Summary
Recognition of pathogens-associated molecular patterns (PAMPs) by Toll-like receptors (TLR), NOD-like receptors (NLR) and RIG-I-like receptors (RLR) plays a critical role in protecting host against pathogens. In addition, TLR and NLR also recognize danger-associated molecular patterns (DAMPs) to initiate limited innate immune responses. While innate immune response to DAMPs may be important for tissue repairs and wound healing, it is normally well controlled to avoid autoimmune destruction. Recent data support a role for sialoside-based pattern recognition by members of the Siglec family to attenuate innate immunity. In particular, since CD24-Siglec 10/G interaction selectively dampens host response to DAMPs but not PAMPs, this sialoside-based pattern recognition may serve as a foundation to discriminate PAMPs from DAMPs.
Introduction
Over 20 years ago, Charles Janeway proposed the revolutionary concept that the immune system discriminates “infectious nonself from non-infectious self” through pattern recognition [1, 2]. With the identification of TLR, NLR, and RLR, pattern recognition is now a major pillar in immunology [3]. However, accumulating data also support Matzinger’s hypothesis [4] that components released during tissue injury, now collectively called DAMPs, also trigger innate immune response, in many cases through TLR [5, 6] and NLR [7]. Unlike the sterilizing immunity that follows most infections, innate responses to tissue injuries may actually promote wound healing and tissue repairs [8–10]. Without additional assumptions, it has become difficult to explain how the activation of the same receptors may result in fundamentally different immune responses.
Finding additional pathways of pattern recognition that regulate innate immune response to DAMPs may help to reconcile this paradox. In this review, we will provide an update on major recent advances on innate response to tissue injury and emphasize the role of sialoside-based pattern recognition in negative regulate of innate immune response. Data emerged from this new field may provide a foundation for a new model of innate discrimination of DAMPs and PAMPs.
New insights from new DAMPs
It has long been demonstrated that tissue injury is associated with inflammation. In the last decade, it has become clear that diverse components from injured tissues or cells are responsible for the inflammatory responses (see [11, 12] for recent reviews). Collectively, recent data demonstrate that when released or accumulated during tissue injuries, components from nuclear, cytoplasm, organelle and extracellular matrix may trigger inflammation [11–13]. As updated in Table 1, two categories of DAMPs were added in last two years. The new sources of DAMPs have not only shed light on a potential evolutional link between DAMPs and PAMPs, but have also provided new insights on the pathogenesis of major diseases.
Table 1.
Two new categories of DAMPs
| DAMPs | Receptors/Sensors |
|---|---|
| I. Evolutionary culprit | |
| Retroelements | ND |
| Mitochondria DNA | TLR9 |
| Formyl peptides | FPRL1/2 |
| II. Disruption of membrane integrity | |
| Monosodium urate crystal | NLRP3 |
| Uric acid | NLRP3 |
| Cholesterol crystals | NLRP3 |
| Alum | NLRP3 |
First, recent studies raised the intriguing possibility that mitochondrial DNAs and retroelement DNA may trigger inflammatory response unless they are properly cleared. Mitochondria are believed to be of bacterial origin and retain two pathogen-associated molecular patterns, N-formyl peptides and unmethylated CpG islands. Zhang et al. [14] reported high levels of mitochondrial DNA in blood stream of trauma patients. The pathologically relevant levels of mitochondrial DNA were capable of eliciting secretion of matrix metalloproteinases from, and inducing migration of, polymorphonuclear neutrophils. In combination with synthetic N-formyl peptide, the mitochondrial DNA induces release of the IL-8 by a TLR9-dependent mechanism. Meanwhile, a major breakthrough was reported on immunological basis of Acardi-Goutieres Syndrome (AGS) which exhibits features of both autoimmune diseases and congenital infections. Mutations of five genes (AGS1-5) have been identified as genetic causes [15]. Surprisingly, these genes are likely involved in nucleic acid metabolism. Stetson et al. demonstrated that targeted mutation of Trex1 (AGS1) results in accumulation of cytosolic DNA and causes massive activation of type I interferon and lethal autoimmunity that can be cured by mutation of IRF3 [16]. Because of a striking enrichment of endogenous retroelements, it is intriguing that the retroelements may constitute another type of endogenous DAMPs. Since the mitochondria DNA and retroelements are ultimately of microbial origin, it is challenging to develop a conceptual framework relying on simple pattern recognition system to discriminate infections from aseptic tissue damages.
