 5 cm H2O intraluminal pressure. Vessel culture conditions up to 9 days did not significantly affect the contraction amplitude, contraction frequency or fractional pump flow in cultured, unpressurized mesenteric lymphatic segments. However, after 7-9 days of culture, lymphatic tone in these segments was significantly decreased – on average by 34.5% at intraluminal pressures from 1-5 cm H2O, and by 25.2% at 7 cm H2O. In the group of mesenteric lymphatic vessels cultured for 14-16 days (data not shown) we also observed significant inhibition of phasic contraction amplitude yet without a complete cessation of spontaneous phasic contractions.
Figure 2.
The parameters of lymphatic contractile activity in isolated segments of the rat mesenteric lymphatic vessels after culture under non-pressurized conditions.
Day 0 – parameters of lymphatic contractile activity in control group of non-cultured lymphatic segments (n=5); Days 7-9 - parameters of lymphatic contractile activity in group of lymphatic segments cultured for 7-9 days (n=10). *Indicates significant differences (P ≤ 0.05) within each group between parameters at initial level of transmural pressure of 1 cm H2O and at all other levels of transmural pressure during the acute functional tests; ◇ Indicates significant differences (P ≤ 0.05) between lymphatic tone index values in Day 0 and Days 7-9 groups.
Cultured pressurized lymphatic vessels
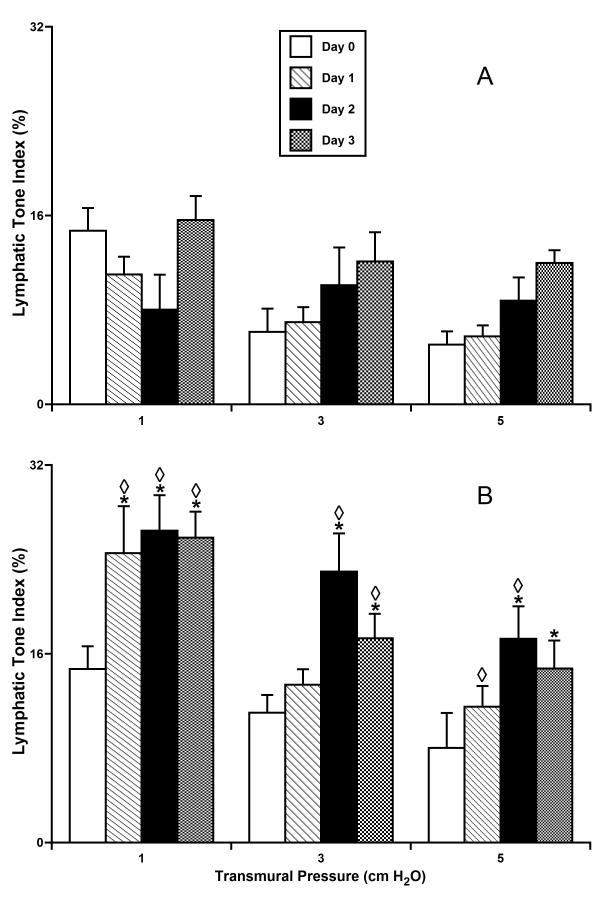
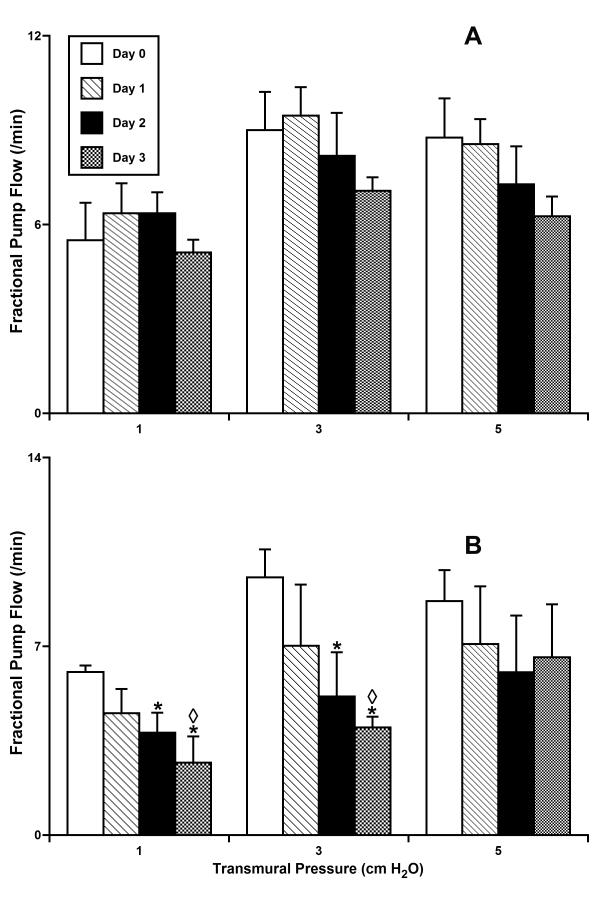
Figs. 3-6 summarize the results of experiments with culture of isolated rat mesenteric lymphatic vessels under pressurized conditions. In each figure the data were analyzed in two groups – vessels maintained at 3 cmH2O for the entire time in culture (panel A) and vessels initially incubated overnight at 3 cmH2O (to verify normal contractile function) and then subsequently maintained at 10 cmH2O (panel B).
Figure 3.
Lymphatic tone in isolated segments of the rat mesenteric lymphatic vessels cultured at normal (A - 3 cm H2O; n=5) or high (B - 10 cm H2O; n=4) intraluminal pressures.
