Abstract
Rationale
Diabetes mellitus is frequently complicated by cardiovascular disease, such as vascular calcification and endothelial dysfunction, which have been associated with bone morphogenetic proteins (BMPs).
Objective
To determine whether hyperglycemia in vitro and diabetes in vivo promote vascular BMP activity and correlate with vascular calcification.
Methods and Results
Increased glucose augmented expression of BMP-2 and BMP-4; the BMP inhibitors matrix Gla protein (MGP) and Noggin; activin-like kinase receptor (ALK)1, -2, -3 and -6; the BMP type 2 receptor; and the vascular endothelial growth factor in human aortic endothelial cells (HAECs). Diabetes induced expression of the same factors in the aortic wall of 3 animal models of diabetes, Ins2Akita/+ mice, db/db mice, and HIP rats (rats transgenic for human islet amyloid polypeptide), representative of types 1 and 2 diabetes. Conditioned media from glucose-treated HAECs increased angiogenesis in bovine aortic endothelial cells, as mediated by BMP-4, and osteogenesis in calcifying vascular cells, as mediated by BMP-2. BMP-4, MGP, ALK1, and ALK2 were predominantly expressed on the endothelial side of the aorta, and small interfering RNA experiments showed that these genes were regulated as a group. Diabetic mice and rats showed a dramatic increase in aortic BMP activity, as demonstrated by SMAD1/5/8 phosphorylation. This was associated with increased osteogenesis and calcium accumulation. These changes were prevented in the Ins2Akita/+ mice by breeding them with MGP transgenic mice, which increased aortic BMP inhibition.
Conclusions
Hyperglycemia and diabetes activate vascular BMP activity, which is instrumental in promoting vascular calcification and may be limited by increasing BMP inhibition.
Keywords: diabetes mellitus, bone morphogenetic protein, vascular calcification, mouse models, endothelial cells
Diabetes mellitus is associated with severe cardiovascular complications, including vascular calcification and accelerated atherosclerosis, leading to increased morbidity and mortality in diabetic patients.1–3 Vascular calcification is frequently seen in 1 of 2 forms, atherosclerotic lesion calcification and medial calcification (also referred to as media sclerosis or Mönckeberg disease). Medial calcification, in particular, is considered to be a characteristic of diabetes4 and occurs along the elastic lamellae. Both types of calcification involve activation of osteogenic cell differentiation.5,6 Diabetes also causes endothelial dysfunction,7–9 which promotes diabetic vascular disease. Even though a number of signaling pathways have been implicated in diabetic vasculopathy,5–7,10 the links between hyperglycemia and vascular disease are still incompletely understood.
Bone morphogenetic protein (BMP)-2 and -4 are inflammatory mediators in vascular endothelium responsive to disturbed flow, increased oxidative stress, and inflammation.11,12 Increased BMP activity enhances atherogenesis and vascular calcification13–15 and may play a role in ocular angiogenesis in diabetic retinopathy.16
The BMPs belong to the transforming growth factor (TGF)-β superfamily and elicit their response via the so-called type I and II receptors. BMP-2 and -4 interact with the activin-like kinase receptor (ALK)2, ALK3, and ALK6, which are type I receptors that form complexes with the BMP type II receptor (BMPRII).17 In canonical BMP signaling, the receptors phosphorylate specific regulated (R)-SMAD proteins, which translocate into the nucleus and regulate gene transcription.17 SMAD1/5/8 mediate BMP-signaling, whereas SMAD2/3 mediate TGF-β signaling. In previous work, we identified a BMP-triggered pathway that modulates expression of vascular endothelial growth factor (VEGF) in endothelial cells (ECs).18,19 In this pathway, BMP-4 interacts with ALK2 to induce expression of ALK1,20 a related type I receptor that is essential for normal angiogenesis.21 BMP-9, which stimulates ALK1,22 induces expression of matrix Gla protein (MGP), a BMP inhibitor known to limit vascular calcification,14,23 and VEGF. Thus, expression of ALK2, ALK1, MGP, and VEGF appears to be closely linked.
Reports from other investigators suggest that vascular BMPs are responsive to hyperglycemia. Bovine vascular smooth muscle cells (SMCs) secrete more BMP-2 when treated with high glucose,24 vascular expression of BMP-2, BMP-7, and BMPRII increases in early autoimmune diabetes in mice,25 and aortic BMP-4 increases as diabetes progresses in db/db mice.26 There is also evidence that diabetes stimulates osteogenic differentiation in aortic myofibroblasts by augmenting the BMP-2/Msx2-Wnt pathway in fat-fed low-density lipoprotein receptor–null mice.10,27 However, the overall effect of hyperglycemia on vascular BMP activity and its association to vascular disease is still poorly understood.
In this study, we demonstrate that high glucose strongly promotes BMP activity in endothelial cells. We further show high aortic BMP activity in 3 diabetic animal models representative of type 1 and 2 diabetes, in which BMP-4, ALK2, ALK1, and MGP were preferentially detected in proximity to the endothelium. The high BMP activity was associated with a remarkable increase in aortic expression of osteogenic markers and calcification. Increased BMP inhibition, as mediated by a MGP transgene, limited these changes.
Methods
The generation of rats transgenic for human islet amyloid polypeptide (HIP rats) has been previously described.28 The Ins2Akita/+ mice (heterozygous for a mutation in one allele of the insulin-2 gene)29,30 and the db/db mice (homozygous for the spontaneous mutation Leprdb),3 both on C57BL/6J background, were obtained from The Jackson Laboratory (Bar Harbor, ME). MGPtg/wt mice, generated in our laboratory on a C57BL/6J background,31 were crossed with Ins2Akita/+ mice to generate MGPtg/wt;Ins2Akita/+ mice. Heterozygous MGPtg/wt mice were used because the phenotype was apparent in MGPtg/wt mice and a low birth rate made it difficult to obtain hemizygous MGPtg/tg mice.31 Genotyping was performed by PCR as previously described.31 The rats were housed individually and fed Rodent Diet 8604 (50% carbohydrate, 24% protein, and 4% fat; Harlan Teklad, Madison, WI) ad libitum; the mice were fed standard chow (Diet 8604, Harlan Teklad) also ad libitum. All animals were subjected to the standard 12-hour light/12-hour dark cycle. The studies were reviewed by the Institutional Review Board and conducted in accordance with the animal care guidelines set by the University of California, Los Angeles.