Second, disruption of membrane integrity by crystals likely constitutes another source of DAMPs. Several laboratories demonstrated that crystals formed by uric acids, alum, silica, and cholesterol activated NLRP3 inflammasome [7, 17–19]. Instead of directly interacting with DAMPs receptors, the crystals change the intracellular environment, such as ROS and possibly intracellular concentration of potassium [7] [20]. The discovery of a critical role for thioredoxin-interacting protein TXNIP in innate response to crystals links oxidative stress to inflammasome activation[20].
The inflammatory potential of diverse crystals has provided new insights to pathogenesis of several major diseases. For instance, the NLRP3-dependent production of IL1β provides a plausible explanation for the lung fibrosis associated with inhalation of asbestos and silica, called asbestosis and silicosis, respectively [7]. Inflammation associated with gout is now attributed to NLRP3-dependent induction of IL1β by monosodium urate crystals [7] [17]. More data suggest that cholesterol crystals may trigger atherosclerosis [19]. Alum crystals, the classic adjuvant used for vaccination, activate IL1β production by similar mechanisms [21–23][24, 25]. Data reported by three independent groups [21–23] showed a critical role for NLRP3 in adjuvant activity of alum. Two other studies [24, 25], however, reported that inactivation of NLRP3 did not have a measurable effect on the adjuvant activity of alum. Additional studies are needed to clarify the inconsistencies.
Sialoside-based pattern recognition as a negative regulator for innate immune responses
Innate immune response initiated by TLR and NLR also consists of negative feedback mechanisms to limit the potential damage to the host. Readers are referred to two recent reviews on the identities and functions of negative feedback mechanisms [13, 26]. However, it is less clear whether a dedicated recognition pathway may serve as negative regulator for innate immune responses.
A long-standing puzzle in leukocyte biology is how desialyation increase their functions, as demonstrated by increased T cell activation [27], NK cytotoxicity [28] and macrophage phagocytosis [29]. Over 20 years ago, Crocker and Gordon [30] identified what has since emerged as the founding member of the family of sialic acid-binding immunoglobulin-like lectins, now called Siglec [31]. At least 13 members in human and 9 members in the mice have been identified [32]. All but one member have been shown to bind sialioside-containing structures with different specificities. In addition, all but 2 members have intracellular domains with ITIM or ITIM-like domains. The Siglec ITIM domains have been shown to be associated with SHP-1 and SHP-2 phophatase [32]. Cross-linking by either antibodies or synthetic oligosaccharides has been shown to inhibit both innate and adaptive immune responses [32].
An important immune function of Siglec is recently demonstrated by a massive increase of B1 B cells and natural IgM antibodies in mice with targeted mutation of Siglec G [33, 34]. However, for the most part, the biological functions and natural ligands of the Siglecs remained elusive. This gap is now being filled by a recent study by Chen et al. who demonstrated interaction between CD24 and Siglec G in mice and Siglec 10 in human [35]. Since the binding of Siglec 10/G to spleen cells is abrogated by targeted mutation of CD24, CD24 is the major ligand for Siglec 10/G on the spleen cells.