*Indicates significant differences (P ≤ 0.05) within each group (A and B) between lymphatic tone index at day 0 of culture (control group) and at all other days of culture; shown separate for each level of transmural pressure during the acute functional tests; ◇ Indicates significant differences (P ≤ 0.05) between lymphatic tone index values in groups A and B for each given pressure/day pair.
Figure 6.
Fractional pump flow in isolated segments of the rat mesenteric lymphatic vessels cultured at normal (A - 3 cm H2O; n=5) or high (B - 10 cm H2O; n=4) intraluminal pressures.
*Indicates significant differences (P ≤ 0.05) within each group (A and B) between fractional pump flow at day 0 of culture (control group) and at all other days of culture; shown separate for each level of transmural pressure during the acute functional tests; ◇ Indicates significant differences (P ≤ 0.05) between fractional pump flow values in groups A and B for each given pressure/day pair.
None of the parameters of lymphatic function - lymphatic tone index, lymphatic phasic contraction amplitude, contraction frequency and fractional pump flow, were significantly changed during 3 days of culture at a pressure of 3 cm H2O. The contractile activity of these vessels initially was in the normal range (compared to previous acute studies of function (16,17,19)) and was not significantly different from that of vessels tested at day 0 of culture at an intraluminal pressure of 3 cm H2O (the control group). In contrast, over the first two days of culture at 10 cm H2O, lymphatic tone was dramatically increased (Fig. 3B) compared to tone at day 0 before exposure to the high pressure conditions. For example, during the first two days of culture at high intraluminal pressure, the vessels demonstrated 2.2- and 2.4-fold increases in tone at an intraluminal test pressure of 1 cm H2O at days 1 and 2, respectively. During these first two days similar trends were observed for the other levels of intraluminal pressure. However, lymphatic tone declined toward normal levels after 3 days in culture at 10 cm H2O (Fig. 3).