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org and provides details on analytic procedures, cell culture and small interfering (si)RNA transfection, RNA analysis, immunoblotting, immunostaining, alkaline phosphatase (ALP) assay, quantification of calcium deposition, proliferation and tube-formation assays, ELISA, determination of media thickness, histochemical staining, and statistical analysis.
Results
Hyperglycemia Induces the Expression of BMP Ligands, Inhibitors, and Receptors in Human Aortic Endothelial Cells
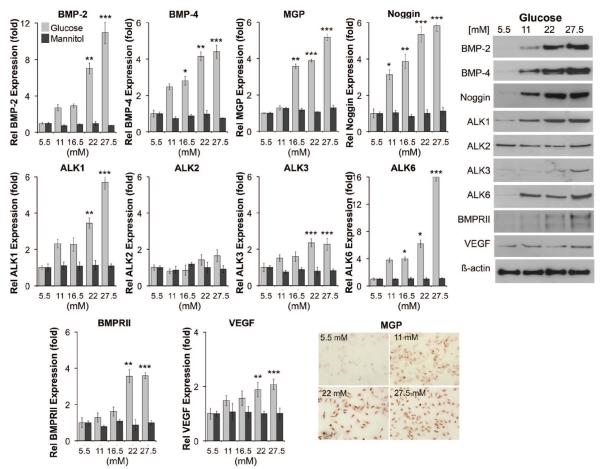
We hypothesized that hyperglycemia promotes expression of BMP-related proteins in human aortic endothelial cells (HAECs). To test our hypothesis, we treated HAECs for 24 hours with increasing concentrations of glucose (5.5 to 27.5 mmol/L; 5.5 mmol/L is the normal media concentration) or D-mannitol (5.5 to 27.5 mmol/L) as a control. The results showed that the ligands BMP-2 and -4; the inhibitors MGP and Noggin; and the receptors ALK1, ALK3, ALK6, and BMPRII dose-dependently increased, as determined by real-time PCR and immunoblotting (Figure 1). Because the MGP antibodies worked poorly for immunoblotting, immunohistochemistry was performed for MGP (Figure 1). In addition, VEGF, which can be regulated through a BMP-triggered pathway,18,19 was 2-fold induced after treatment with 27.5 mmol/L glucose (Figure 1). Human aortic smooth muscle cells (HASMCs) that were treated similarly with glucose or d-mannitol for 24 hours did not show induction of the BMP-related proteins (data not shown). The results suggested that aortic EC respond readily to hyperglycemia with an increase in BMP ligands, inhibitors and receptors.
Figure 1. Expression of BMP components in HAECs in response to increasing concentrations of glucose.
HAECs were treated with increasing concentrations of glucose (5.5 to 27.5 mmol/L) or control D-mannitol for 24 hours. Expression of the indicated genes was determined by real-time PCR (left) and immunoblotting or immunohistochemistry (for MGP) (right). Asterisks indicate statistically significant differences compared with 5.5 mmol/L glucose: *P<0.05, **P<0.01, ***P<0.001 (Tukey test).
Increased BMP Levels and BMP Activity in Media From HAECs Treated With High Glucose
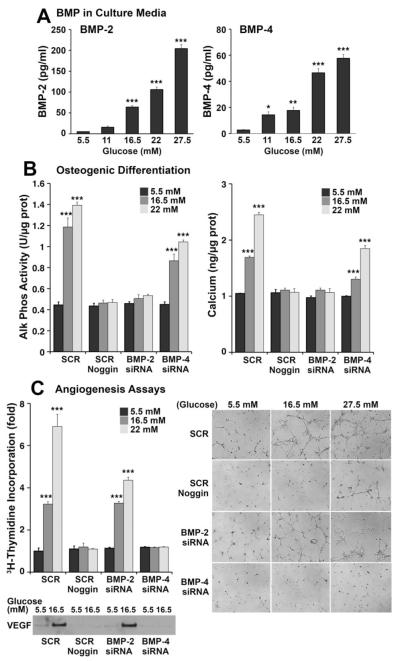
To determine whether the increase in BMP expression resulted in higher secretion of BMP-2 and -4, we determined BMP-2 and -4 by ELISA in the media from HAECs treated with high glucose for 24 hours. The results showed a dose-dependent increase in both BMP-2 and -4 (Figure 2A). We then examined the effect of BMP-2 and -4 in four assays sensitive to BMP activity, BMP-stimulated ALP activity (early osteogenic marker) and calcium accumulation (late osteogenic marker) in calcifying vascular cells (CVCs), and proliferation and tube formation in bovine aortic endothelial cells (BAECs). We prepared conditioned media from glucose-treated (5.5 to 22 mmol/L) HAECs that had been transfected with scrambled control siRNA or siRNA for BMP-2 or -4 to specifically deplete the respective BMP in the media. CVCs were incubated with the conditioned media from the glucose-treated HAECs, and AP activity and calcium accumulation were measured after 2 and 8 days, respectively. The results showed that both ALP activity and calcium accumulation progressively increased with media from control HAECs and that the effect was abolished by Noggin, an inhibitor of both BMP-2 and -4 (Figure 2B). Depletion of BMP-2, but not BMP-4, abolished the effect (Figure 2B, left), suggesting that BMP-2 is a stronger stimulator of osteogenesis than BMP-4. CVCs treated directly with increasing concentrations of glucose did not show increases in ALP activity or calcium accumulation compared with controls (data not shown).
Figure 2. Increased BMP activity in conditioned media from glucose-treated HAECs.
A, Levels of BMP-2 (left) and BMP-4 (right) in conditioned media from HAECs treated with glucose (5.5 to 27.5 mmol/L) for 24 hours, as determined by ELISA. B, Alkaline phosphate activity (left) and calcium accumulation (right) in CVCs incubated with conditioned media from HAECs treated with glucose (5.5 to 27.5 mmol/L) were determined after 2 and 8 days, respectively. Before glucose treatment, HAECs were transfected with siRNA for BMP-2 or BMP-4 to selectively deplete these in the conditioned media. Scrambled siRNA (SCR) was used as control. Noggin was added to inhibit the activity of both BMP-2 and BMP-4. C, Cell proliferation (left) and tube formation (right) in BAECs incubated with the same conditioned media from siRNA-transfected and glucose-treated HAECs as above. VEGF in the conditioned media was examined by immunoblotting. Asterisks indicate statistically significant differences compared with control 5.5 mmol/L glucose: *P<0.05, **P<0.01, ***P<0.001 (Tukey test).