Mature CD24 consists of 27–30 amino acids and is anchored to the plasma membrane through a glycosylphosphatidylinositol tail. However, with approximately 50% of the amino acids as potential glycosylation sites, more than 80% of molecular mass are derived from glycosylation [36, 37]. The diverse and heterogenous glycosylation may allow CD24 to present a diverse array of DAMPs. Indeed, massspectrometry of CD24-associated protein identifies several prominent DAMPs, including HSP70, 90, HMGB1, Nucleolin and others [35]. Two lines of genetic evidence demonstrate that CD24-Siglec G interaction is an important negative regulator in host response to DAMPs [35]. First, targeted mutation of either CD24 or Siglec G greatly increased NFκb activation and production of inflammatory cytokines by bone marrow-derived dendritic cells to a variety of DAMPs, including HMGB1, HSP70 and HSP90. Second, mice with targeted mutations of either CD24 or Siglecg genes exhibit much higher susceptibility to acetaminophen-induced liver injury. Since the increased susceptibility is abrogated by anti-HMGB1 mAb, CD24-Siglec G interaction is a negative regulator for host response to DAMPs.
Discriminating DAMPs from PAMPs by sialoside-based pattern recognition
It has been largely overlooked that identification of endogenous ligands for TLR and NLR makes it difficult for one to use these receptor to discriminate “infectious nonself from noninfectious self”, as originally envisioned by Charles Janeway. One way to reconcile the Janeway concept with host response to DAMPs is to propose a selective regulatory pathway to discriminate DAMPs from PAMPs. Chen et al. [35] reported that CD24-Siglec G interaction repressed inflammatory responses to HMGB1 and HSP70, 90, but not LPS and PolyI:C, the prototypic PAMPs that stimulate TLR4 and TLR3, respectively. If this selectivity can be extended to other DAMPs and PAMPs, the sialoside-based pattern recognition may be used to discriminate DAMPs from PAMPs.
An important feature of sialoside-based pattern recognition is its susceptibility to sialidase. Since sialidasesare is commonly expressed by pathogens [38], the host may mount a stronger inflammatory response during infection as the sialoside-based negative regulatory mechanism may be disarmed by pathogen sialidase. In addition, at least 4 sialidases, Neu1-4, are expressed in mammalian cells. Neu3, the only known cell-surface sialidase, is over-expressed in a number of cancer tissues [39–43]. Over-expression of sialidase in cancer cells may provide a mechanism to exacerbate inflammatory response under pathological conditions, thus providing a second mechanism to discriminate “infectious nonself from noninfectious self”.
Since mimicry is a sincere form of flattery, one may get a glimpse of the significance of sialoside-based negative regulation through the molecular mimicry by survivors of the immune system, i.e. pathogens and cancer cells. While machinery of sialyation is believed to be evolved in higher organisms, a number of pathogenic bacteria are known to have acquired either the enzymes to synthesize the sialosides or pre-synthesized sialosides from the host [32]. Carlin et al. [49] demonstrated that interaction between sialyated capsular polysaccharide of group B Streptococcus and Siglec 9 dampened the neutrophil defense. Moreover, cancers are known to over-express sialyated glycan either on cell surface (STn, MUC1) or as secreted forms (Soluble mucin 16, CA125). Since these markers have been used for cancer diagnosis, it is of interest to consider whether they may represent a mechanism of immune evasion by cancer. Belisle et al demonstrated [50] that mucin secreted by cancer cells also binds to Siglec 9, a putative negative regulator for activation of neutrophil [49], monocytes and NK cells [51].
Concluding remarks
Taken together, the new model, as illustrated in Fig. 1, envisions two mechanisms to explain how a stronger inflammatory response may be induced following infection. On the one hand, the sialoside-based pattern recognition down-regulates host responses to DAMPs without affecting those to PAMPs. On the other hand, pathogen sialdases may exacerbate inflammation during infection by disrupting CD24-Siglec G/10 interaction. Both mechanisms, working together, enable the pattern recognition receptors to distinguish “infectious nonself from noninfectious self”. The recent discoveries of genetic control of autoimmune diseases either by CD24 [44–47] or by sialic acid acetylesterase [48] suggest dysregulation of this pathway as a cause for autoimmune diseases.