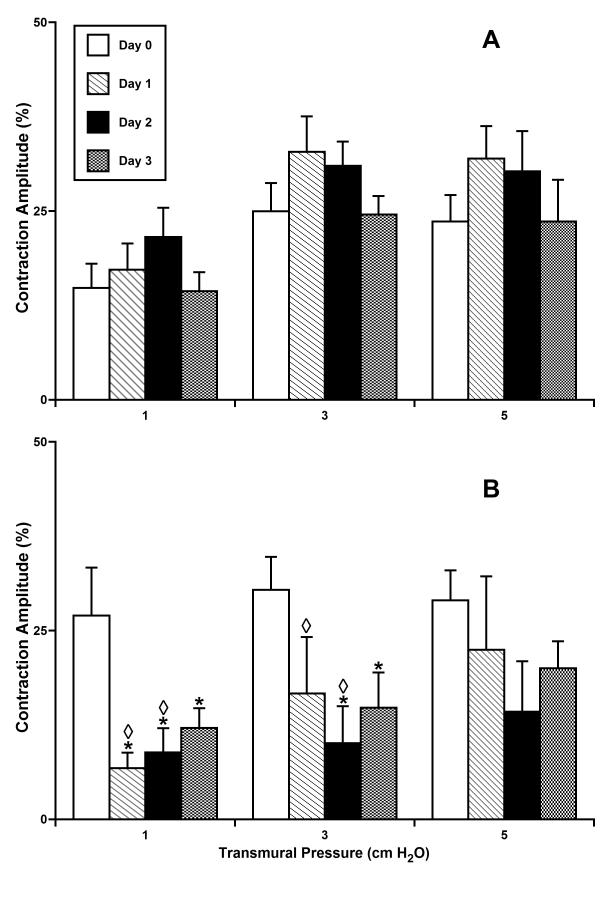
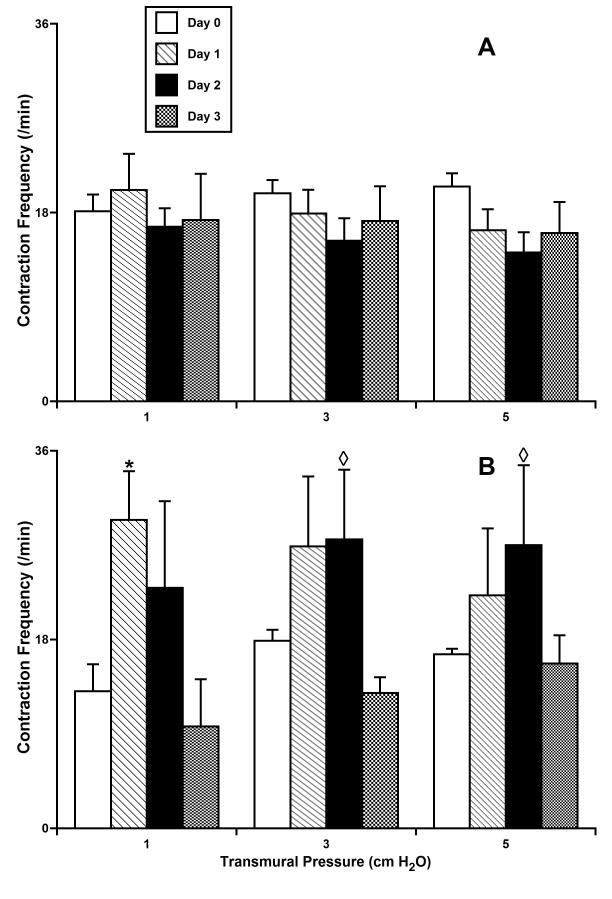
Fig. 4 shows the phasic contraction amplitude for the same vessel groups pressurized and cultured for 3 days at 3 or 10 cm H2O. Contraction amplitude was significantly diminished in lymphatic vessels cultured at 10 cm H2O (Fig. 4B). For instance, at a test pressure of 1 cm H2O we observed 75% and 67% decreases in contraction amplitude for high-pressure days 1 and 2, respectively, compared to their amplitude before culture at high pressure. However, by day 3 of culture at 10 cm H2O, we observed a slight trend toward the restoration of normal contraction amplitude. High-pressure culture, opposite to culture at 3 cm H2O, produced a rise in contraction frequency, as shown in Figure 5. The maximal increases in contraction frequency were observed after days 1 and 2 under high-pressure, whereas at day 3 of high pressure this positive chronotropic effect virtually disappeared (Fig. 5B). As a result of the counterbalancing effect of reduced amplitude but increased frequency after chronic exposure to high pressure, fractional pump flow did not dramatically decline in overall function up to day 3 of culture at 10 cm H2O (Fig. 6B). However, there was a consistent, albeit not significant, trend toward the decompensation of lymphatic pumping over time under high-pressure culture conditions.