We used the same conditioned media for the angiogenesis assays. BAECs were incubated with the conditioned media and cell proliferation was determined by 3H-thymidine incorporation. The results showed that proliferation progressively increased with media from control HAECs and that the effect was abolished by Noggin (Figure 2C, left). Depletion of BMP-4, but not BMP-2, also abolished the effect (Figure 2C, left), suggesting that BMP-4 is a stronger angiogenic factor than BMP-2. Depletion of BMP-4 also depleted VEGF in the conditioned media (Figure 2C, left), suggesting that the angiogenic effect of BMP-4 was mediated by VEGF. The same conditioned media were used for tube formation assays in Matrigel. Tube formation increased in media from control HAECs, but was abolished with Noggin and depletion of BMP-4 in the glucose-treated HAECs (Figure 2C, right). Together, the results suggest increased BMP secretion in glucose-treated ECs, and that BMP-2 and -4 have differential effects on osteogenesis and angiogenesis, respectively.
Effect of the Hyperglycemia on Expression of ALK1, ALK2, MGP, and VEGF Is Coordinated
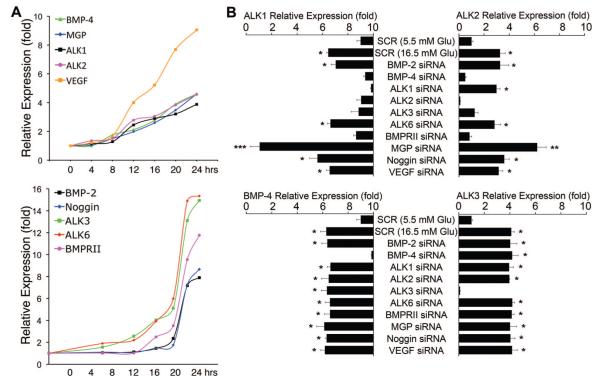
We previously found that BMP-4 stimulates expression of MGP and VEGF by increasing expression of ALK1 and ALK2 receptors,18–20 and that ALK1 is induced through ALK2, but not the other way around.20 We compared the time course and coordination of BMP-4, ALK1, ALK2, MGP and VEGF to those of BMP-2, ALK3, ALK6, BMPRII and Noggin. First, we performed time course experiments by treating HAECs with 16.5 mmol/L glucose for up to 24 hours. Samples were collected every 4 hours, and gene expression was determined by real-time PCR. This revealed that glucose progressively stimulated expression of BMP-4, ALK1, ALK2, MGP, and VEGF for up to 24 hours (Figure 3A). However, expression of BMP-2, ALK3, ALK6, BMPRII, and Noggin exhibited a different time course with a rapid increase between 16 and 20 hours (Figure 3A).
Figure 3. Coordinated regulation of expression of BMP-4, ALK2, ALK1, MGP, and VEGF in glucose-treated HAECs.
A, HAECs were treated with glucose (16.5 mmol/L) for up to 24 hours. Samples were taken every 4 hours and expression of the indicated genes was determined by real-time PCR. B, HAECs were transfected with scrambled (SCR) or specific siRNA as indicated in the figure and treated with glucose (5.5 or 16.5 mmol/L) for 24 hours starting 24 hours after transfection. Expression of ALK1, ALK2, BMP-4, and ALK3 was determined by real-time PCR. Asterisks indicate statistically significant differences compared with SCR control (5.5 mmol/L glucose): *P<0.05, **P<0.01, ***P<0.001 (Tukey test).
We next used siRNA techniques to selectively deplete BMP components and examine the effect of glucose stimulation on the expression of ALK1, ALK2 (both induced by BMP activity18,20), ALK3, and BMP-4. We used a glucose concentration of 16.5 mmol/L, which gave significant stimulation in previous experiments without being excessively high. Scrambled siRNA was used as control. Depletion of BMP-4, ALK1, ALK2, ALK3 and BMPRII abolished the glucose-induced ALK1 expression, whereas depletion of BMP-2, ALK6, Noggin and VEGF did not (Figure 3B). Depletion of the same factors, except for ALK1, also abolished the glucose-induced ALK2 expression (Figure 3B). Depletion of MGP increased expression of ALK1 and ALK2, consistent with its role as a BMP inhibitor.32 Although Noggin is also a BMP inhibitor, its depletion did not alter the expression of ALK1 and ALK2, suggesting differences in BMP affinity or cell-specific function between Noggin and MGP. Glucose-induced expression of BMP-4 and ALK3, however, was unaffected by depletion of any of the genes except for BMP-4 and ALK3, respectively. Thus, the induction of ALK2, ALK1, MGP, and VEGF is coordinated in glucose-treated HAECs, where it is stimulated by BMP-4, but not BMP-2. Furthermore, our results suggest that ALK3 mediates the induction of ALK2, which in turn mediates the induction of ALK1. BMPRII signals together with ALK1, ALK2, ALK3, and ALK6,17,19 and the depletion of BMPRII may affect signaling through all these receptors.