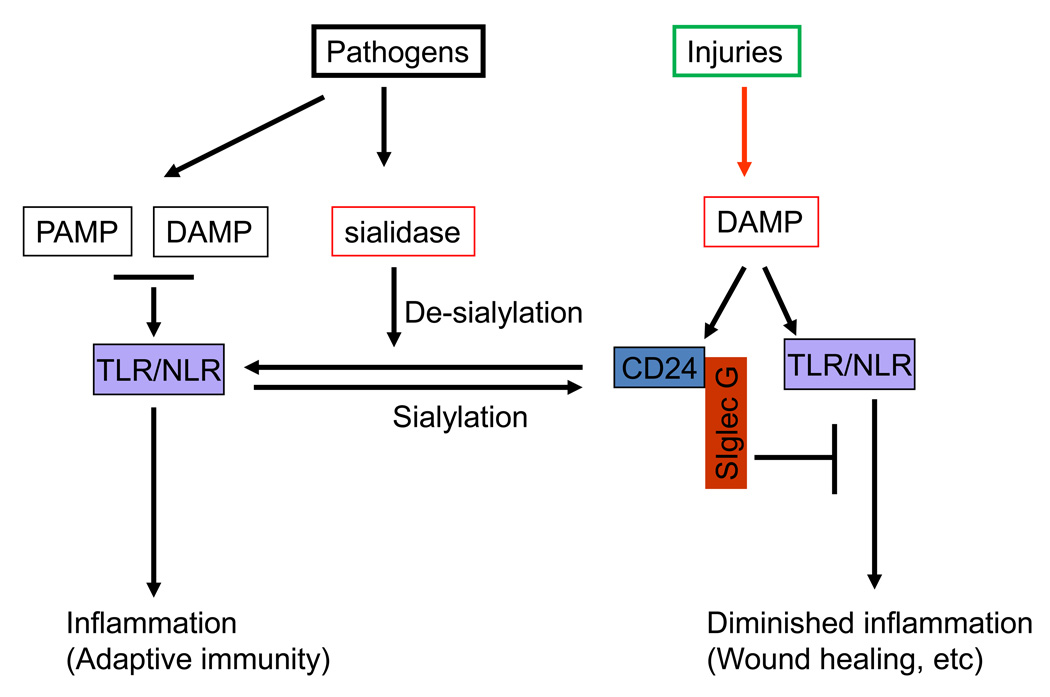
Fig. 1.
Sialoside-based pattern recognition discriminates infections from aseptic tissue injuries by two possible mechanisms. First, CD24 forms trimolecular complex with DAMPs and Siglec G that inhibits activation of TLR/NLR. Second, pathogen-encoded sialidases prevent CD24 from interacting with Siglec G. As a result, DAMPs and PAMPs become indistinguishable during infection.
Acknowledgement
We thank Ms. Darla Kroft for editorial assistance. This work is supported by grants from National Institutes of Health, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest.
References
- 1.Janeway CA. Approaching the asymptote? Evolution and revolution in immunology; Cold Spring Harb Symp Quant Biol; 1989. pp. 1–13. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunology today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Matzinger P. Tolerance, danger, and the extended family. Annual review of immunology. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 5.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 6.Millar DG, Garza KM, Odermatt B, Elford AR, Ono N, Li Z, et al. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nature medicine. 2003;9:1469–1476. doi: 10.1038/nm962. [DOI] [PubMed] [Google Scholar]
- 7. Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. The authors showed that a variety of crystals, including those of silican, asbestos, monosodium urate, activate inflammasome and induces intracellular ROS levels. This is the first demonstration that crystals are a new form of DAMPs.
- 8.Germani A, Limana F, Capogrossi MC. Pivotal advances: high-mobility group box 1 protein--a cytokine with a role in cardiac repair. J Leukoc Biol. 2007;81:41–45. doi: 10.1189/jlb.0306165. [DOI] [PubMed] [Google Scholar]
- 9.Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, et al. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. The Journal of cell biology. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, et al. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008;8:372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. The authors reported high levels of mitochondrial DNA in trauma patients and demonstrate that the DNA activate neutrophil by TLR9-dependent pathway. The study demonstrate mitochondrial DNA as a new category of DAMPs.