Figure 4.
Contraction amplitude in isolated segments of the rat mesenteric lymphatic vessels cultured at normal (A - 3 cm H2O; n=5) or high (B - 10 cm H2O; n=4) intraluminal pressures.
*Indicates significant differences (P ≤ 0.05) within each group (A and B) between contraction amplitude at day 0 of culture (control group) and at all other days of culture; shown separate for each level of transmural pressure during the acute functional tests; ◇ Indicates significant differences (P ≤ 0.05) between contraction amplitude values in groups A and B for each given pressure/day pair.
Figure 5.
Contraction frequency in isolated segments of the rat mesenteric lymphatic vessels cultured at normal (A - 3 cm H2O; n=5) or high (B - 10 cm H2O; n=4) intraluminal pressures.
*Indicates significant differences (P ≤ 0.05) within each group (A and B) between contraction frequency at day 0 of culture (control group) and at all other days of culture; shown separate for each level of transmural pressure during the acute functional tests; ◇ Indicates significant differences (P ≤ 0.05) between contraction frequency values in groups A and B for each given pressure/day pair.
Adenoviral transfection of cultured isolated mesenteric lymphatic vessels by AdGFP constructs
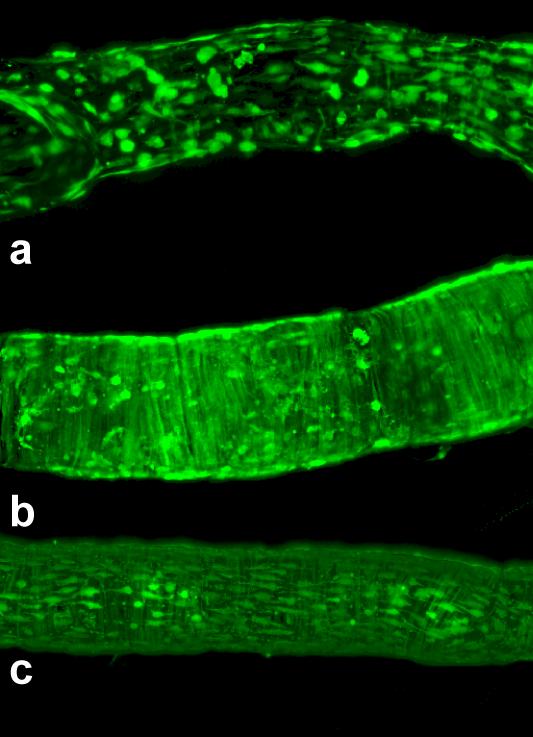
Figure 7 shows images of AdGFP-treated isolated rat mesenteric lymphatic vessels, illustrating successful transfection of both major types of cells in the lymphatic wall - endothelial and muscle cells - separately as well as together, depending on variations in the experimental procedure. After several tests, we found that for optimal AdGFP transfection, the incubation time was ~66-74 hours. By varying the concentration of viral particles inside and outside the cultured vessels and by using denuded or non-denuded vessels, we could achieve combined or separate GFP expression in lymphatic endothelial and/or muscle cells. For fairly selective transfection of lymphatic endothelial cells in isolated rat mesenteric lymphatic vessels, it was sufficient to inject DMEMF12a/b containing 1.1*1010 viral particles/ml only into the lumen of a non-denuded vessel (Fig. 7A). To transfect only lymphatic muscle cells in endothelium-denuded vessels, a concentration of 1.5*1010 viral particles/ml only in the lumen of a denuded vessel was generally sufficient (Fig. 7B), whereas transfection of both lymphatic endothelial and muscle cells required at least 1.1*1010 viral particles/ml-containing solution outside of the vessel together with 1.5*1010 viral particles/ml-containing solution into the lumen of the vessel (Fig. 7 C).