Diabetes Mellitus Induces Aortic Expression of BMP Ligands, Inhibitors, and Receptors in Ins2Akita/+ and db/db Mice
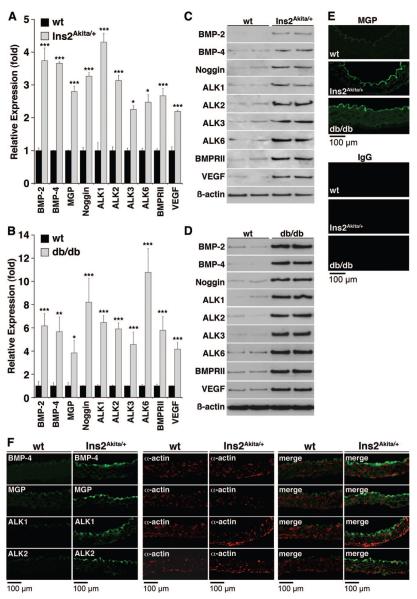
To examine whether hyperglycemia activates vascular expression of BMP ligands, inhibitors and receptors in vivo, we took advantage of the Ins2Akita/+ and db/db diabetic mouse models. Heterozygous Ins2Akita+/− mice become spontaneously diabetic because of a mutation in one allele of the insulin-2 gene with a progressive decrease in insulin levels,29,30 whereas homozygous db/db mice develop obesity and diabetes because of a mutation in the leptin receptor.3 To determine aortic BMP expression, we prepared aortas from heterozygous Ins2Akita/+ mice, homozygous db/db mice and wild-type (wt) littermates aged 20 weeks, when serum glucose was significantly increased in both types of mice (Online Table I). Aortic expression of BMP ligands, inhibitors and receptors was determined. The results showed that expression of BMP-2, BMP-4, MGP, Noggin, ALK1, ALK2, ALK3, ALK6, BMPRII, and VEGF was significantly increased in both Ins2Akita/+ mice and db/db mice, as determined by real-time PCR (Figure 4A and 4B), immunoblotting (Figure 4C and 4D), and immunofluorescence for MGP (Figure 4E). To examine the localization of the expression of the different BMP components in the vascular wall, we selected the Ins2Akita/+ mouse and compared it to littermate controls. We colocalized expression of BMP-2, BMP-4, ALK1, ALK2, ALK3, ALK6, BMPRII, Noggin, MGP, and VEGF with that of smooth muscle α-actin, a marker of the medial SMCs, using immunofluorescence. The results revealed that BMP-4, ALK2, ALK1, and MGP, which showed coordination in HAECs, were detected mainly in the endothelium (Figure 4F), whereas BMP-2, Noggin, ALK3, ALK6 and BMPRII were detected throughout the aortic wall (Online Figure I). VEGF was detected throughout the aortic wall, suggesting induction through multiple pathways. Together, the results suggest that diabetes induces vascular BMP expression, which may contribute to vascular disease.
Figure 4. Aortic expression of BMP components in Ins2Akita/+ and db/db mice.
Aortas were obtained from Ins2Akita/+ and db/db mice aged 20 weeks. A and B, Expression of the indicated genes was determined by real-time PCR and compared with wild-type (n=4 animals in each group). C through E, Protein levels were determined by immunoblotting or immunofluorescence (for MGP). F, Aortas were obtained from Ins2Akita/+ mice aged 20 weeks and used for immunofluorescence. BMP-4, MGP, ALK1, and ALK2 (green) were detected on the luminal side (up) by the endothelium and did not colocalize with smooth muscle α-actin (red). Asterisks indicate statistically significant differences compared with wild type: *P<0.05, **P<0.01, ***P<0.001 (Tukey test).
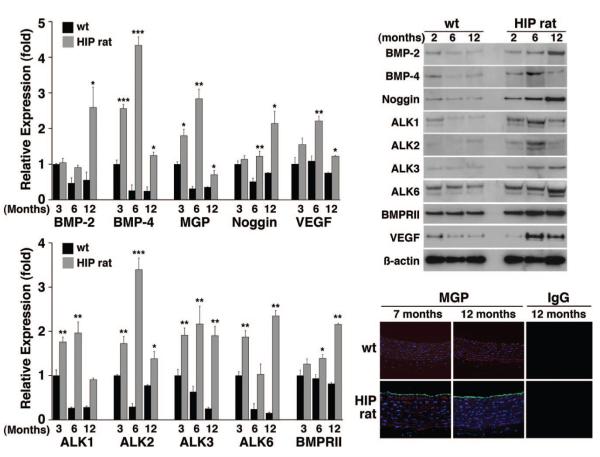
Diabetes Mellitus Induces Aortic Expression of BMP Ligands, Inhibitors, and Receptors in HIP Rats
The so-called HIP rats progressively develop diabetes because of overexpression of the human islet amyloid polypeptide in the pancreatic β-cells, leading to a progressive apoptosis of β-cells, and is a model of type 2 diabetes.28 To determine whether aortic BMP expression is induced also in these animals, and how expression correlates with the development of diabetes, we prepared aortas from HIP rats and littermate controls aged 3, 6, and 12 months. We found that expression of BMP-2, BMP-4, MGP, Noggin, ALK1, ALK2, ALK3, ALK6, BMPRII, and VEGF progressively increased as the glucose levels increased, as determined by real-time PCR, immunoblotting and immunofluorescence for MGP (Figure 5A and 5B). The glucose levels were already significantly increased after 3 months compared with controls, and peaked after 12 months (Online Table II). In the case of BMP-4, ALK2, ALK1, and MGP, the expression was lower at 12 months than at 6 months, but still increased compared with control (Figure 5A and 5B). The results support that vascular induction of BMP components is part of the development of diabetes also in rats and occurs at relatively modest levels of hyperglycemia.
Figure 5. Aortic expression of BMP components in HIP rats.
Aortas were obtained from HIP rats aged 3 to 12 months. Left, Expression of the indicated genes was determined by real-time PCR and compared with wild type rats (n=6 animals in each group). Right, Protein levels were determined by immunoblotting or immunofluorescence (for MGP). Asterisks indicate statistically significant differences; each group was compared with wild type at 3 months of age: *P<0.05, **P<0.01, ***P<0.001 (Tukey test).
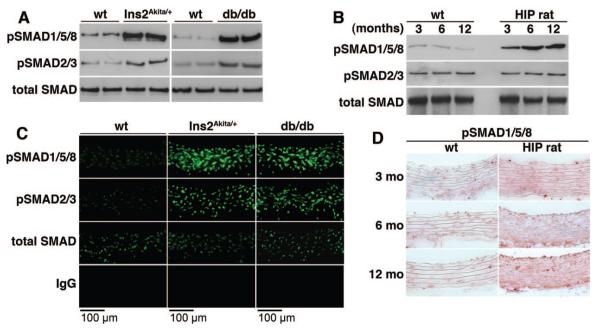
Increased BMP Activity in Diabetic Vascular Wall and Serum
Because aortic expression of BMPs, receptors and inhibitors was induced to varying degrees, we determined the overall BMP activity in tissue using immunoblotting for phosphorylated (p)SMAD1/5/8 and compared with pSMAD2/3, which show TGF-β activation, and total SMAD. Protein extracts were prepared from aortas of Ins2Akita/+ and db/db mice aged 20 weeks, and from HIP rats aged 3, 6 and 12 month, and subjected to immunoblotting. The results showed that pSMAD1/5/8 levels were increased in both mouse models (Figure 6A), and in HIP rats where they increased with age (Figure 6B). Furthermore, we confirmed the levels of pSMAD1/5/8 in mice and HIP rats using immunofluorescence and immunohistochemistry, respectively, and found that the levels increased similarly throughout the vascular wall (Figure 6C and 6D). The results support that the vascular BMP activity is increased in diabetes. In addition, serum levels of BMP-2 and -4 increased progressively in the HIP rats, resulting in greater ability to induce BMP-dependent osteogenesis in CVCs (Online Figure II).