- 15.Crow YJ, Rehwinkel J. Aicardi-Goutieres syndrome and related phenotypes: linking nucleic acid metabolism with autoimmunity. Hum Mol Genet. 2009;18:R130–R136. doi: 10.1093/hmg/ddp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. The authors demonstrated the the Acardi-Goutieres Syndrome is an autoimmune disease initiated by IRF3-dependent pathway. More importantly, the authors showed that Trex1 prevents autoimmune diseases by degradation of retroviral elements in normal cells.
- 17.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature immunology. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. The authors demonstrated the existence of cholesterol crystals at early stage of atheroclerosis in animal and demonstrated the pathogenic role of the crystal-induced activation of inflammasome. This study provides a new insights in the pathogenesis of atherosclerosis.
- 20. Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. An interesting opinion piece that proposes cellular ROS as the common cause of inflammasome activation.
- 21. Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. This work suggests activation of inflammasome as the basis of adjuvant activity of alum. Since the alum is the adjuvant of choice for human vaccines, the new hypothesis provides a direct link between pattern recognition and immunogenicity.
- 22.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 24.McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N, Kappler JW, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxidemediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptormediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 27.Powell LD, Whiteheart SW, Hart GW. Cell surface sialic acid influences tumor cell recognition in the mixed lymphocyte reaction. J Immunol. 1987;139:262–270. [PubMed] [Google Scholar]
- 28.Jarahian M, Watzl C, Fournier P, Arnold A, Djandji D, Zahedi S, et al. Activation of natural killer cells by Newcastle disease virus hemagglutinin-neuraminidase. J Virol. 2009;83:8108–8121. doi: 10.1128/JVI.00211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller E, Schroder C, Schauer R, Sharon N. Binding and phagocytosis of sialidase-treated rat erythrocytes by a mechanism independent of opsonins. Hoppe Seylers Z Physiol Chem. 1983;364:1419–1429. doi: 10.1515/bchm2.1983.364.2.1419. [DOI] [PubMed] [Google Scholar]
- 30.Crocker PR, Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989;169:1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crocker PR, Clark EA, Filbin M, Gordon S, Jones Y, Kehrl JH, et al. Siglecs: a family of sialic-acid binding lectins. Glycobiology. 1998;8:v. doi: 10.1093/oxfordjournals.glycob.a018832. [DOI] [PubMed] [Google Scholar]
- 32.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 33. Ding C, Liu Y, Wang Y, Park BK, Wang CY, Zheng P, et al. Siglecg limits the size of B1a B cell lineage by down-regulating NFkappaB activation. PloS one. 2007;2 doi: 10.1371/journal.pone.0000997. e997. This paper demonstrates a physiological function of Siglec G in development of B1B cells and provides the first link between Siglec G and NFkappaB activation.
- 34. Hoffmann A, Kerr S, Jellusova J, Zhang J, Weisel F, Wellmann U, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nature immunology. 2007;8:695–704. doi: 10.1038/ni1480. These papers provide the first demonstration that Siglec G mediates inhibitory signaling and suppresses B1 B cell development.
- 35. Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 Selectively Repress Tissue Damage-Induced Immune Responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. Using a liver injury model, the authors showed that mice with targeted mutations of either CD24 or Siglecg genes mounted fatal inflammatory response to acetaminophen-induced liver injury. Moreover, the authors demonstrated that CD24 formed trimolecular complex with Siglec G to inhibit inflammatory response to DAMPs. By ignoring host response to PAMPs, the data suggest a role for CD24-Siglec G interaction in discriminating DAMPs from PAMPs.