Figure 7.
Examples of effective adenoviral/GFP transfection of lymphatic endothelial and muscle cells in isolated rat mesenteric lymphatic vessels. Vessel diameters are ~ 120-140 μm. Average 2-D projections of the stacks of confocal images, taken at steps of 0.5 μm through the vessels pressurized at 5 cm H2O in the standard isolated vessel chamber.
a. Transfection of mostly lymphatic endothelial cells was performed using solution containing 1.1*1010 viral particles/ml which was injected only inside of non-denuded vessel; b. Transfection of only lymphatic muscle cells in endothelium-denuded vessels using a concentration of 1.5*1010 viral particles/ml only intraluminally; c. Transfection of both lymphatic endothelial and muscle cells required a solution outside the vessel containing 1.1*1010 viral particles/ml together with an intraluminal solution containing 1.5*1010 viral particles/ml.
We also performed functional tests on AdGFP-transfected vessels (n=4), prior to confocal detection of the GFP signal in lymphatic muscle cells. Figure 8 presents an example of the normal phasic contraction pattern that was observed at three different pressures and the corresponding GFP expression in the muscle layer of the same vessel transfected with AdGFP ablumenally. The parameters of contractile activity of these vessels were similar to recent descriptions of acute function (16).
Figure 8.
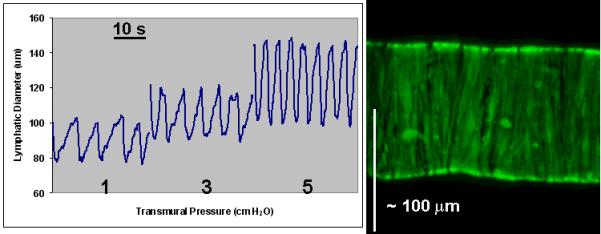
An example of the normal phasic contraction patterns at three different pressures was observed prior to demonstration of GFP expression by lymphatic muscle cells in the same isolated rat mesenteric lymphatic vessel. Average 2-D projections of the stacks of confocal images, taken at steps of 0.5 μm through the vessel while pressurized at 5 cm H2O.
DISCUSSION
In this study we performed for the first time the maintenance of non-pressurized and pressurized rat isolated lymphatic vessels in tissue culture conditions for periods of 3-12 days with preservation of normal function, and with the option of continuously monitoring their contractility at different intralymphatic pressures. The vessels could be maintained for up to 10-12 days without substantial impairment of regular spontaneous contractions. Finally, we also report for the first time effective gene transfection of lymphatic endothelial and muscle cells in isolated, cultured intact lymphatic vessels.
During the present studies we were able for the first time to develop experimental protocols to perform non-pressurized isolated lymphatic vessel culture for up to 9 days without substantial compromise of the normal contractile patterns. Such conditions may be applicable to subsequent transfection experiments to overexpress or knockdown genes involved in the regulation of lymphatic contractility. We believe that daily changes of the outside solution may be used to further improve the technique to effectively maintain the contractile function of the lymphatic vessels cultured for longer periods of time. However, to avoid the potential influence of moderate changes in lymphatic tone on phasic contractile activity of transfected lymphatic segments, we performed subsequent transfections on pressurized lymphatic segments (as described in Methods). We concluded that culture and transfection protocols of isolated lymphatic segments should utilize a constantly open, pressurized vessel lumen.