Figure 6. Increased vascular BMP signaling in diabetic mice and rats.
A through D, Aortic BMP activity in Ins2Akita/+ and db/db mice aged 20 weeks (A and C) and HIP rats aged 3 to 12 months (B and D), as determined by immunoblotting for pSMAD1/5/8 (A and B), immunofluorescence (C), and immunohistochemistry (D) and compared with wild-type animals. PSMAD1/5/8 was compared with pSMAD2/3, which show TGF-β activation, and total SMAD.
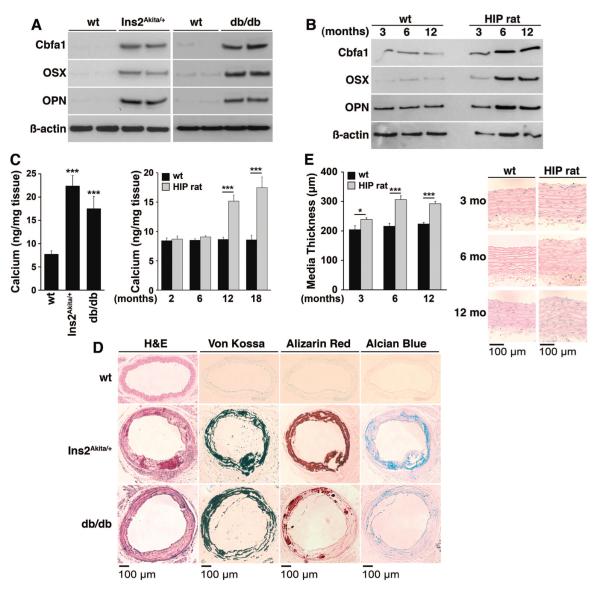
Diabetes and Increased BMP Activity Is Associated With Vascular Calcification
To determine whether the diabetic animals had vascular calcification, we compared expression of osteogenic markers and calcium accumulation in diabetic and control aortas. We found strong induction of Cbfa1 and Osterix, essential transcription factors in bone,33 in both Ins2Akita/+ and db/db mice aged 20 weeks, and in HIP rats aged 3, 6, and 12 months, as determined by immunoblotting (Figure 7A and 7B). Expression of osteopontin, a multifunctional glycoprotein in bone,33 was also increased (Figure 7A and 7B). Total aortic calcium was significantly increased in the mouse models, and at 12 and 18 months of age in HIP rats compared with controls (Figure 7C). The calcium mineral and cartilage-associated mucopolysaccharides were easily visualized in the mouse models using Von Kossa and Alizarin Red staining for mineral, and Alcian blue staining for mucopolysaccharides (Figure 7D). The calcium was observed along the elastic lamellae, resembling medial calcification. In the HIP rats, however, only small punctate calcifications were seen (data not shown) even though the thickness of the aortic media had increased significantly at 6 and 12 months of age compared with controls (Figure 8E). Together, the results support that the increased BMP activity in diabetic mice and rats is associated with increased vascular calcification.
Figure 7. Increased osteogenic differentiation in the aortas of diabetic mice and rats.
A and B, Aortic expression of osteogenic markers in Ins2Akita/+ and db/db mice aged 20 weeks (A) and HIP rats aged 3 to 12 months (B), as determined by immunoblotting. C, Total calcium accumulation Ins2Akita/+ and db/db mice aged 20 weeks and HIP rats aged 3 to 18 months. D, Calcium and cartilage-associated mucopolysaccharides in Ins2Akita/+ and db/db mice aged 20 weeks, as determined by histochemical staining. E, Media thickness in HIP rats aged 3 to 12 months (n=6 animals in each group). Asterisks indicate statistically significant differences compared with wild type: *P<0.05, ***P<0.001 (Tukey test).
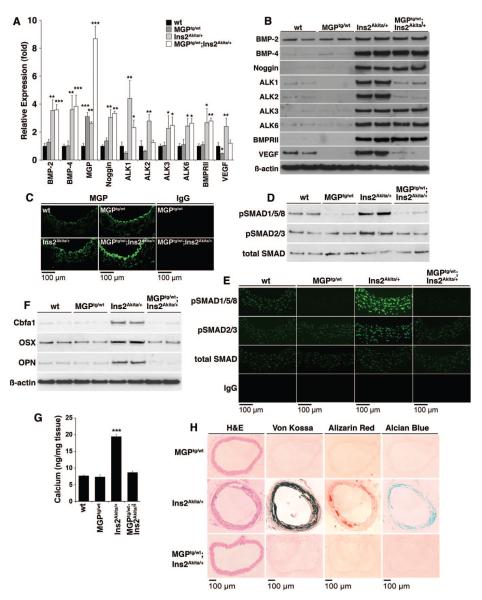
Figure 8. Enhanced BMP inhibition limits diabetic vascular disease in Ins2Akita/+ mice.