- 36.Motari E, Zheng X, Su X, Liu Y, Kvaratskhelia M, Freitas M, et al. Analysis of Recombinant CD24 Glycans by MALDI-TOF-MS Reveals Prevalence of Sialyl-T Antigen. American journal of biomedical sciences. 2009;1:1–11. doi: 10.5099/aj090100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bleckmann C, Geyer H, Reinhold V, Lieberoth A, Schachner M, Kleene R, et al. Glycomic analysis of N-linked carbohydrate epitopes from CD24 of mouse brain. J Proteome Res. 2009;8:567–582. doi: 10.1021/pr800729r. [DOI] [PubMed] [Google Scholar]
- 38.Vimr ER. Microbial sialidases: does bigger always mean better? Trends Microbiol. 1994;2:271–277. doi: 10.1016/0966-842x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 39.Kakugawa Y, Wada T, Yamaguchi K, Yamanami H, Ouchi K, Sato I, et al. Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10718–10723. doi: 10.1073/pnas.152597199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyagi T, Wada T, Yamaguchi K, Shiozaki K, Sato I, Kakugawa Y, et al. Human sialidase as a cancer marker. Proteomics. 2008;8:3303–3311. doi: 10.1002/pmic.200800248. [DOI] [PubMed] [Google Scholar]
- 41.Ueno S, Saito S, Wada T, Yamaguchi K, Satoh M, Arai Y, et al. Plasma membrane-associated sialidase is up-regulated in renal cell carcinoma and promotes interleukin-6-induced apoptosis suppression and cell motility. J Biol Chem. 2006;281:7756–7764. doi: 10.1074/jbc.M509668200. [DOI] [PubMed] [Google Scholar]
- 42.Valaperta R, Valsecchi M, Rocchetta F, Aureli M, Prioni S, Prinetti A, et al. Induction of axonal differentiation by silencing plasma membrane-associated sialidase Neu3 in neuroblastoma cells. Journal of neurochemistry. 2007;100:708–719. doi: 10.1111/j.1471-4159.2006.04279.x. [DOI] [PubMed] [Google Scholar]
- 43.Nomura H, Tamada Y, Miyagi T, Suzuki A, Taira M, Suzuki N, et al. Expression of NEU3 (plasma membrane-associated sialidase) in clear cell adenocarcinoma of the ovary: its relationship with T factor of pTNM classification. Oncology research. 2006;16:289–297. doi: 10.3727/000000006783981035. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez E, Abelson AK, Sabio JM, Gonzalez-Gay MA, Ortego-Centeno N, Jimenez-Alonso J, et al. Association of a CD24 gene polymorphism with susceptibility to systemic lupus erythematosus. Arthritis Rheum. 2007;56:3080–3086. doi: 10.1002/art.22871. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez E, Fernandez-Gutierrez B, Gonzalez-Gay MA, Balsa A, Garcia A, Rodriguez L, et al. Investigating the role of CD24 gene polymorphisms in rheumatoid arthritis. Ann Rheum Dis. 2008;67:1197–1198. doi: 10.1136/ard.2007.084475. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Lin S, Rammohan K, Liu Z, Liu J, Liu R-H, et al. A di-nucleotide deletion in CD24 confers protection against autoimmune diseases. PLoS genetics. 2007;3 doi: 10.1371/journal.pgen.0030049. e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Q, Rammohan K, Lin S, Robinson N, Li O, Liu X, et al. CD24 is a genetic modifier for risk and progression of multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15041–15046. doi: 10.1073/pnas.2533866100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surolia I, Pirnie SP, Chellappa V, Taylor KN, Cariappa A, Moya J, et al. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature. 2010;466:243–247. doi: 10.1038/nature09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and DAMPsen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. The authors showed that sialyated bacterial capsular polysaccharide inhibits neutrophil responses to group B Streptococcus. The molecular mimicry provides another evidence for the power of sialoside-based negative regulation in innate immune response.
- 50.Belisle JA, Horibata S, Jennifer GA, Petrie S, Kapur A, Andre S, et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol Cancer. 2010;9:118. doi: 10.1186/1476-4598-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Avril T, Floyd H, Lopez F, Vivier E, Crocker PR. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol. 2004;173:6841–6849. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]