We also developed experimental techniques to culture pressurized rat isolated lymphatic vessels, with the option of continuously monitoring their contractility at different intralymphatic pressures. We found that after one day at a constantly elevated intralymphatic pressure, a profound restructuring of the contractile pattern in lymphatic muscle cells was underway. While the mechanisms involved in such regulatory reactions of lymphatic vessels will be the subject of a separate research project, we have already demonstrated the feasibility of this approach to develop an experimental model of the high lymph pressure conditions that characterize several lymphatic pathologies. In addition, the ability to continuously monitor lymphatic diameter and contractile function during organ culture of isolated lymphatic vessels offers the opportunity to simulate different disorders of lymphatic transport that are characterized by increases in intralymphatic pressure. Such an experimental model would be helpful to probe mechanisms of pathogenesis in these diseases and to develop the potential preventive measures and treatment for several kinds of lymphedema.
Finally we performed for the first time gene transfection of lymphatic muscle and endothelial cells in isolated whole vessel lymphatic segments without significantly altering function via the transfection procedure per se. Future improvements of our transfection approach will likely provide powerful tools to lymphologists to precisely target genes involved in the regulation of lymphatic contractility and provide an alternative to the use of genetically modified mouse models to investigate the mechanisms of lymphatic contractile function.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants HL-070308, HL-075199, AG-030578, HL-080526, HL-089784 and KO2 HL-086650.
The authors thank Aleksey Gashev and Sheng (Max) Yu for their help in primary data analysis of functional tests of lymphatic vessels and thank Dr. Andreea Trache and the Texas A&M Health Science Center Microscopy Imaging Facility for their help with the confocal imaging studies.
REFERENCES
- 1.Abels C, Fickweiler S, Weiderer P, Baumler W, Hofstadter F, Landthaler M, Szeimies RM. Indocyanine green (icg) and laser irradiation induce photooxidation. Arch Dermatol Res. 2000;292:404–411. doi: 10.1007/s004030000147. [DOI] [PubMed] [Google Scholar]
- 2.Aukland K. Arnold heller and the lymph pump. Acta Physiol Scand. 2005;185:171–180. doi: 10.1111/j.1365-201X.2005.01470.x. [DOI] [PubMed] [Google Scholar]
- 3.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am. J. Physiol. 1989;257:H2059–2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- 4.Borisov AV. The theory of the design of the lymphangion. (in russian) Morfologiia. 1997;112:7–17. [PubMed] [Google Scholar]
- 5.Browse N, Burnand KG, Mortimer PS. Diseases of the lymphatics. Arnold, Hodder Headline Group; London: 2003. [Google Scholar]
- 6.Cai S, Alp NJ, McDonald D, Smith I, Kay J, Canevari L, Heales S, Channon KM. Gtp cyclohydrolase i gene transfer augments intracellular tetrahydrobiopterin in human endothelial cells: Effects on nitric oxide synthase activity, protein levels and dimerisation. Cardiovasc Res. 2002;55:838–849. doi: 10.1016/s0008-6363(02)00460-1. [DOI] [PubMed] [Google Scholar]
- 7.Cammalleri M, Cervia D, Dal Monte M, Martini D, Langenegger D, Fehlmann D, Feuerbach D, Pavan B, Hoyer D, Bagnoli P. Compensatory changes in the hippocampus of somatostatin knockout mice: Upregulation of somatostatin receptor 2 and its function in the control of bursting activity and synaptic transmission. Eur J Neurosci. 2006;23:2404–2422. doi: 10.1111/j.1460-9568.2006.04770.x. [DOI] [PubMed] [Google Scholar]