A through C, Aortic expression of BMP components in wild-type, MGPtg/wt, Ins2Akita/+, and MGPtg/wt; Ins2Aktiat/+ mice aged 20 weeks, as determined by real-time PCR (n=3 mice in each group) (A), immunoblotting (B), and immunofluorescence (for MGP) (C). Asterisks indicate statistically significant differences in MGPtg/wt and MGPtg/wt; Ins2Aktiat/+ mice compared with wild-type and Ins2Akita/+ mice, respectively: *P<0.05, ***P<0.001 (Tukey test). D, Aortic BMP activity in wild-type, MGPtg/wt, Ins2Akita/+, and MGPtg/wt;Ins2Aktiat/+ mice, as determined by immunoblotting for pSMAD1/5/8. E, Aortic BMP activity in wild-type, MGPtg/wt, Ins2Akita/+, and MGPtg/wt;Ins2Aktiat/+ mice aged 20 weeks, as determined by immunofluorescence for pSMAD1/5/8, and compared with pSMAD2/3 and total SMAD. F, Aortic expression of osteogenic markers in wild-type, MGPtg/wt, Ins2Akita/+, and MGPtg/wt; Ins2Aktiat/+ mice, as determined by immunoblotting. G, Total calcium accumulation in wild-type, MGPtg/wt, Ins2Akita/+, and MGPtg/wt;Ins2Aktiat/+ mice (n=3 mice in each group). Asterisks indicate a statistically significant difference between the MGPtg/wt;Ins2Aktiat/+ mice and the Ins2Akita/+mice. ***P<0.001 (Tukey test). H, Calcium mineral and cartilage-associated mucopolysaccharides in MGPtg/wt, Ins2Akita/+, and MGPtg/wt; Ins2Aktiat/+ mice, as determined by histochemical staining.
Because high phosphate levels are known to enhance vascular calcification,6 we determined serum phosphate in all animals. The phosphate levels did not differ significantly between animals (Online Tables I and II).
Enhanced BMP Inhibition Limits Diabetic Vascular Disease in the Ins2Akita/+ Mice
To determine whether enhanced BMP inhibition would limit diabetic vascular disease, we generated MGPtg/wt;Ins2Akita/+ mice with a transgene for MGP, which efficiently inhibits BMP-2 and -4.14,32 We compared them with the wild type, MGPtg/wt, and Ins2Akita/+ mice at age 20 weeks. The MGP transgene did not affect serum glucose or phosphate levels (Online Table III). We compared aortic expression of BMPs, receptors and inhibitors, BMP activity, osteogenic expression, and calcification. The results revealed increased aortic expression of MGP, as determined by real-time PCR and immunofluorescence, ≈3-fold in the MGPtg/wt mice and the Ins2Akita/+ mice, and 8-fold in the MGPtg/wt;Ins2Akita/+ mice as compared with wild type (Figure 8A and 8C). Immunofluorescence also revealed that the increased MGP was predominantly found in proximity to the endothelium, suggesting that the endothelium is important in limiting calcification. The expression of ALK1, ALK2, and VEGF was significantly decreased in the MGPtg/wt;Ins2Akita/+ mice compared with Ins2Akita/+ mice, as determined by real-time PCR and immunoblotting (Figure 8A and 8B). The high MGP level also led to less aortic BMP activity, as determined by immunoblotting and immunofluorescence for pSMAD1/5/8 9 (Figure 8D and 8E), demonstrating that more BMP inhibition shifted the balance in overall vascular BMP activity. We also detected less expression of Cbfa1, osterix, and osteopontin when MGP was high, as determined by immunoblotting (Figure 8F). Finally, there was less aortic calcium mineral and cartilage-associated mucopolysaccharides, as determined by total aortic calcium (Figure 8G) and histochemical staining (Figure 8H). Thus, increased BMP inhibition as mediated by enhanced MGP expression decreased total BMP activity and prevented vascular osteogenesis and calcification caused by diabetes.
Discussion
In this study, we demonstrate that hyperglycemia and diabetes are strong activators of the BMP signaling system in endothelial cells in vitro and the aortic wall in vivo. High vascular BMP activity was found in 3 diabetic animal models and was associated with a remarkable increase in expression of osteogenic markers and medial calcification. This was largely prevented by enhancing BMP inhibition using a MGP transgene, which caused a decrease in total BMP activity.
Our results are consistent with previous reports on increased vascular expression of BMP-2, -7, and the BMPRII in early autoimmune diabetes in mice,25 and increased aortic expression of BMP-4 as diabetes progresses in db/db mice.26 An increase in endothelial BMP-2 in the adventitia may also be instrumental in promoting osteogenic differentiation in aortic myofibroblasts by augmenting the BMP-2/Msx2-Wnt pathway as has been reported for diabetic low-density lipoprotein receptor–null mice by Al-Aly et al.27 In contrast, we did not detect increased expression of BMP components in HASMCs when treated with high glucose, even though previous studies show increased BMP-2 secretion from bovine SMCs treated with high glucose for 48 hours.24 In addition, CVCs did not show enhanced ALP activity or calcium accumulation even if treated with high glucose for up to 10 days. The differences may be attributable to variations in cell systems or timing of treatment.
Our results point to a role for the endothelium in preventing vascular calcification. The enhanced MGP expression in MPGtg/wt and MPGtg/wt;Ins2Akita/+ mice was mainly found in the endothelium even though calcification was prevented in the media. Endothelial BMP-4 has been shown to be important in mediating atherogenic stimuli such as disturbed flow and increased oxidative stress,11,12 and it is possible that it is also an initiating factor for medial calcification. However, the exact mechanisms of endothelial-medial crosstalk responsible for such effects will require further studies. Other investigators have found that BMP-2, which in our experiments is expressed in both the endothelium and the media, is also an important inflammatory cytokine in the endothelium,12 which also would be inhibited by MGP. However, it is possible that Noggin is more important for inhibiting BMP-2 based on similar distributions of BMP-2 and Noggin in the vascular wall. The findings that depletion of BMP-4 abolished the proangiogenic effects of conditioned media from glucose-treated HAECs, whereas depletion of BMP-2 abolished the procalcific effects further support that BMP-2 and -4 have distinct vascular roles despite their molecular similarities.
There appears to be a hierarchy in how BMP/TGF-β receptors are used in vascular cells. The finding that ALK3 is responsible for induction of ALK2 adds to our previous observations that ALK2 and ALK1 are responsible for induction of ALK1 and ALK5, respectively.19,20 Thus, the BMP/TGF-β receptors appears to be used in the order ALK3, ALK2, ALK1 and ALK5, where each receptor may correlate to a specific stage in vascular growth and development.
Aortic calcium increased in all animals as diabetes developed. It occurred later in the HIP rats, suggesting that rats are more resistant to vascular calcification. The calcification pattern in Ins2Akita/+ and db/db mice was distinct, following the elastic lamellae and resembling that of medial calcification considered characteristic for diabetes. Indeed, our studies showed that both Ins2Akita/+ and db/db mice are new models of diabetic vascular calcification and would be suitable for testing interventions aimed at diabetic vascular disease. We did not detect any significant development of atherosclerotic lesions during the study period. An advantage with the animal models used in this study is that they develop diabetes spontaneously, thus avoiding toxic effects of streptozotocin or other agents used to induce diabetes, which might interfere with organ or stem cell function and make the results difficult to interpret.
Considering that the vascular endothelium altogether is a very large organ, it is interesting to speculate whether it also provides feedback for the pancreatic islet cells. Goulley et al34 recently reported that BMP-4 improves insulin sensitivity in pancreatic islet cells. Autocrine BMP-4 maintained healthy insulin secretion and could be replaced by BMP-4 infusion. Thus, BMP-4 derived from the endothelium may have a physiological role in modulating insulin secretion in the pancreas, and persistently high levels of BMP-4 in vascular tissues or serum may be early signs of diabetes.
In summary, BMP signaling is strongly activated in the diabetic vessel wall and associated with vascular calcification. Lowering BMP activity by means of an MGP transgene limited the calcification, suggesting that vascular BMP inhibition may be a new strategy for treating diabetic vascular disease.
Novelty and Significance.
What Is Known?
Diabetes mellitus is associated with the development of several cardiovascular complications.
Bone morphogenetic protein (BMP)-2 and BMP-4, multifunctional growth factors, have been associated with atherosclerosis and vascular calcification.
Matrix Gla protein (MGP) binds BMP-2 and -4 and inhibits BMP activity.
What New Information Does This Article Contribute?
High glucose levels enhance BMP activity in vascular endothelial cells in culture.
Diabetes mellitus enhances vascular BMP activity, medial calcification and expression of bone-related genes in mice and rats with type 1 or 2 diabetes.
Suppression of vascular BMP activity by an MGP transgene limits diabetic vascular disease in mice, including medial calcification and expression of bone-related genes.
Enhanced MGP expression is predominantly detected in the vascular endothelium, but BMP activity is inhibited throughout the vascular wall.
The BMPs, a family of multifunctional growth factors and morphogens, have been associated with atherosclerosis and vascular calcification. We demonstrate that high glucose levels enhance BMP activity in endothelial cells in culture, and that diabetes enhances vascular BMP activity in mice and rats with type 1 or 2 diabetes mellitus. Suppression of the BMP activity by high expression of MGP, an inhibitor of BMP-2 and -4, limits medial calcification and expression of bone-related genes in diabetic mice. This is the first study to show that an intervention that decreases BMP activity prevents diabetic vascular disease. Highest induction of MGP expression was observed in the endothelium; however, BMP activity was inhibited throughout the vascular media. Thus, BMP activity appears to be an important determinant of diabetic vascular disease, and suppression of BMP activity may be a new strategy to treat vascular disease in diabetic patients.
Supplementary Material
Supplemental Table I. Serum levels of glucose and phosphate in wild type and diabetic mice (C57BL6/J background), 20 weeks of age.
Supplemental Table II. Serum levels of glucose and phosphate in wild type and HIP rats, aged 3-18 months.
Supplemental Table III. Serum levels of glucose and phosphate in wild type and diabetic mice (C57BL6/J background) with or without the MGP transgene, 20 weeks of age.
Supplemental Figure I A. Localization of BMP components in the aortic wall of Ins2 Akita/+.
To examine the localization of the expression of the different BMP components in the aortic wall, we selected the Ins2Akita/+ mouse and compared it to littermate controls at age 20 weeks. We co-localized expression of BMP-2, BMP-4, ALK1, ALK2, ALK3, ALK6, BMPRII, Noggin, MGP and VEGF with that of smooth muscle α-actin, a marker of the medial SMC, using immunofluorescence.
(Top) BMP-4, MGP, ALK1 and ALK2 (green) were predominantly detected in on the luminal side (up) in proximity to the endothelium, and did not co-localize with smooth muscle alpha-actin (red).
(Bottom) BMP-2, Noggin, ALK3, ALK6, BMPRII and VEGF (green) were detected throughout the vascular wall, and co-localized with alpha-actin (red).
B. High magnification of MGP immunofluorescent staining in wild type mouse aorta.
High magnification of MGP staining in wild type mice (aged 20 weeks), with and without DAPI nuclear stain, demonstrates that the MGP staining (green) is associated with the endothelial layer. Autofluorescence of the elastic lamellae was minimal with green fluorescence in most of our stained specimens, but visible with blue fluorescence.
Supplemental Figure II Increased serum levels of BMP-2 and BMP-4 in diabetic rats.
(A) Levels of BMP-2 (left) and BMP-4 (right) in serum from HIP rats aged 3-12 months, as determined by ELISA (n=6 animals in each group).
(B) Osteogenic differentiation in CVC incubated with serum from HIP rats and wt rats aged 3-12 months. The cells were treated with medium containing 10% serum from the HIP rats or controls, and alkaline phosphatase activity (left) and calcium accumulation (right) were determined in absence and presence of Noggin (300 ng/ml) after 2 and 8 days, respectively.
Asterisks indicate statistically significant differences compared to wt for the respective age. *<0.05, ***<0.001, Tukey’s test.
Acknowledgments
Sources of Funding This work was supported in part by NIH grants HL30568, HL81397, and DK057303; the American Heart Association (Western Affiliate); and a Norman S. Coplon Award (to S.B.N.). A Larry Hillblom Foundation grant and NIH grant DK061539 were used to develop and maintain a HIP rat colony at the University of California, Los Angeles.
Non-standard Abbreviations and Acronyms
- ALK
activin-like kinase receptor
- ALP
alkaline phosphatase
- BAEC
bovine aortic endothelial cell
- BMP
bone morphogenetic protein
- BMPRII
bone morphogenetic protein type II receptor
- Cbfa1
core binding factor α1
- CVC
calcifying vascular cell
- Gla
γ-carboxyglutamic acid
- HAEC
human aortic endothelial cells
- HASMC
human aortic smooth muscle cell
- HIP
rats transgenic for human islet amyloid polypeptide
- MGP
matrix Gla protein
- pSMAD
phosphorylated SMAD
- siRNA
small interfering RNA
- SMAD
homolog of the drosophila protein, mothers against decapentaplegic (MAD) and the Caenorhabditis elegans protein SMA
- SMC
smooth muscle cell
- tg
transgenic
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
- wt
wild type
Footnotes
Disclosures None.