- 8.Cho JH, Klein WH, Tsai MJ. Compensational regulation of bhlh transcription factors in the postnatal development of beta2/neurod1-null retina. Mech Dev. 2007;124:543–550. doi: 10.1016/j.mod.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis MJ. An improved, computer-based method to automatically track internal and external diameter of isolated microvessels. Microcirculation. 2005;12:361–372. doi: 10.1080/10739680590934772. [DOI] [PubMed] [Google Scholar]
- 10.Davis MJ, Zawieja DC, Gashev AA. Automated measurement of diameter and contraction waves of cannulated lymphatic microvessels. Lymphat Res Biol. 2006;4:3–10. doi: 10.1089/lrb.2006.4.3. [DOI] [PubMed] [Google Scholar]
- 11.Denofrio JC, Yuan W, Temple BR, Gentry HR, Parise LV. Characterization of calcium- and integrin-binding protein 1 (cib1) knockout platelets: Potential compensation by cib family members. Thromb Haemost. 2008;100:847–856. [PMC free article] [PubMed] [Google Scholar]
- 12.Foldi M, Foldi E. Foldi’s texbook of lymphology. Elsevier; Munich, Germany: 2006. [Google Scholar]
- 13.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Gashev AA. Physiologic aspects of lymphatic contractile function: Current perspectives. Ann. N .Y. Acad. Sci. 2002;979:178–187. doi: 10.1111/j.1749-6632.2002.tb04878.x. discussion 188-196. [DOI] [PubMed] [Google Scholar]
- 15.Gashev AA. Lymphatic vessels: Pressure- and flow-dependent regulatory reactions. Ann. N .Y. Acad. Sci. 2008;1131:100–109. doi: 10.1196/annals.1413.009. [DOI] [PubMed] [Google Scholar]
- 16.Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation. 2004;11:477–492. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- 17.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J. Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gashev AA, Delp MD, Zawieja DC. Inhibition of active lymph pump by simulated microgravity in rats. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2295–2308. doi: 10.1152/ajpheart.00260.2005. [DOI] [PubMed] [Google Scholar]
- 19.Gasheva OY, Knippa K, Nepiushchikh ZV, Muthuchamy M, Gashev AA. Age-related alterations of active pumping mechanisms in rat thoracic duct. Microcirculation. 2007;14:827–839. doi: 10.1080/10739680701444065. [DOI] [PubMed] [Google Scholar]
- 20.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated no-dependent lymphatic relaxation: A self-regulatory mechanism in rat thoracic duct. J. Physiol. 2006;575:821–832. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlai R. Gene targeting: Technical confounds and potential solutions in behavioral brain research. Behav Brain Res. 2001;125:13–21. doi: 10.1016/s0166-4328(01)00282-0. [DOI] [PubMed] [Google Scholar]
- 22.Gnepp DR. Lymphatics. In: Staub NC, Taylor AE, editors. Edema. Raven Press; New York: 1984. pp. 263–298. [Google Scholar]
- 23.Granger HJ, Kovalcheck S, Zweifach BW, Barnes GE. Quantitative analysis of active lymphatic pumping. Proceedings of the VII Summer Computer Simulation Conference; Simulation Council, La Jolla, CA. 1977. pp. 562–565. [Google Scholar]
- 24.Gratton JP, Yu J, Griffith JW, Babbitt RW, Scotland RS, Hickey R, Giordano FJ, Sessa WC. Cell-permeable peptides improve cellular uptake and therapeutic gene delivery of replication-deficient viruses in cells and in vivo. Nat. Med. 2003;9:357–362. doi: 10.1038/nm835. [DOI] [PubMed] [Google Scholar]
- 25.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon S, Sevick-Muraca EM. Noninvasive quantitative imaging of lymph function in mice. Lymphat Res Biol. 2007;5:219–231. doi: 10.1089/lrb.2007.1013. [DOI] [PubMed] [Google Scholar]
- 27.Miosge N, Sasaki T, Timpl R. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol. 2002;21:611–621. doi: 10.1016/s0945-053x(02)00070-7. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno R, Ono N, Nojiri H, Ikomi F, Ohhashi T. Spontaneous pumping activity of rat and mice isolated lymph microvessels. Microcirculation Annual. 1998;14:69–70. [Google Scholar]