References
- 1.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14(Suppl 5):S1–85. [PubMed] [Google Scholar]
- 2.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation. 2003;108:1655–1661. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 3.Wu KK, Huan Y. Diabetic atherosclerosis mouse models. Atherosclerosis. 2007;191:241–249. doi: 10.1016/j.atherosclerosis.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–E696. doi: 10.1152/ajpendo.00552.2003. [DOI] [PubMed] [Google Scholar]
- 5.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Abbadi M, Giachelli CM. Mechanisms of vascular calcification. Adv Chronic Kidney Dis. 2007;14:54–66. doi: 10.1053/j.ackd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853–876. [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 9.Boyd NL, Dhara SK, Rekaya R, Godbey EA, Hasneen K, Rao RR, West FD, III, Gerwe BA, Stice SL. BMP4 promotes formation of primitive vascular networks in human embryonic stem cell-derived embryoid bodies. Exp Biol Med (Maywood) 2007;232:833–843. [PubMed] [Google Scholar]
- 10.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 11.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 12.Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006;168:629–638. doi: 10.2353/ajpath.2006.050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97:105–114. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- 16.Vogt RR, Unda R, Yeh LC, Vidro EK, Lee JC, Tsin AT. Bone morphogenetic protein-4 enhances vascular endothelial growth factor secretion by human retinal pigment epithelial cells. J Cell Biochem. 2006;98:1196–1202. doi: 10.1002/jcb.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 18.Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281:33921–33930. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
- 19.Shao ES, Lin L, Yao Y, Bostrom KI. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood. 2009;114:2197–2206. doi: 10.1182/blood-2009-01-199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao Y, Shao ES, Jumabay M, Shahbazian A, Ji S, Bostrom KI. High-density lipoproteins affect endothelial bmp-signaling by modulating expression of the activin-like kinase receptor 1 and 2. Arterioscler Thromb Vasc Biol. 2008;28:2266–2274. doi: 10.1161/ATVBAHA.108.176958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20:203–212. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 22.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102:914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 24.Chen NX, Duan D, O’Neill KD, Moe SM. High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol Dial Transplant. 2006;21:3435–3442. doi: 10.1093/ndt/gfl429. [DOI] [PubMed] [Google Scholar]
- 25.Nett PC, Ortmann J, Celeiro J, Haas E, Hofmann-Lehmann R, Tornillo L, Terraciano LM, Barton M. Transcriptional regulation of vascular bone morphogenetic protein by endothelin receptors in early autoimmune diabetes mellitus. Life Sci. 2006;78:2213–2218. doi: 10.1016/j.lfs.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Martin A San, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H2073–H2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 27.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 28.Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC. Diabetes due to a progressive defect in beta-cell mass in rats transgenic for human islet amyloid polypeptide (HIP Rat): a new model for type 2 diabetes. Diabetes. 2004;53:1509–1516. doi: 10.2337/diabetes.53.6.1509. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 30.Breyer MD, Bottinger E, Brosius FC, III, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 31.Yao Y, Nowak S, Yochelis A, Garfinkel A, Bostrom KI. Matrix GLA protein, an inhibitory morphogen in pulmonary vascular development. J Biol Chem. 2007;282:30131–30142. doi: 10.1074/jbc.M704297200. [DOI] [PubMed] [Google Scholar]
- 32.Yao Y, Shahbazian A, Bostrom KI. Proline and gamma-carboxylated glutamate residues in matrix gla protein are critical for binding of bone morphogenetic protein-4. Circ Res. 2008;102:1065–1074. doi: 10.1161/CIRCRESAHA.107.166124. [DOI] [PubMed] [Google Scholar]
- 33.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 34.Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. bMP4-BMPR1a signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007;5:207–219. doi: 10.1016/j.cmet.2007.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table I. Serum levels of glucose and phosphate in wild type and diabetic mice (C57BL6/J background), 20 weeks of age.
Supplemental Table II. Serum levels of glucose and phosphate in wild type and HIP rats, aged 3-18 months.
Supplemental Table III. Serum levels of glucose and phosphate in wild type and diabetic mice (C57BL6/J background) with or without the MGP transgene, 20 weeks of age.
Supplemental Figure I A. Localization of BMP components in the aortic wall of Ins2 Akita/+.
To examine the localization of the expression of the different BMP components in the aortic wall, we selected the Ins2Akita/+ mouse and compared it to littermate controls at age 20 weeks. We co-localized expression of BMP-2, BMP-4, ALK1, ALK2, ALK3, ALK6, BMPRII, Noggin, MGP and VEGF with that of smooth muscle α-actin, a marker of the medial SMC, using immunofluorescence.
(Top) BMP-4, MGP, ALK1 and ALK2 (green) were predominantly detected in on the luminal side (up) in proximity to the endothelium, and did not co-localize with smooth muscle alpha-actin (red).
(Bottom) BMP-2, Noggin, ALK3, ALK6, BMPRII and VEGF (green) were detected throughout the vascular wall, and co-localized with alpha-actin (red).
B. High magnification of MGP immunofluorescent staining in wild type mouse aorta.
High magnification of MGP staining in wild type mice (aged 20 weeks), with and without DAPI nuclear stain, demonstrates that the MGP staining (green) is associated with the endothelial layer. Autofluorescence of the elastic lamellae was minimal with green fluorescence in most of our stained specimens, but visible with blue fluorescence.
Supplemental Figure II Increased serum levels of BMP-2 and BMP-4 in diabetic rats.
(A) Levels of BMP-2 (left) and BMP-4 (right) in serum from HIP rats aged 3-12 months, as determined by ELISA (n=6 animals in each group).
(B) Osteogenic differentiation in CVC incubated with serum from HIP rats and wt rats aged 3-12 months. The cells were treated with medium containing 10% serum from the HIP rats or controls, and alkaline phosphatase activity (left) and calcium accumulation (right) were determined in absence and presence of Noggin (300 ng/ml) after 2 and 8 days, respectively.
Asterisks indicate statistically significant differences compared to wt for the respective age. *<0.05, ***<0.001, Tukey’s test.