- 29.Mizuno R, Ono N, Ohhashi T. Parathyroid hormone-related protein-(1-34) inhibits intrinsic pump activity of isolated murine lymph vessels. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H60–66. doi: 10.1152/ajpheart.2001.281.1.H60. [DOI] [PubMed] [Google Scholar]
- 30.Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. 2003;17:920–922. doi: 10.1096/fj.02-0626fje. [DOI] [PubMed] [Google Scholar]
- 31.Muthuchamy M, Zawieja D. Molecular regulation of lymphatic contractility. Ann. N .Y. Acad. Sci. 2008;1131:89–99. doi: 10.1196/annals.1413.008. [DOI] [PubMed] [Google Scholar]
- 32.Nojiri H, Ono N, Mizuno R, Ikomi F, Ohhashi T. Spontaneous contractions in mice mesenteric lymph vessels: With special reference to arrangement of smooth muscle layers in the walls. Microcirculation Annual. 1998;14:67–68. [Google Scholar]
- 33.Ohhashi T, Mizuno R, Ikomi F, Kawai Y. Current topics of physiology and pharmacology in the lymphatic system. Pharmacol Ther. 2005;105:165–188. doi: 10.1016/j.pharmthera.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Olszewski W. Surgical pathophysiology of the lymphatic system. Z Exp Chir. 1981;14:9–20. [PubMed] [Google Scholar]
- 35.Olszewski WL. Contractility patterns of normal and pathologically changed human lymphatics. Ann. N .Y. Acad. Sci. 2002;979:52–63. doi: 10.1111/j.1749-6632.2002.tb04867.x. discussion 76-59. [DOI] [PubMed] [Google Scholar]
- 36.Olszewski WL. Contractility patterns of human leg lymphatics in various stages of obstructive lymphedema. Ann. N .Y. Acad. Sci. 2008;1131:110–118. doi: 10.1196/annals.1413.010. [DOI] [PubMed] [Google Scholar]
- 37.Ono N, Mizuno R, Nojiri H, Ohhashi T. Development of an experimental apparatus for investigating lymphatic pumping activity of murine mesentery in vivo. Jpn J Physiol. 2000;50:25–31. doi: 10.2170/jjphysiol.50.25. [DOI] [PubMed] [Google Scholar]
- 38.Rockson SG. Lymphedema. Curr Treat Options Cardiovasc Med. 2006;8:129–136. doi: 10.1007/s11936-006-0005-y. [DOI] [PubMed] [Google Scholar]
- 39.Schmid-Schonbein G, Zweifach B. Fluid pump mechanisms in initial lymphatics. News Physiol Sci. 1994;9:67. [Google Scholar]
- 40.Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiological Reviews. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- 41.Sharma R, Wendt JA, Rasmussen JC, Adams KE, Marshall MV, Sevick-Muraca EM. New horizons for imaging lymphatic function. Ann. N .Y. Acad. Sci. 2008;1131:13–36. doi: 10.1196/annals.1413.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi E, Ino M, Miyamoto N, Nagasu T. Increased expression of p/q-type ca2+ channel alpha1a subunit mrna in cerebellum of n-type ca2+ channel alpha1b subunit gene-deficient mice. Brain Res Mol Brain Res. 2004;124:79–87. doi: 10.1016/j.molbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Hummler E, Maillard M, Nussberger J, Rossier BC, Brunner HR, Burnier M. Compensatory up-regulation of angiotensin ii subtype 1 receptors in alpha enac knockout heterozygous mice. Kidney Int. 2001;59:2216–2221. doi: 10.1046/j.1523-1755.2001.00739.x. [DOI] [PubMed] [Google Scholar]
- 44.Yoffey JM, Courtice FC. Lymphatics, lymph and the lymphomyeloid complex. Academic Press; London, New York: 1970. [Google Scholar]
- 45.Zawieja DC, Davis KL. Inhibition of the active lymph pump in rat mesenteric lymphatics by hydrogen peroxide. Lymphology. 1993;26:135–142. [PubMed] [Google Scholar]
- 46.Zawieja DC, Greiner ST, Davis KL, Hinds WM, Granger HJ. Reactive oxygen metabolites inhibit spontaneous lymphatic contractions. Am. J. Physiol. 1991;260:H1935–1943. doi: 10.1152/ajpheart.1991.260.6.H1935. [DOI] [PubMed] [Google Scholar]